Abstract
Several studies have shown that electroconvulsive therapy (ECT) results in increased hippocampal volume. It is likely that a multitude of mechanisms including neurogenesis, gliogenesis, synaptogenesis, angiogenesis, and vasculogenesis contribute to this volume increase. Neurotrophins, like vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) seem to play a crucial mediating role in several of these mechanisms. We hypothesized that two regulatory SNPs in the VEGF and BDNF gene influence the changes in hippocampal volume following ECT. We combined genotyping and brain MRI assessment in a sample of older adults suffering from major depressive disorder to test this hypothesis. Our results show an effect of rs699947 (in the promotor region of VEGF) on hippocampal volume changes following ECT. However, we did not find a clear effect of rs6265 (in BDNF). To the best of our knowledge, this is the first study investigating possible genetic mechanisms involved in hippocampal volume change during ECT treatment.
Similar content being viewed by others
Introduction
Electroconvulsive therapy (ECT) is the most effective biological treatment for major depressive disorder1. A higher age is associated with a better outcome2,3. Despite the proven excellent effectiveness of this treatment, much of the exact working mechanisms of ECT remain largely unknown4.
Several studies, both in late-life depression and younger adults have shown that ECT results in an increased hippocampal volume5,6. This increase seems to be dose-dependent, with a higher number of ECT sessions resulting in more pronounced enlargement7. Several mechanisms may play a role in these hippocampal volume changes. Animal models have shown an electroconvulsive shock (ECS)-related increase in neurogenesis and synapse formation8,9,10, angiogenesis and proliferation of endothelial cells11,12,13,14,15,16,17 and gliogenesis18,19. Human studies have shown changes in regional blood flow20,21 and immunological and inflammatory responses following ECT22. Rather than one single mechanism, it is more likely that a multitude of mechanisms including neurogenesis, gliogenesis, synaptogenesis, angiogenesis, and vasculogenesis contribute to the increase in hippocampal volume following ECT, as there is a close interconnection between these systems23. For example, endothelial cells can influence neurogenesis by secreting factors, which act on neural stem and progenitor cells15. An increase in the number of synapses and changes in dendritic structures might be accompanied by growth of both capillaries and glia24, perhaps to support increased energy demand of the new synapses25.
Neurotrophins, like vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) seem to play a crucial mediating role in several of these mechanisms that result in hippocampal volume changes. VEGF is the most potent inducer of blood vessel growth26. It is also expressed in the brain, and has an important role in promoting neurogenesis, neuronal migration, neuronal survival and axon guidance27,28,29. VEGF has been linked to antidepressant treatment response30. An association between low baseline serum VEGF levels and non-response to ECT has been found31. In a recent study a moderate negative correlation between the change in VEGF plasma levels and change in depression severity following bitemporal (BT) ECT was identified, however, no other associations between VEGF and mood or responder/remitter status were found32.
The expression of VEGF can be regulated by certain single nucleotide polymorphisms (SNPs). The common SNP rs699947 in the promotor region of the VEGF gene (also known as 2578 C/A) significantly affects serum levels of VEGF33. Moreover, an association between the VEGF 2578 C/A polymorphism and treatment-resistant depression was reported34. An impact of this polymorphism on total grey and total white matter volume and on total arterial blood volume in the brain was shown35.
BDNF is an important neurotrophin and plays a key role in neuronal development and neuroplasticity36. BDNF is consistently low in patients with major depression37, nevertheless, it lacks diagnostic specificity as it can be reduced in other psychiatric disorders as well38,39,40,41. In animal studies it was shown that chronic ECS induces hippocampal mossy fiber sprouting, and that the expression of BDNF seems to play a crucial role in the induction of this sprouting42,43.
Rs6265 is a widely studied SNP in the BDNF gene. As a result of a G(uanine) to A(denine) substitution in the gene at this position, a valine (val) to methionine (met) substitution in the 5’ proregion of the BDNF protein occurs, resulting in a reduced mature BDNF expression in met-carriers44. The met-variant is more common in late-life depression45,46 and is associated with smaller hippocampal volumes in both patients with major depression and healthy controls47,48. Peripheral BDNF levels have shown to increase after ECT in some but not all studies49.
