Abstract
Genetic variation in the human serotonin transporter (5-HTT) has been linked to altered fear learning but the data are inconsistent and the mechanism is unclear. The present study investigated conditioned aversive learning in 5-HTT knockout (KO) mice while simultaneously recording neural network activity (theta oscillations) and hemodynamic responses (tissue oxygen delivery) from the amygdala, a brain region necessary for forming fearful memories. Conditioned aversive learning was measured using a discrimination learning task in which one auditory cue was paired with foot-shock, whereas a second auditory cue was not. Compared with wild-type mice, 5-HTTKO mice exhibited faster discrimination learning. This effect was associated with stronger theta frequency oscillations and greater hemodynamic changes in the amygdala in response to both the emotionally relevant cues and the unconditioned foot-shock stimulus. Furthermore, hemodynamic responses to the unconditioned stimulus predicted behavioral discrimination performance the following day. Acute pharmacological 5-HTT blockade in wild-type mice produced a similar effect, to the extent that administration of citalopram during the fear conditioning sessions enhanced fear memory recall. Collectively, our data argue that loss of 5-HTT function enhances amygdala responsivity to aversive events and facilitates learning for emotionally relevant cues.
Similar content being viewed by others
Introduction
The serotonin transporter (5-HTT) regulates serotonin (5-HT) availability at the synapse, and is the target of selective serotonin reuptake inhibitors (SSRIs), which are currently the mainline treatment for depression and anxiety disorders. A substantial research effort has examined the influence of variation in the human 5-HTT gene on the risk for developing anxiety and depression, as well as other aspects of emotional function. These studies have focused on the 5-HTT gene-linked polymorphic region (5-HTTLPR), which is an insertion/deletion polymorphic site that generates long and short alleles. The latter allele is linked to reduced 5-HTT expression (relative to the long allele, specifically the LA subtype), which is thought to predispose individuals to higher neuroticism (an anxiety-related personality trait), and increased risk of depression and anxiety, especially when combined with aversive life experiences1,2,3,4,5. Moreover, the risk of psychiatric disorders associated with reduced 5-HTT expression is thought to be underpinned by elevated amygdala responsivity to fearful stimuli as detected in functional Magnetic Resonance Imaging (fMRI) Blood Oxygen Level Dependent (BOLD) studies4,5.
However, collectively these findings remain controversial, with large sample studies and meta-analyses being inconsistent in their outcome6,7,8,9,10. Interpretation is further complicated by the difficulty establishing whether 5-HTTLPR genotype influences 5-HTT expression (and thereby synaptic 5-HT availability) in human brain in vivo11,12. Nevertheless, unraveling associations between 5-HTT expression and anxiety/fear is highly relevant in the context of recent theories proposing that altered 5-HT availability modulates environmental sensitivity through altering the plasticity of synapses involved in emotional learning13,14,15,16,17.
In comparison with human studies, animal experiments demonstrate more reliable evidence that altered 5-HTT expression influences anxiety. In particular, variation in the 5-HTT has been modeled in mice by both 5-HTT knockout (5-HTTKO) and 5-HTT overexpression (5-HTTOE), which demonstrate elevated and reduced extracellular 5-HT levels, respectively18. Importantly, and as predicted by human 5-HTT gene association studies (see above), 5-HTTKO mice exhibit greater anxiety in unconditioned tasks such as the elevated plus maze and novelty suppressed feeding, whereas 5-HTTOE mice exhibit reduced anxiety in these tasks19,20,21.
The influence of altered 5-HTT expression on conditioned aversive learning has also been investigated in rodents although the data are less clear. Thus, 5-HTTKO mice and rats are reported to exhibit normal fear learning22,23,24 rather than increased fear learning that human data and the above 5-HT emotional plasticity theory predict. However, these 5-HTTKO rodent studies used “single-cue” conditioning paradigms (i.e., several exposures to one auditory cue paired with foot-shock); and since wild-type (WT) animals typically display high levels of fear-related behavior (“freezing”) in such paradigms, it is difficult to detect further increases.
In contrast, 5-HTTOE mice exhibit impaired fear learning25,26. Moreover, 5-HTTOE mice exhibit reduced amygdala tissue oxygen (TO2) responses to aversive cues as measured by voltammetry, as well as reduced cue-evoked theta oscillations in the amygdala local field potential (LFP)25. Voltammetric TO2 measurements are considered a hemodynamic parameter that reflects on-going neuronal activity, analogous to the BOLD signal in fMRI25,27,28. Moreover, theta oscillations in the amygdala are thought to be a neurophysiological substrate for the acquisition of fearful memories27,29.
To clarify the impact of 5-HTT loss on emotional learning, the present study investigated conditioned aversive learning and amygdala activity in 5-HTTKO mice using a discriminative fear learning paradigm rather than the “single-cue” fear paradigm used in previous investigations of 5-HTTKO rodents. Mice were trained to discriminate between two distinct auditory stimuli, one that was paired with foot-shock (CS+) and another that was never paired with foot-shock (CS–). In a subset of mice, hemodynamic responses and LFPs were simultaneously recorded from the amygdala during different stages of fear learning. An additional experiment tested the effect of acute pharmacological 5-HTT blockade on aversive learning in WT mice because of reports that the anxiety phenotype of 5-HTTKO mice is of developmental origin rather than an on-going loss of 5-HTT function30,31.