We hypothesize that these two regulatory SNPs in two important neurotrophins influence the changes in hippocampal volume during ECT treatment. We combined genotyping and brain MRI assessment in a sample of older adults suffering from major depressive disorder to test this hypothesis.
Materials and methods
Subjects and characteristics
We included all subjects that participated in the Mood Disorders in Elderly treated with ECT (MODECT) study50 (clinical trial NCT02667353) and from whom genetic material as well as MRI scans pre- and post-ECT were available (61 subjects of total MODECT sample (n = 110), University Psychiatric Center KU Leuven, Belgium (n = 31) and GGZ inGeest Amsterdam, the Netherlands (n = 30)). All subjects were 55 or older and had a DSM-IV diagnosis of major depressive disorder, with or without psychotic features. The diagnosis was made by a psychiatrist and confirmed by the Mini International Neuropsychiatric Interview (MINI)51. Exclusion criteria were other major psychiatric illness, a major neurological illness (including Parkinson’s disease, stroke and dementia) and contraindications for MRI. Psychotropic medication was discontinued at least 1 week prior to ECT, or if deemed impossible, kept stable from 6 weeks prior to ECT and during the ECT-course50.
Depressive symptoms were monitored 1 week prior to ECT and 1 week after the last ECT using the Montgomery-Åsberg Depression Rating Scale (MADRS)52. MADRS scores before and after ECT were available for 58 subjects.
All patients provided written informed consent. The ethical committees of the two centers approved this study.
ECT procedure
ECT was administered twice a week with a constant-current brief pulse device (Thymaton System IV, Somatics, IL, USA). Anesthesia was achieved with intravenous administration of etomidate (0.2 mg/kg) and succinylcholine (1 mg/kg). Seizures were monitored to ensure adequate duration and quality. All subjects were treated with right unilateral (RUL) ECT with stimulus intensity 6 times the initial seizure threshold (ST), as determined by empirical dose titration at the first treatment, until remission. Subjects who failed to respond to RUL after the sixth treatment were switched to bitemporal (BT) ECT (1.5xST)50. All patients were treated with brief pulse (0.5–1.0 ms) ECT.
MRI procedure
Structural MRI was performed 1 week prior to ECT and 1 week after the last ECT. High resolution 3D T1-weighted images were acquired using an 8-channel head-coil with a 3D turbo field echo sequence on a 3T Philips Intera scanner in Leuven and on a 3T GE Signa HDxt scanner in Amsterdam (TR = 9.6 s, TE = 4.6 s, flip angle = 8°, slice thickness = 1.2 mm, in-plane voxel-size = 0.98 × 1.2 mm3, 182 slices, acquisition time = 383 s).
Hippocampal volume
A trained rater blinded to time-point manually delineated the hippocampus in native space following an initial automatic segmentation step53. Manual editing was performed using ITK-SNAP version 2.4 (http://www.itksnap.org/pmwiki/pmwiki.php) in accordance with the HarP guidelines54. Hippocampal volumes were normalized based on total brain volume55, using a standard approach56.
Genotyping of polymorphisms
Genomic DNA was extracted from peripheral blood using standard methods. The SNP rs6265 in BDNF and rs699947 in the promotor region of VEGF were genotyped by a real-time polymerase chain reaction with melting curve analysis using a LichtCycler 480 II instrument (Roche, Penzberg, Germany). For each SNP a LightSNiP kit (TIB Molbiol, Berlin, Germany) was used with pre-mixed primer and probes specific for each SNP. Melting temperatures were called using Lightcycler 480 Software 1.5.1 (Roche, Penzberg, Germany).
Statistical analyses
Statistical analyses were performed using SPSS Statistics 25 (IBM, Chicago, IL, USA).
Differences in demographic or clinical variables (age, sex, RUL and BT ECT, late onset of depression, baseline hippocampal volume) between rs699947 and rs6265 genotype groups were analyzed using a t-test (for age and baseline hippocampal volume) or chi-square test (for sex, RUL/BT ECT, late onset of depression).