Materials and methods
Detailed methods can be found in the Supplementary Material.
Subjects
Experiment 1 (study of 5-HTTKO mice) used 31 WT mice (15 males and 16 females) and 29 5-HTTKO mice (15 males and 14 females). Mice were generated as described previously32 and backcrossed onto a C57BL/6J background for at least eight generations. Experiment 2 (study of 5-HTT blockade) used 96 female C57BL/6J mice (Charles River Laboratories, Kent, UK). Mice were 4–10 months (5-HTTKO and WT) or ~2 months old (C57BL/6J) at the start of testing. All experiments were conducted in accordance with the United Kingdom Animals Scientific Procedures Act (1986) under project licenses PPL 30/2561 and 30/3068, and were approved by local ethical review for the University of Oxford.
Surgery
For Experiment 1, a subset of mice (WT 8 females and 6 males: 5-HTTKO 7 females and 7 males) were stereotaxically implanted (under isoflurane anesthesia) with electrodes into the basolateral amygdala (BLA) to record TO2 and LFPs. Co-ordinates were −1.35 mm anterior/posterior, ±3.15 mm medial/lateral and −5.00 mm dorsal/ventral, relative to bregma (Supplementary Fig. S1).
Amygdala TO2 and LFP recordings
Signals for TO2 were measured using constant potential amperometry, as described in detail previously28,33. A constant potential (−650 mV relative to a reference electrode) was applied to the electrode, which results in the electrochemical reduction of O2 on the electrode surface such that local changes in O2 concentration produce directly proportional changes in the measured Faradaic current. LFPs were recorded using a differential amplifier (DP-301, Warner Instruments, CT, USA) and sampled continuously at 4 kHz.
Fear conditioning
Fear conditioning was conducted in one of two operant chambers (ENV-307A, Med Associates Inc., IN, USA), each with distinct visual and olfactory cues. Mice (total of 60 including 28 with implanted BLA electrodes) underwent a pre-exposure day, three training days (T1, T2, and T3), and a fear memory recall day (FMR; Fig. 1a). Training was performed in one context (e.g., context A) and recall/extinction was performed in a different context (e.g., context B if trained in context A). Contexts were counterbalanced across mice.
a Schematic of the training paradigm. b Each session contained a 300-s lead-in period followed by five CS+ and five CS– presentations, pseudo-randomly interleaved, and with a different presentation sequence on each day. On training days (T1–T3), the CS+ co-terminated with foot-shock (lightening icon), whereas the CS– was never paired with foot-shock. No shocks were given during pre-exposure (PE) or fear memory recall (FMR) sessions. c, d Illustration of how percentage freezing scores during pre-CS and CS periods (shown in c) were used to create Δfreezing difference scores [CS minus pre-CS] (shown in d). e A discrimination index was generated by subtracting CS– from CS+ Δfreezing scores (e.g., +20 – (–15) = +35)
Mice were trained to discriminate between two distinct auditory cues (tone and white noise), with one conditioned stimulus (e.g., CS+ = tone) always paired with foot-shock during the training sessions and the other never paired with shock (e.g., CS– = white noise). Allocation of tone and white noise to the CS+/CS– was fully counterbalanced across mice. During each session, mice were presented with 10 auditory cues (5 × 2900 Hz tone, 5 × white noise; all 72 dB and 30-s duration) in a pseudo-randomly interleaved order with a mean inter-cue interval of 80 s (range 60–100 s). On training days, each of the five CS+ trials co-terminated with mild foot-shock (0.3 mA, 0.5 s; Fig. 1b). No shocks were given during the pre-exposure or recall sessions.
Data analyses
Behavior
Freezing responses were assessed by automated movement detection using software running in NIH image34. To calculate cue-evoked freezing responses (Δfreezing), % freezing in the 30 s before cue onset was subtracted from % freezing during cue presentation (Fig. 1c, d). Cue-evoked freezing greater than the pre-cue baseline yields positive scores and cue-evoked freezing less than pre-cue period yields negative scores (Fig. 1c, d). A discrimination index was calculated by subtracting the Δfreezing evoked by the CS– from the Δfreezing evoked by the CS+ (Fig. 1e). Note that there were no genotypic differences in freezing during pre-cue or cue periods during the pre-exposure session (all F-values < 1.4, all p-values > 0.2; Supplementary Fig. S2). Also freezing levels during the pre-cue periods did not differ between genotypes during training or recall (no main effect of genotype or genotype × day interaction: F < 1, p > 0.8). Freezing responses during pre-cue and cue periods for all days are shown in Supplementary Fig. S3.
Cue-evoked TO2 responses
Cue-evoked TO2 responses were calculated by subtracting the mean TO2 signal during the 5 s before cue onset (i.e., pre-cue baseline) from the TO2 signal during the 30-s cue presentation. Then, the 30-s signal was divided into 15 2-s time bins (i.e., 0–2 s, 2–4 s, 4–6 s…28–30 s) with each data point equal to the mean value during each 2-s time bin. The foot-shock (US) was 0.5-s duration, and US-evoked signals were measured in the 30 s after the shock (excluding 0.5 s of US administration). TO2 responses were averaged over the five CS+, CS–, and US trials of each session. In addition, a regression analysis was used to investigate whether TO2 signals predicted behavioral discrimination. For this analysis, the maximum TO2 signal (i.e., the peak value in one of the 15 time bins) was calculated during the CS+, CS–, and US periods. The regression model used these values (TO2_CS+, TO2_CS–, and TO2_shock) on training days T1, T2, and T3 to predict behavioral discrimination on subsequent training days T2 and T3 and the FMR session, respectively. Stepwise linear regression was used to determine the maximum variance explained with the fewest independent variables.
LFPs
LFPs were band-pass filtered between 1 and 80 Hz. Power spectra were calculated during the 10 s after cue onset for each trial and then averaged over the five CS+ or five CS– trials in each session. Spectra were computed in MATLAB (The Mathworks, MA, USA). For statistical analysis, the power spectral density in each frequency bin (Φi,) was transformed into a proportion of the total power between 1 and 40 Hz:
where Pi = proportional power, Φi = raw power density (mV2/Hz).
From these proportional spectra, we determined the peak power in the theta frequency range (5–10 Hz).
Histology
At the end of the experiments, mice implanted with electrodes were injected with sodium pentobarbitone; 200 mg/kg) and perfused transcardially with physiological saline followed by either 10% formol saline or 4% paraformaldehyde. Coronal sections (40 μm) were stained with cresyl violet to determine that electrodes were in the BLA (Supplementary Fig. S1).
Drug treatment
In Experiment 2, a separate group of mice (n = 96) were injected with saline or citalopram (10 mg/kg, i.p.; Tocris, UK) 30 min before each training and/or recall session using a fully counterbalanced design (Table 1). The behavioral paradigm was identical to that used in Experiment 1, except there was no pre-exposure session and only 2 days of training. There were four separate treatment groups: mice given saline during training and recall (Sal_Sal), mice given citalopram during training and saline during recall (Cit_Sal), mice given saline during training and citalopram during recall (Sal_Cit), and mice given citalopram during both training and recall (Cit_Cit).
Statistical procedures
Data were analyzed using analysis of variance (ANOVA) in SPSS (version 22, IBM, USA). ANOVAs are described in the form: A2 × B3, where A is a factor with two levels and B a factor with three levels. All graphs show the mean ± 1 standard error of the mean (SEM). The family-wise error was set at α = 0.05, with all tests performed as two tailed, and the Dunn–Sidak method employed to correct for multiple comparisons.
Results
Facilitated discriminative aversive learning in 5-HTTKO mice
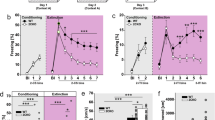
Both genotypes learned to discriminate between the aversive cue (CS+) and non-aversive cue (CS–) by training day 2 (T2) but this discrimination was markedly stronger in 5-HTTKO mice (Fig. 2a, b). Analysis of Δfreezing scores (ANOVA model: genotype2 × sex2 × day2 × CS type2 × trial5, n = 60 mice) revealed greater freezing evoked by the CS+ than CS– (main effect of CS type: F(1, 56) = 23.4, p < 0.001) and superior discrimination in 5-HTTKO compared with WT mice (genotype × CS type interaction: F(1, 56) = 7.6, p = 0.01). Moreover, 5-HTTKO mice exhibited significant discrimination during training day 1 (T1), whereas WT mice did not (p = 0.01 versus p = 0.7; see Supplementary Fig. S3). In addition, analysis of the discrimination index (calculated as [CS+] – [CS–] Δfreezing scores), revealed significantly stronger discrimination in 5-HTTKO versus WT mice during T2 (F(1, 56) = 8.0, p = 0.006; Fig. 2b), thus 5-HTTKO mice exhibited better discriminative aversive learning. However, with one additional training day (T3), discrimination now did not differ between WT and 5-HTTKO mice, and there was no effect of genotype or genotype × CS type interaction during T3 or during FMR (F < 2.5, p > 0.1; see Fig. 2a, b).
a 5-HTTKO mice exhibited larger differences between CS+ and CS– evoked Δfreezing scores during training. Note that positive Δfreezing scores indicate an increase in freezing compared with the pre-CS period whereas negative scores indicate a decrease in freezing compared with the pre-CS period. b The discrimination index (CS+Δfreezing – CS– Δfreezing scores) was significantly higher in 5-HTTKO than WT mice during T2. c–e Mean amygdala hemodynamic responses before, during, and after CS+ presentation in WT and 5-HTTKO mice on T1, T2, and fear memory recall (FMR). Note that TO2 signals were higher in 5-HTTKO mice following foot-shock on T1 and higher during CS+ presentations on T2. a, b Mean ± SEM, n = 60 mice. c–e Mean ± SEM, n = 22–24 mice. *p < 0.05, **p < 0.01
Stimulus-evoked amygdala TO2 responses are greater in 5-HTTKO mice
Voltammetric TO2 signals are considered hemodynamic responses, which reflect on-going neuronal activity, similar to the BOLD signal in fMRI, and in the amygdala provide a neural correlate of aversive learning27. During the pre-exposure session, TO2 responses did not differ between genotypes (no effect of genotype, stimulus type or interaction: F < 1.5, p > 0.2; Supplementary Fig. S2).