Differences in hippocampal volume and MADRS scores pre- and post- ECT were analyzed with a paired samples t-test.
Association between rs699947 and rs6265 genotypes and changes in normalized total hippocampal volume and association between SNP genotypes and change in MADRS scores, were analyzed using one-way ANOVA, followed by one-way analysis of covariance (ANCOVA) to control for number of ECTs. Kolmogorov–Smirnov test showed normal distribution of residuals and Brown–Forsythe test showed homogeneity of variance across different genotype groups for both SNPs. Bonferroni correction was applied for multiple testing on the number of SNPs tested ( = 2). As our group has identified lateralization of volume changes following ECT57, post-hoc we also analyzed changes in right and left hippocampal volume separately.
Association between hippocampal volume changes and change in MADRS scores was analyzed using linear regression. Assumptions for linear regression were met.
The significance level for statistical tests was set at p < 0.05.
Results
Demographic and clinical characteristics
The mean age of the 61 subjects included was 72.3 (range 55–90; SD = 8.6) with 37 female and 24 male subjects. 17 subjects, who failed to respond initially to RUL ECT were switched after the sixth session to BT ECT. 29 subjects had a first depressive episode after the age of 55 (“late onset”). The distribution of the genotypes were: for rs699947 (SNP in the promotor region of VEGF) C/C in 15 subjects, C/A in 29 subjects and A/A in 17 subjects; for rs6265 (SNP in BDNF) G/G in 30 subjects, G/A in 25 subjects, A/A in 6 subjects. There were no significant group differences, in sex, age, RUL/BT ECT, onset of depression and baseline hippocampal volumes, between the 3 genotype groups of both SNPs.
Association between SNP genotypes and change in hippocampal volume
There was a significant increase in normalized total hippocampal volume following ECT (pre-ECT: M = 6794.18 mm3, SD = 767.98; post-ECT: M = 6978.56 mm3, SD = 864.55; t(60) = 4.344, p = 0.000055).
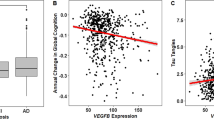
A significant association was observed between rs699947 (SNP in the promotor region of VEGF) genotype and change in normalized total hippocampal volume (F(2,58) = 4.799, p = 0.012). When corrected for number of ECT sessions this association remained significant (F(2,57) = 3.516, p = 0.021). After Bonferroni correction for testing two SNPs, the association remained significant (p < 0.025). The effect size of the association between rs699947 genotype and changes in normalized total hippocampal volume was η2 = 0.14, and after correction for number of ECT sessions η2 = 0.16. Post hoc pairwise comparisons using the Tukey HSD test revealed that normalized total hippocampal volume increased significantly less following ECT (p = 0.009) in the A/A genotype group (M = 33.00 mm3, SD = 315.57), compared to the C/C genotype group (M = 374.80 mm3, SD = 344.42). Increase in normalized total hippocampal volume in the C/A genotype group was intermediate (M = 174.62 mm3, SD = 293.01) between, but not significantly different from, the C/C and A/A genotype group (p = 0.118 and p = 0.306, respectively). Post hoc comparison of the estimated marginal means after ANCOVA (with the number of ECT sessions as covariate) with Bonferroni correction again showed significantly lower increase of normalized total hippocampal volume in the A/A group compared to the C/C group (p = 0.013) and increase in the C/A group being intermediate between, but not statistically significantly different from, the C/C and A/A group (p = 0.142 and p = 0.545, respectively). The association between rs699947 genotype and normalized total hippocampal volume change is illustrated in Fig. 1.
We could not find a significant association between rs6265 (SNP in BDNF) and change in normalized total hippocampal volume (F(2,58) = 3.066, p = 0.054). When corrected for number of ECT sessions this association weakened further (F(2,57) = 2.263, p = 0.091).
As the volume increase was more pronounced on the right side (pre-ECT: M = 3431.24 mm3, SD = 411.80; post-ECT: M = 3553.68 mm3, SD = 465.23; t(60) = 5.095, p = 0.000004) versus the left side (pre-ECT: M = 3362.94 mm3, SD = 400.62; post-ECT: M = 3424.88 mm3, SD = 428.39; t(60) = 2.373, p = 0.021), we then looked at the association with volume changes of the right and left hippocampus separately. For rs699947 we found a strong association with changes in normalized right hippocampal volume (F(2,58) = 6.132, p = 0.004), which remained significant after correction for number of ECT sessions (F(2,57) = 4.043, p = 0.011), but no significant association with left hippocampal volume changes (F(2,58) = 1.943, p = 0.152; after correction for number of ECT sessions: F(2,57) = 2.393, p = 0.078). The effect size of the association between rs699947 genotype and changes in normalized right hippocampal volume was η2 = 0.18, and after correction for number of ECT sessions remained η2 = 0.18. Post hoc pairwise comparisons using the Tukey HSD test revealed that normalized right hippocampal volume increased significantly less (p = 0.003) in the A/A genotype group (M = 12.29 mm3, SD = 194.88), compared to the C/C genotype group (M = 225.40 mm3, SD = 198.17). Increase in normalized right hippocampal volume in the C/A genotype group was intermediate (M = 133.76 mm3, SD = 144.84) between, but not significantly different from, the C/C and A/A genotype group (p = 0.229 and p = 0.065, respectively). Post hoc comparison of the estimated marginal means after ANCOVA (with the number of ECT sessions as covariate) with Bonferroni correction again showed significantly lower increase of normalized right hippocampal volume in the A/A group compared to the C/C group (p = 0.003) and increase in the C/A group being intermediate between, but not statistically significantly different from, the C/C and A/A group (p = 0.317 and p = 0.078, respectively).
For rs6265 we did not find an association between changes in normalized right (F(2,58) = 1.799, p = 0.175; after correction for number of ECT sessions: F(2,57) = 1.199, p = 0.318) nor left (F(2,57) = 2.493, p = 0.091; after correction for number of ECT sessions: F(2,57) = 2.546, p = 0.065) hippocampal volume.
Clinical outcome and association with SNP genotypes and hippocampal volume changes
There was a significant decrease in MADRS scores following ECT (pre-ECT: M = 34.31, SD = 9.40; post-ECT: M = 9.93, SD = 9.83; t(57) = 14.806, p = 3.45 × 10−21).
No association between genotypes of the 2 SNPs and change in MADRS score prior versus after ECT could be found (for rs699947: F(2,55) = 0.262, p = 0.770; after correction for number of ECT sessions F(2,53) = 0.778, p = 0.545; for rs6265: F(2,55) = 0.166, p = 0.847; after correction for number of ECT sessions F(2,53) = 0.717, p = 0.584).
Simple linear regression showed that changes in normalized hippocampal volumes did not significantly predict change in MADRS score (for total hippocampal volume R2 = 0.001, F(1,56) = 0.042, p = 0.838; for right hippocampal volume: R2 = 0.0001, F(1,56) = 0.007, p = 0.933; for left hippocampal volume: R2 = 0.003, F(1,56) = 0.165, p = 0.686).
Discussion
To the best of our knowledge, this is the first study investigating possible genetic mechanisms involved in hippocampal volume change following ECT.
In line with our hypothesis, our results show an effect of rs699947 genotype on hippocampal volume changes during ECT treatment. Patients with more C alleles tend to have more hippocampal volume increase. The C allele is regarded as the “wild-type” allele, and it was suggested previously that C-carriers have higher VEGF expression than non-carriers33.
Animal models of ECT (ECS) have shown upregulation of VEGF and this upregulation was most prominent in the dentate gyrus of the hippocampus58. Interestingly, several recent studies highlighted that ECT seems to consistently lead to volume increase in the dentate gyrus subfield59,60,61. It was demonstrated before that VEGF-signaling is necessary and sufficient for ECS-related induction of quiescent neural progenitor cell proliferation in rats16. VEGF expression strongly stimulates angiogenesis. VEGF is known to also increase proliferation of other cells like neurons, astroglia and endothelial cells27. An animal study showed that VEGF is an essential mediator in restoration of neurogenesis by electroconvulsive seizure after irradiation exposure17. Considering these previous findings, our results suggest that the more C alleles at rs699947, the higher the expression of VEGF during ECT treatment, which in turn leads to angiogenesis and neurogenesis, thus leading to more increase in hippocampal volume.