However, during fear learning, amygdala TO2 responses were greater in 5-HTTKO than WT mice. Analysis of TO2 signals during the first 2 training days (ANOVA: genotype2 × stimulus type3 (CS–, CS+, US), × day2 × time bin15, n = 24 mice) revealed that during T1, shock-evoked TO2 responses were larger in 5-HTTKO mice (Fig. 2c). The higher amygdala TO2 signal in 5-HTTKO mice was not because the foot-shock evoked greater locomotor activity in 5-HTTKO versus WT mice (Supplementary Fig. S4). During T2, both CS+ evoked and CS– evoked TO2 responses were larger in 5-HTTKO versus WT mice (Fig. 2d; interactions between genotype, stimulus type and day: F(2, 44) = 3.6, p = 0.035, and genotype, stimulus type, day, and time bin: F(28, 616) = 2.4, p < 0.001; CS– responses are shown in Supplementary Fig. S5). Note that both genotypes had larger TO2 responses to the CS+ than the CS–, but this was not apparent in WT mice until training day T3 (Supplementary Fig. S5). Moreover, CS+ evoked amygdala TO2 responses were greater in 5-HTTKO compared with WT mice during FMR (main effect of genotype: F(1, 20) = 4.4, p = 0.049; genotype × CS type × time bin interaction: F(14, 280) = 2.2, p = 0.009), even though there were no genotype differences in behavioral responses on this day. Overall, cue-evoked amygdala TO2 responses were greater in 5-HTTKO than WT mice.
Amygdala TO2 responses to foot-shock predict subsequent fear-related behavior
Multiple linear regression was used to investigate whether TO2 signals on day n predicted behavioral discrimination between the CS+ and CS– on day n + 1, as observed previously27. The dependent variable was the behavioral discrimination index and three independent variables were used: maximum TO2 signal during CS+ presentations (TO2_CS+), maximum TO2 signal during CS– presentations (TO2_CS–), and maximum TO2 signal in the 30 s following foot-shock (TO2_shock). The optimum regression model contained only TO2_shock and accounted for 7% of the variance in behavioral discrimination, which was significantly greater than zero (F(1, 68) = 5.1, p = 0.03, R2 = 0.07). There was a significant positive correlation between TO2_shock and behavioral discrimination (standardized β coefficient = 0.27; t(68) = 2.4, p = 0.03).
Overall, and consistent with our previous report27, mice that exhibited larger TO2 responses to foot-shock exhibited stronger behavioral discrimination the following day. The larger shock-evoked responses in 5-HTTKO versus WT mice during T1 (Fig. 2c) may therefore explain, at least in part, the superior discrimination in 5-HTTKO mice during T2.
Cue-evoked theta frequency neuronal oscillations are greater in 5-HTTKO mice
Next, experiments investigated whether the enhanced discrimination learning in 5-HTTKO mice was accompanied by changes in amygdala oscillatory neuronal activity. Previous work suggests that theta frequency (5–10 Hz) oscillations in the amygdala are a neurophysiological substrate of aversive learning27,29,35. Consistent with these previous reports, during training and FMR the onset of the CS+ increased theta oscillations and decreased delta oscillations (1–4 Hz). This increase in theta was evident in both genotypes but was stronger in 5-HTTKO than WT mice (Fig. 3).
a Raw local field potential trace showing theta activity evoked by CS+ onset. b, c Time-frequency spectrograms from one WT (b) and one 5-HTTKO (c) mouse during T2 illustrating how CS+ onset evoked a shift from delta-dominant (1–4 Hz) to theta-dominant (5–10 Hz) oscillations. d, e Peak CS+ evoked theta power was higher in 5-HTTKO than WT mice (d-f). Mean ± SEM, n = 24 mice. *p < 0.05
Analysis of cue-evoked theta responses during T1, T2, and FMR (ANOVA: genotype2 × day3 × CS type2, n = 24 mice) revealed that theta oscillations were stronger during CS+ than CS– trials (main effect of CS type: F(1, 22) = 10.8, p = 0.003), and were stronger in 5-HTTKO than WT mice (genotype × CS type interaction: F(1, 22) = 7.1, p = 0.01). Pairwise comparisons revealed that CS+ evoked theta power was significantly greater in 5-HTTKO than WT mice (p = 0.02), with no genotypic difference for CS– trials (p = 0.15). Thus, 5-HTTKO mice exhibited enhanced amygdala theta oscillations during the aversive conditioned cues.
5-HTT blockade before training sessions facilitates subsequent FMR
5-HTTKO mice will experience loss of 5-HTT (and presumably greater synaptic 5-HT availability) throughout their entire lifetime. Hence, the facilitated fear discrimination in 5-HTTKO mice could be driven by loss of 5-HTT during development rather than adulthood30,31. To test whether the enhanced discrimination performance reflects loss of 5-HTT at the time of learning, WT mice were tested for discriminative fear conditioning, and administered citalopram (10 mg/kg, i.p.) or saline 30 min before each session in a fully counterbalanced design (see Table 1).