The effect of rs699947 genotype on hippocampal volume change following ECT seems to be largest on the right hippocampus. This is probably due to all patients initially receiving RUL ECT. The changes in total hippocampal volume during ECT in this sample are mainly due to changes in the volume of the right hippocampus. This lateralization could be explained by higher electric field/exposure leading to more structural changes62.
Contrary to our hypothesis, we did not find a clear effect of the rs6265 (in BDNF) genotype on hippocampal volume changes.
The association of the BDNF Val66Met polymorphism with major depressive disorder remains unclear63. In a previous study in a geriatric population no significant association between the BDNF Val66Met polymorphism and hippocampal volume or function was found64. The authors hypothesized that other factors may have a stronger effect on hippocampal structure in older individuals and that the association between the Val66Met polymorphism and geriatric depression is mediated through other mechanisms. Our group and other groups also found no significant changes in mean serum BDNF levels following ECT treatment65,66,67,68,69. On the other hand it is possible that our sample was too small to detect an effect, with a low number of patients with the A/A genotype (n = 6).
In line with what we reported in the larger MODECT study69, we did not find an association between hippocampal volume change and change in MADRS score following ECT in this sample. However, some studies did establish a positive correlation between hippocampal volume change and clinical outcome61,70,71. Several other studies did not find this effect or identified even a negative correlation7,69,72,73,74,75. It is possible that hippocampal volume increase following ECT is related more to the electrical current or to the seizure aspect of the treatment, rather than to its therapeutic effects7. Larger studies are therefore needed to elucidate the clinical effects of hippocampal volume change following ECT and the role of genetic mechanisms in mediating these effects.
To conclude, our study provides first-in-human evidence that VEGF genotype is involved in structural brain changes following ECT and warrants further research into the role of genetic mediators of neural effects induced by brain stimulation techniques.
References
Kho, K. H., van Vreeswijk, M. F., Simpson, S. & Zwinderman, A. H. A meta-analysis of electroconvulsive therapy efficacy in depression. J. Ect 19, 139–147 (2003).
Haq, A. U., Sitzmann, A. F., Goldman, M. L., Maixner, D. F. & Mickey, B. J. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J. Clin. Psychiatry 76, 1374–1384 (2015).
van Diermen, L. et al. Prediction of electroconvulsive therapy response and remission in major depression: meta-analysis. Br. J. Psychiatry 212, 71–80 (2018).
Sienaert, P. Mechanisms of ECT: reviewing the science and dismissing the myths. J. Ect 30, 85–86 (2014).
Gbyl, K. & Videbech, P. Electroconvulsive therapy increases brain volume in major depression: a systematic review and meta-analysis. Acta Psychiatr. Scand. 138, 180–195 (2018).
Takamiya, A. et al. Effect of electroconvulsive therapy on hippocampal and amygdala volumes: systematic review and meta-analysis. Br. J. Psychiatry 212, 19–26 (2018).
Oltedal, L. et al. Volume of the Human Hippocampus and Clinical Response Following Electroconvulsive Therapy. Biol. Psychiatry 84, 574–581 (2018).
Chen, F. H., Madsen, T. M., Wegener, G. & Nyengaard, J. R. Repeated electroconvulsive seizures increase the total number of synapses in adult mate rat hippocampus. Eur. Neuropsychopharm 19, 329–338 (2009).
Scott, B. W., Wojtowicz, J. M. & Burnham, W. M. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp. Neurol. 165, 231–236 (2000).
Olesen, M. V., Wortwein, G., Folke, J. & Pakkenberg, B. Electroconvulsive stimulation results in long-term survival of newly generated hippocampal neurons in rats. Hippocampus 27, 52–60 (2017).