Analysis of the discrimination index revealed that citalopram treatment did not affect discrimination during the training days (no effect of day, drug treatment or interaction, F < 1, p > 0.5; Fig. 4). However, mice that received citalopram during training exhibited significantly better discrimination during FMR, regardless of whether they received saline or citalopram before the FMR session (main effect of treatment during training: F(1, 94) = 8.5, p = 0.005; Fig. 4a).
a Citalopram treatment 30 min before training sessions had no effect on CS+/CS– discrimination during the training sessions (T1, T2) but mice that received citalopram during training exhibited significantly better discrimination during the fear memory recall session (FMR). Note that during recall, the saline bar comprises the Sal_Sal and Sal_Cit groups and the citalopram bar comprises the Cit_Sal and Cit_Cit groups, so the effect was not due to citalopram on fear expression per se (n = 96 mice). b Discrimination during recall was strongest in those mice that received citalopram before training and saline before recall (Cit_Sal) and weakest in mice that received saline before both training and recall (Sal_Sal). ***p = 0.001, **p = 0.005, *p = 0.03
Comparison of the four treatment groups specifically during FMR (Fig. 4b) revealed that the Cit_Sal and the Cit_Cit groups exhibited significantly better discrimination than the Sal_Sal group (p = 0.001 and p = 0.03, respectively), and the Cit_Sal group discriminated better than the Sal_Cit group (p = 0.05). There were no other significant group differences. Citalopram did not affect freezing during pre-CS periods (Supplementary Fig. S6), nor responsivity to the foot-shock, except on the very first conditioning trial (Supplementary Fig. S7). Thus, mice that received citalopram during training exhibited enhanced FMR. These results suggest that 5-HTT blockade, and a likely increase in 5-HT availability at the time of conditioning, enhances aversive discrimination learning.
Discussion
Summary of results
The current study found that 5-HTTKO mice exhibited enhanced fear learning in a discriminative learning task in which one auditory cue (CS+) was paired with foot-shock, whereas a second auditory cue (CS–) was not. Importantly, it was found that this learning effect in 5-HTTKO mice was accompanied by larger amygdala tissue oxygen (TO2) responses to the conditioned stimuli (CS+, CS–), as well as the unconditioned stimulus (foot-shock). Tissue oxygen responses were predictive of subsequent learning, with larger shock-evoked responses predicting better behavioral discrimination between CS+ and CS– on the following day. Moreover, CS+ evoked theta oscillations in amygdala, a neurophysiological substrate for fear learning and the associated synaptic plasticity, were stronger in 5-HTTKO than WT mice. Finally, acute pharmacological blockade of the 5-HTT with citalopram during the fear conditioning sessions enhanced subsequent fear memory recall. These data are consistent with the idea that loss of 5-HTT function increases sensitivity to emotionally relevant stimuli and thereby enhances memory of those events.
5-HTTKO mice have superior aversive learning
Previous studies have reported normal fear learning in 5-HTTKO mice and rats22,23,24,36 but these studies used “single-cue” fear conditioning and not the discriminative approach used in the present study. Single-cue fear paradigms typically produce strong fear responses (i.e., high levels of freezing), which likely reduces the chance of detecting enhanced fear learning because of ceiling effects. Moreover, discrimination tasks require the mice to attend to the specific sensory features of the auditory cues to a greater extent than single-cue paradigms. In this respect, it is worth noting that the enhanced discrimination performance shown here in the 5-HTTKO mice was driven by responses to both the CS+ and CS– (Fig. 2), and was mirrored by larger amygdala TO2 responses to both the CS+ and CS– cues.
The present finding of improved aversive learning in 5-HTTKO mice complements recent studies showing impaired aversive learning in 5-HTTOE mice25,26,37. 5-HTTOE mice have ~3-fold greater 5-HTT expression than their WT counterparts, and lower extracellular 5-HT18,20,25. Our results are also consistent with data from human 5-HTT gene association studies showing superior discriminative aversive learning in putative low 5-HTT expressing individuals38,39, as well as a positron emission tomography (PET) study showing that individuals with lower amygdala 5-HTT expression show enhanced discriminative aversive learning40. Collectively, these data argue that aversive learning is influenced across a continuum of 5-HTT expression levels in humans and mice, with superior learning in low- or null-expressing 5-HTT variants.
5-HTTKO mice have larger fear-evoked amygdala TO2 responses
We found that conditioned aversive cues evoked larger amygdala TO2 responses in 5-HTTKO than WT mice. These findings are supported by human fMRI studies showing larger amygdala BOLD responses to emotional faces in carriers of the short allele of the 5-HTTLPR variant5. Our TO2 data are also consistent with an ex vivo auto-radiographic study, which reported a biomarker of blood-flow to be higher in the amygdala of 5-HTTKO mice following fear recall41. Moreover, we found that larger TO2 responses to the unconditioned stimulus (foot-shock) were predictive of better behavioral discrimination between the CS+ and CS–, arguing that these signals have a meaningful relationship with the observed behavioral output27.
Collectively these data support enhanced amygdala responses to the unconditioned and conditioned stimuli being the neural substrate for the superior aversive learning in association with loss of 5-HTT function. Moreover, we have recently shown lower fear-evoked amygdala TO2 responses in 5-HTTOE mice25, indicating that amygdala responsivity to fearful stimuli is bi-directionally modulated by changes in 5-HTT expression levels.