Ekstrand, J., Hellsten, J., Wennstrom, M. & Tingstrom, A. Differential inhibition of neurogenesis and angiogenesis by corticosterone in rats stimulated with electroconvulsive seizures. Prog. Neuro-Psychoph 32, 1466–1472 (2008).
Hellsten, J., Wennstrom, M., Bengzon, J., Mohapel, P. & Tingstrom, A. Electroconvulsive seizures induce endothelial cell proliferation in adult rat hippocampus. Biol. Psychiat 55, 420–427 (2004).
Hellsten, J. et al. Electroconvulsive seizures induce angiogenesis in adult rat hippocampus. Biol. Psychiat 58, 871–878 (2005).
Jansson, L., Hellsten, J. & Tingstrom, A. Region specific hypothalamic neuronal activation and endothelial cell proliferation in response to electroconvulsive seizures. Biol. Psychiat 60, 874–881 (2006).
Newton, S. S., Girgenti, M. J., Collier, E. F. & Duman, R. S. Electroconvulsive seizure increases adult hippocampal angiogenesis in rats. Eur. J. Neurosci. 24, 819–828 (2006).
Segi-Nishida, E., Warner-Schmidt, J. L. & Duman, R. S. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc. Natl. Acad. Sci. USA. 105, 11352–11357 (2008).
Warner-Schmidt, J. L., Madsen, T. M. & Duman, R. S. Electroconvulsive seizure restores neurogenesis and hippocampus-dependent fear memory after disruption by irradiation. Eur. J. Neurosci. 27, 1485–1493 (2008).
Jansson, L., Wennstrom, M., Johanson, A. & Tingstrom, A. Glial cell activation in response to electroconvulsive seizures. Prog. Neuro-Psychoph 33, 1119–1128 (2009).
Wennstrom, M., Hellsten, J. & Tingstrom, A. Electroconvulsive seizures induce proliferation of NG2-expressing glial cells in adult rat amygdala. Biol. Psychiat 55, 464–471 (2004).
Milo, T. J. et al. Changes in regional cerebral blood flow after electroconvulsive therapy for depression. J. Ect 17, 15–21 (2001).
Takano, H. et al. Changes in regional cerebral blood flow during acute electroconvulsive therapy in patients with depression - Positron emission tomographic study. Brit J. Psychiat 190, 63–68 (2007).
Yrondi, A. et al. Electroconvulsive therapy, depression, the immune system and inflammation: a systematic review. Brain Stimul. 11, 29–51 (2018).
Bouckaert, F. et al. ECT: its brain enabling effects: a review of electroconvulsive therapy-induced structural brain plasticity. J. Ect 30, 143–151 (2014).
Anderson, B. J. et al. Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia 11, 73–80 (1994).
Lovden, M., Wenger, E., Martensson, J., Lindenberger, U. & Backman, L. Structural brain plasticity in adult learning and development. Neurosci. Biobehav Rev. 37, 2296–2310 (2013).
Olsson, A. K., Dimberg, A., Kreuger, J. & Claesson-Welsh, L. VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 7, 359–371 (2006).
Mackenzie, F. & Ruhrberg, C. Diverse roles for VEGF-A in the nervous system. Development 139, 1371–1380 (2012).
Storkebaum, E., Lambrechts, D. & Carmeliet, P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays 26, 943–954 (2004).
Greenberg, D. A. & Jin, K. Experiencing VEGF. Nat. Genet 36, 792–793 (2004).
Warner-Schmidt, J. L. & Duman, R. S. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc. Natl Acad. Sci. USA 104, 4647–4652 (2007).
Minelli, A. et al. Association between baseline serum vascular endothelial growth factor levels and response to electroconvulsive therapy. Acta Psychiat Scand. 129, 461–466 (2014).
Ryan, K. M. & McLoughlin, D. M. Vascular endothelial growth factor plasma levels in depression and following electroconvulsive therapy. Eur. Arch. Psy Clin. N. 268, 839–848 (2018).