5-HTTKO mice have enhanced fear-evoked theta oscillations
We found that alongside TO2 responses, aversive cue-evoked theta oscillations were also enhanced in the amygdala of 5-HTTKO mice. This result is consistent with a previous study showing increased fear-evoked theta frequency synchronization between the amygdala and prefrontal cortex in 5-HTTKO mice, although this study did not report whether amygdala theta oscillations were enhanced per se24. Moreover, we have previously reported that fear-evoked theta oscillations are reduced in the amygdala of 5-HTTOE mice25, in association with impaired aversive learning25,26,37.
A large body of work suggests that theta oscillations are an important neurophysiological substrate for synaptic plasticity and learning in general, and for the encoding of fear memories in amygdala in particular25,27,35. Theta oscillations are thought to be modulated by the activity of parvalbumin (PV)-expressing inhibitory interneurons42, and amygdala PV interneurons are activated by cue-evoked fear43 and required for successful fear conditioning44. Recently we found that amygdala PV interneurons are activated by 5-HT in an in vitro preparation, and that this response was reduced in the amygdala of 5-HTTOE mice43. It is an interesting possibility that increased 5-HT sensitivity of amygdala PV interneurons underpins the increased fear learning in 5-HTTKO mice.
Pharmacological blockade of the 5-HTT enhances fear learning
We tested whether the effects of genetic 5-HTTKO on fear learning could be phenocopied by pharmacological blockade of the 5-HTT, using the SSRI citalopram. 5-HTTKO rodents have elevated 5-HT availability, as shown using microdialysis45 and fast cyclic voltammetry18. Nevertheless, it has been argued that the negative emotionality phenotype seen in 5-HTTKO mice is of neurodevelopmental origin rather than due to elevated 5-HT availability at the time of behavioral testing30,31,46. Citalopram causes a marked elevation of 5-HT availability, as shown by microdialysis47, and here we tested whether this elevation was sufficient to affect fear learning or memory. We did not use chronic SSRI administration because this can cause widespread changes to neuronal circuitry (e.g., increasing neurogenesis, altering the expression/function of other 5-HT receptors)48,49, and we wanted to test the effects of increasing 5-HT availability on fear learning/memory without these potential confounds. We found that acute citalopram given before the fear learning sessions did not affect discrimination during learning but it produced significantly better discrimination during subsequent fear memory recall.
Our results are consistent with previous studies in rats50,51 but extends these findings by showing a specific effect of 5-HTT blockade on discrimination learning. Using a non-discriminative fear conditioning task, Burghardt and colleagues found that an acute SSRI given before training increased freezing in rats. However, they interpreted their result in terms of a short-term increase in anxiety due to SSRI administration, an interpretation that does not fit with our data for two reasons. First, we found that superior discrimination was most evident in mice that did not receive citalopram before fear memory recall and these mice showed markedly better discrimination than a group of mice that did receive citalopram before fear memory recall. Second, anxiety is often characterized by stimulus generalization, which is the tendency to conflate aversive and benign cues, treating both as if they signal threat52. Thus, if citalopram were to increase anxiety, it would predict impaired discrimination between the CS+ and CS–, opposite to what we observed. Our results are consistent with the hypothesis asserted by Branchi and others16,17 that SSRIs amplify the impact of emotionally relevant cues (and environments) that the animal or human encounters. Our data argue that SSRIs result in increased sensitivity to both aversive (e.g., CS+) and non-aversive (e.g., CS–) cues (see Supplementary Fig. S8). Indeed, our results suggest that reducing generalization between aversive and non-aversive cues or events (i.e., enhancing their discrimination), could be a mechanism through which SSRIs reduce anxiety in human patients. Thus, SSRIs may reduce anxiety by helping patients to learn to discriminate between cues that signal aversive and benign outcomes, which in turn will reduce uncertainty and therefore reduce anxiety. However, if the living environment is dominated by aversive events then SSRIs may even cause a worsening of symptoms, as recently reported by Branchi and colleagues.
Fear learning and memory after 5-HTTKO and 5-HTT blockade
Although the present data demonstrate that both 5-HTTKO and 5-HTT blockade enhance discriminative aversive learning, this effect was seen at different time-points in the two experiments. Thus, 5-HTTKO mice showed enhanced discrimination during training but not during recall, whereas the opposite pattern was seen in citalopram-treated mice. These data demonstrate that 5-HTT blockade does not precisely phenocopy the effects of 5-HTTKO, when studied under the current conditions. This finding may reflect the consequences of lifelong loss of 5-HTT in the KO mice versus acute blockade of the 5-HTT after citalopram. Specifically, deletion of the 5-HTT throughout life may increase 5-HT availability to cause neurodevelopmental changes to key neural circuits involved in fear that increase vigilance/sensitivity to environmental cues in adulthood, as has been suggested by previous studies on the developmental origin of the 5-HTTKO phenotype30,31. In comparison, acute 5-HTT blockade appears to be insufficient to cause immediate behavioral changes during training, but, in combination with the aversive experience, may produce stronger learning, as evidenced by superior discrimination during the recall sessions. Further experiments combining 5-HTT blockade and aversive experiences prior to fear conditioning could test this hypothesis.
Conclusion
In summary, here we provide evidence that both genetic and pharmacological loss of 5-HTT function increases emotional learning in a discriminative fear conditioning paradigm. The increase in fear learning observed as a consequence of genetic 5-HTTKO was accompanied by increased amygdala responsivity in the form of enhanced cue-evoked TO2 responses and theta oscillations. In comparison with these effects in 5-HTTKO mice, we recently reported parametrically opposite behavioral and neurophysiological effects in 5-HTTOE mice25.