Steffensen, K. D., Waldstrom, M., Brandslund, I. & Jakobsen, A. The relationship of VEGF polymorphisms with serum VEGF levels and progression-free survival in patients with epithelial ovarian cancer. Gynecol. Oncol. 117, 109–116 (2010).
Viikki, M. et al. Vascular endothelial growth factor (VEGF) polymorphism is associated with treatment resistant depression. Neurosci. Lett. 477, 105–108 (2010).
Takeuchi, H. et al. The VEGF gene polymorphism impacts brain volume and arterial blood volume. Hum. Brain Mapp. 38, 3516–3526 (2017).
Sheikh, H. I., Hayden, E. P., Kryski, K. R., Smith, H. J. & Singh, S. M. Genotyping the BDNFrs6265 (val66met) polymorphism by one-step amplified refractory mutation system PCR. Psychiat Genet 20, 109–112 (2010).
Molendijk, M. L. et al. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol. Psychiatry 19, 791–800 (2014).
Green, M. J., Matheson, S. L., Shepherd, A., Weickert, C. S. & Carr, V. J. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol. Psychiatr. 16, 960–972 (2011).
Fernandes, B. S. et al. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis. J. Psychiatr. Res 45, 995–1004 (2011).
Monteleone, P. et al. Circulating brain-derived neurotrophic factor is decreased in women with anorexia and bulimia nervosa but not in women with binge-eating disorder: relationships to co-morbid depression, psychopathology and hormonal variables. Psychol. Med. 35, 897–905 (2005).
Molendijk, M. L. et al. Gender specific associations of serum levels of brain-derived neurotrophic factor in anxiety. World J. Biol. Psychiatry 13, 535–543 (2012).
Vaidya, V. A., Siuciak, J. A., Du, F. & Duman, R. S. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience 89, 157–166 (1999).
Chen, A. C., Shin, K. H., Duman, R. S. & Sanacora, G. ECS-Induced mossy fiber sprouting and BDNF expression are attenuated by ketamine pretreatment. J. Ect 17, 27–32 (2001).
Egan, M. F. et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 (2003).
Taylor, W. D. et al. Allelic differences in the brain-derived neurotrophic factor Val66Met polymorphism in late-life depression. Am. J. Geriatr. Psychiatry 15, 850–857 (2007).
Hwang, J. P. et al. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiol. Aging 27, 1834–1837 (2006).
Frodl, T. et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch. Gen. Psychiat 64, 410–416 (2007).
Pezawas, L. et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J. Neurosci. 24, 10099–10102 (2004).
Polyakova, M. et al. Brain-derived neurotrophic factor and antidepressive effect of electroconvulsive therapy: systematic review and meta-analyses of the preclinical and clinical literature. PLoS ONE 10, e0141564 (2015).
Dols, A. et al. Early- and late-onset depression in late life: a prospective study on clinical and structural brain characteristics and response to electroconvulsive therapy. Am. J. Geriatr. Psychiatry 25, 178–189 (2017).
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59(Suppl 20), 22–33 (1998). quiz 34–57.
Montgomery, S. A. & Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134, 382–389 (1979).
van der Lijn, F., den Heijer, T., Breteler, M. M. & Niessen, W. J. Hippocampus segmentation in MR images using atlas registration, voxel classification, and graph cuts. Neuroimage 43, 708–720 (2008).
Frisoni, G. B. et al. The EADC-ADNI Harmonized Protocol for manual hippocampal segmentation on magnetic resonance: evidence of validity. Alzheimers Dement 11, 111–125 (2015).
Jain, S. et al. Automatic segmentation and volumetry of multiple sclerosis brain lesions from MR images. Neuroimage-Clin. 8, 367–375 (2015).
Jack, C. R. et al. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology 172, 549–554 (1989).
Bouckaert, F. et al. Grey matter volume increase following electroconvulsive therapy in patients with late life depression: a longitudinal MRI study. J. Psychiatry Neurosci. 41, 105–114 (2016).
Newton, S. S. et al. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J. Neurosci. 23, 10841–10851 (2003).
Cao, B. et al. Predicting individual responses to the electroconvulsive therapy with hippocampal subfield volumes in major depression disorder. Sci. Rep. 8, 5434 (2018).
Gryglewski, G. et al. Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br. J. Psychiatry 214, 1–9 (2018).
Takamiya, A. et al. Acute and long-term effects of electroconvulsive therapy on human dentate gyrus. Neuropsychopharmacology 44, 1805–1811 (2019).
Lee, W. H. et al. Regional electric field induced by electroconvulsive therapy in a realistic finite element head model: Influence of white matter anisotropic conductivity. Neuroimage 59, 2110–2123 (2012).
Groves, J. O. Is it time to reassess the BDNF hypothesis of depression? Mol. Psychiatr. 12, 1079–1088 (2007).
Benjamin, S. et al. The brain-derived neurotrophic factor Val66Met polymorphism, hippocampal volume, and cognitive function in geriatric depression. Am. J. Geriatr. Psychiatry 18, 323–331 (2010).
Fernandes, B. et al. Serum brain-derived neurotrophic factor (BDNF) is not associated with response to electroconvulsive therapy (ECT): a pilot study in drug resistant depressed patients. Neurosci. Lett. 453, 195–198 (2009).
Gronli, O., Stensland, G. O., Wynn, R. & Olstad, R. Neurotrophic factors in serum following ECT: a pilot study. World J. Biol. Psychiatry 10, 295–301 (2009).
Gedge, L. et al. Effects of electroconvulsive therapy and repetitive transcranial magnetic stimulation on serum brain-derived neurotrophic factor levels in patients with depression. Front Psychiatry 3, 12 (2012).
Lin, C. H. et al. Electroconvulsive therapy improves clinical manifestation with plasma BDNF levels unchanged in treatment-resistant depression patients. Neuropsychobiology 68, 110–115 (2013).
Bouckaert, F. et al. Relationship between hippocampal volume, serum BDNF, and depression severity following electroconvulsive therapy in late-life depression. Neuropsychopharmacology 41, 2741–2748 (2016).
Dukart, J. et al. Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc. Natl. Acad. Sci. USA 111, 1156–1161 (2014).
Joshi, S. H. et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol. Psychiatry 79, 282–292 (2016).
Nordanskog, P. et al. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J. Ect 26, 62–67 (2010).
Nordanskog, P., Larsson, M. R., Larsson, E. M. & Johanson, A. Hippocampal volume in relation to clinical and cognitive outcome after electroconvulsive therapy in depression. Acta Psychiatr. Scand. 129, 303–311 (2014).
Abbott, C. C. et al. Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl. Psychiatry 4, e483 (2014).
Sartorius, A. et al. Electroconvulsive therapy induced gray matter increase is not necessarily correlated with clinical data in depressed patients. Brain Stimul. 12, 335–343 (2019).
Acknowledgements
This research was supported by Research Foundation Flanders (FWO) grant G0C0319N, KU Leuven Fund C24/18/095 and the Sequoia Fund for Research on Ageing and Mental Health. The authors thank Prof. Dr. Gert Matthijs for his support in genotyping of the polymorphisms.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Van Den Bossche, M.J.A., Emsell, L., Dols, A. et al. Hippocampal volume change following ECT is mediated by rs699947 in the promotor region of VEGF. Transl Psychiatry 9, 191 (2019). https://doi.org/10.1038/s41398-019-0530-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-019-0530-6
This article is cited by
-
How electroconvulsive therapy works in the treatment of depression: is it the seizure, the electricity, or both?
Neuropsychopharmacology (2024)
-
Electroconvulsive therapy, electric field, neuroplasticity, and clinical outcomes
Molecular Psychiatry (2022)
-
Neuromodulation-Based Stem Cell Therapy in Brain Repair: Recent Advances and Future Perspectives
Neuroscience Bulletin (2021)
-
Novel candidate genes for ECT response prediction—a pilot study analyzing the DNA methylome of depressed patients receiving electroconvulsive therapy
Clinical Epigenetics (2020)