Moreover, although the 5-HTTLPR association with negative emotionality phenotypes in humans remains highly controversial6, our results show that 5-HTT expression does influence fear learning and amygdala function. In this respect, our data provide important validation for human studies that report increased fear responses in low 5-HTT-expressing individuals as measured using PET 40,53.
An important current theory proposes that 5-HT availability does not regulate emotional state directly but rather it regulates plasticity in key neural networks underlying emotional learning and therefore influences sensitivity to emotional life events13,14,15. Thus, emotional experiences, both positive and negative, may be encoded more strongly in individuals with higher 5-HT availability. A prediction of this theory is that loss of 5-HTT expression, thereby resulting in increased synaptic 5-HT availability18, will facilitate aversive learning, whereas gain of 5-HTT expression will have the opposite effects. The finding of the present study and our previous work25 therefore provides supporting evidence for this theory.
References
Lesch, K. P. et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531 (1996).
Caspi, A., Hariri, A. R., Holmes, A., Uher, R. & Moffitt, T. E. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am. J. Psychiatry 167, 509–527 (2010).
Caspi, A. et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389 (2003).
Hariri, A. R. et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch. Gen. Psychiatry 62, 146–152 (2005).
Hariri, A. R. et al. Serotonin transporter genetic variation and the response of the human amygdala. Science 297, 400–403 (2002).
Culverhouse, R. C. et al. Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Mol. Psychiatry 23, 133–142 (2018).
Willis-Owen, S. A. et al. The serotonin transporter length polymorphism, neuroticism, and depression: a comprehensive assessment of association. Biol. Psychiatry 58, 451–456 (2005).
Murphy, S. E. et al. The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a meta-analysis. Mol. Psychiatry 18, 512–520 (2013).
Kendler, K. S., Kuhn, J. W., Vittum, J., Prescott, C. A. & Riley, B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch. Gen. Psychiatry 62, 529–535 (2005).
Sen, S., Burmeister, M. & Ghosh, D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 127B, 85–89 (2004).
Murthy, N. V. et al. Serotonin transporter polymorphisms (SLC6A4 insertion/deletion andrs25531) do not affect the availability of 5-HTT to [11C] DASB binding in the living human brain. Neuroimage 52, 50–54 (2010).
Parsey, R. V. et al. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am. J. Psychiatry 163, 48–51 (2006).
Castren, E. Is mood chemistry? Nat. Rev. Neurosci. 6, 241–246 (2005).
Branchi, I. The double edged sword of neural plasticity: increasing serotonin levels leads to both greater vulnerability to depression and improved capacity to recover. Psychoneuroendocrinology 36, 339–351 (2011).
Homberg, J. R. & Lesch, K. P. Looking on the bright side of serotonin transporter gene variation. Biol. Psychiatry 69, 513–519 (2011).
Alboni, S. et al. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol. Psychiatry 22, 552–561 (2017).
Chiarotti, F., Viglione, A., Giuliani, A. & Branchi, I. Citalopram amplifies the influence of living conditions on mood in depressed patients enrolled in the STAR*D study. Transl. Psychiatry 7, e1066 (2017).
Jennings, K. A., Lesch, K. P., Sharp, T. & Cragg, S. J. Non-linear relationship between 5-HT transporter gene expression and frequency sensitivity of 5-HT signals. J. Neurochem. 115, 965–973 (2010).
Holmes, A., Murphy, D. L. & Crawley, J. N. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol. Psychiatry 54, 953–959 (2003).
Jennings, K. A. et al. Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J. Neurosci. 26, 8955–8964 (2006).
Line, S. J. et al. Opposing alterations in anxiety and species-typical behaviours in serotonin transporter overexpressor and knockout mice. Eur. Neuropsychopharmacol. 21, 108–116 (2011).
Muller, J. M., Morelli, E., Ansorge, M. & Gingrich, J. A. Serotonin transporter deficient mice are vulnerable to escape deficits following inescapable shocks. Genes. Brain. Behav. 10, 166–175 (2011).
Wellman, C. L. et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J. Neurosci. 27, 684–691 (2007).
Narayanan, V. et al. Social defeat: impact on fear extinction and amygdala-prefrontal cortical theta synchrony in 5-HTT deficient mice. PLoS ONE 6, e22600 (2011).
Barkus, C. et al. Variation in serotonin transporter expression modulates fear-evoked hemodynamic responses and theta-frequency neuronal oscillations in the amygdala. Biol. Psychiatry 75, 901–908 (2014).
Line, S. J. et al. Reduced sensitivity to both positive and negative reinforcement in mice over-expressing the 5-hydroxytryptamine transporter. Eur. J. Neurosci. 40, 3735–3745 (2014).
McHugh, S. B. et al. Aversive prediction error signals in the amygdala. J. Neurosci. 34, 9024–9033 (2014).
McHugh, S. B. et al. Hemodynamic responses in amygdala and hippocampus distinguish between aversive and neutral cues during Pavlovian fear conditioning in behaving rats. Eur. J. Neurosci. 37, 498–507 (2013).
Pape, H. C. & Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 90, 419–463 (2010).
Ansorge, M. S., Zhou, M., Lira, A., Hen, R. & Gingrich, J. A. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306, 879–881 (2004).
Ansorge, M. S., Morelli, E. & Gingrich, J. A. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J. Neurosci. 28, 199–207 (2008).
Bengel, D. et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 53, 649–655 (1998).
Lowry, J. P. et al. Real-time electrochemical monitoring of brain tissue oxygen: a surrogate for functional magnetic resonance imaging in rodents. Neuroimage 52, 549–555 (2010).
Richmond, M. A. et al. A computer controlled analysis of freezing behaviour. J. Neurosci. Methods 86, 91–99 (1998).
Seidenbecher, T., Laxmi, T. R., Stork, O. & Pape, H. C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science 301, 846–850 (2003).
Shan, L., Schipper, P., Nonkes, L. J. & Homberg, J. R. Impaired fear extinction as displayed by serotonin transporter knockout rats housed in open cages is disrupted by IVC cage housing. PLoS ONE 9, e91472 (2014).
McHugh, S. B. et al. SERT and uncertainty: serotonin transporter expression influences information processing biases for ambiguous aversive cues in mice. Genes. Brain. Behav. 14, 330–336 (2015).
Lonsdorf, T. B. et al. Genetic gating of human fear learning and extinction: possible implications for gene-environment interaction in anxiety disorder. Psychol. Sci. 20, 198–206 (2009).
Garpenstrand, H., Annas, P., Ekblom, J., Oreland, L. & Fredrikson, M. Human fear conditioning is related to dopaminergic and serotonergic biological markers. Behav. Neurosci. 115, 358–364 (2001).
Ahs, F., Frick, A., Furmark, T. & Fredrikson, M. Human serotonin transporter availability predicts fear conditioning. Int. J. Psychophysiol. 98(3 Pt 2), 515–519 (2015).
Pang, R. D. et al. Mapping functional brain activation using [14C]-iodoantipyrine in male serotonin transporter knockout mice. PLoS ONE 6, e23869 (2011).
Amilhon, B. et al. Parvalbumin interneurons of hippocampus tune population activity at theta frequency. Neuron 86, 1277–1289 (2015).
Bocchio, M. et al. Increased serotonin transporter expression reduces fear and recruitment of parvalbumin interneurons of the amygdala. Neuropsychopharmacology 40, 3015–3026 (2015).
Wolff, S. B. et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature 509, 453–458 (2014).
Shen, H. W. et al. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology 29, 1790–1799 (2004).
Kalueff, A. V., Olivier, J. D., Nonkes, L. J. & Homberg, J. R. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci. Biobehav. Rev. 34, 373–386 (2010).
Antoniadou, I. et al. Ebselen has lithium-like effects on central 5-HT2A receptor function. Br. J. Pharmacol. 175, 2599–2610 (2018).
Segi-Nishida, E. The effect of serotonin-targeting antidepressants on neurogenesis and neuronal maturation of the hippocampus mediated via 5-HT1A and 5-HT4 receptors. Front. Cell. Neurosci. 11, 142 (2017).
Tilakaratne, N., Yang, Z. & Friedman, E. Chronic fluoxetine or desmethylimipramine treatment alters 5-HT2 receptor mediated c-fos gene expression. Eur. J. Pharmacol. 290, 263–266 (1995).
Burghardt, N. S., Sullivan, G. M., McEwen, B. S., Gorman, J. M. & LeDoux, J. E. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol. Psychiatry 55, 1171–1178 (2004).
Ravinder, S., Burghardt, N. S., Brodsky, R., Bauer, E. P. & Chattarji, S. A role for the extended amygdala in the fear-enhancing effects of acute selective serotonin reuptake inhibitor treatment. Transl. Psychiatry 3, e209 (2013).
Dunsmoor, J. E. & Paz, R. Fear generalization and anxiety: behavioral and neural mechanisms. Biol. Psychiatry 78, 336–343 (2015).
Rhodes, R. A. et al. Human 5-HT transporter availability predicts amygdala reactivity in vivo. J. Neurosci. 27, 9233–9237 (2007).
Acknowledgements
This study was supported by the Wellcome Trust (Grant No. 087736 to D.M.B.), FCT Portugal (doctoral grant SFRH/BD/87496/2012 to J.L.), and Merton College, Oxford (Graduate Research Grants to J.L.). S.B.M. receives support from Biotechnology and Biological Sciences Research Council UK (award: BB/N002547/1) and Medical Research Council UK (award: MC_UU_12024/3). We would like to thank Amy Taylor for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lima, J., Sharp, T., Bannerman, D.M. et al. Enhanced discriminative aversive learning and amygdala responsivity in 5-HT transporter mutant mice. Transl Psychiatry 9, 139 (2019). https://doi.org/10.1038/s41398-019-0476-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-019-0476-8
This article is cited by
-
Metabolite profiling of Althaea officinalis by HPLC-DAD-MS with in silico and in vitro analysis for therapeutic potential
Chemical Papers (2023)
-
Enhanced ultrasonic adsorption of pesticides onto the optimized surface area of activated carbon and biochar: adsorption isotherm, kinetics, and thermodynamics
Biomass Conversion and Biorefinery (2023)