Abstract
Post-traumatic stress disorder (PTSD) is a trauma- and stress-related disorder with dysregulated fear responses and neurobiological impairments, notably at neurotrophic and inflammation levels. Understanding the mechanisms underlying this disease is crucial to develop PTSD models that meet behavioral and neurobiological validity criteria as well as innovative therapeutic approaches. Serotonin 2C receptors (5-HT2CR) are known for their important role in anxiety, and mice having only the fully edited VGV isoform of 5-HT2CR, which thereby overexpressed brain 5-HT2CR, are of special interest to study PTSD predisposition. Innate and conditioned fear-related behaviors were assessed in VGV and wild-type mice. mRNA expression of brain-derived neurotrophic factor (BDNF), tissue-plasminogen activator (tPA), and pro-inflammatory cytokines (IL-6, IL-1β, and calcineurin) were measured by qRT-PCR. The effect of acute and chronic paroxetine was evaluated on both behavior and gene expression. VGV mice displayed greater fear expression, extensive fear extinction deficits, and fear generalization. Paroxetine restored fear extinction in VGV mice when administered acutely and decreased innate fear and fear generalization when administered chronically. In parallel, Bdnf, tPA, and pro-inflammatory cytokines mRNA levels were dysregulated in VGV mice. Bdnf and tPA mRNA expression was decreased in the hippocampus but increased in the amygdala, and chronic paroxetine normalized Bdnf mRNA levels both in the amygdala and the hippocampus. Amygdalar calcineurin mRNA level in VGV mice was also normalized by chronic paroxetine. VGV-transgenic mice displayed behavioral and neurobiological features that could be accessory to the investigation of PTSD and its treatment. Furthermore, these data point out to the role of 5-HT2CR in neuroplasticity and neuroinflammation.
Similar content being viewed by others
Introduction
Post-traumatic stress disorder (PTSD) is a prevalent trauma- and stress-related disorder caused by exposure to a strong psychological trauma. This disorder is characterized by hyperarousal and dysregulated fear responses triggered by contexts and cues reminding the traumatic event. PTSD patients also suffer from fear memory extinction deficits and contextual fear generalization1,2. Chronic treatment with selective serotonin reuptake inhibitors (SSRIs, such as paroxetine) is the first-line pharmacological approach, while behavioral therapies include the prolonged exposure therapy that generates fear extinction. However, no more than 30% of patients reached full remission with pharmacological therapies, while at least 40% of patients are non-responders to behavioral approaches1,2. Combining pharmacological and prolonged exposure therapies could theoretically present increased benefits. Nevertheless, clinical studies on cognitive behavioral therapy and SSRI are sparse and non-conclusive3,4. There are indications that chronic antidepressant treatment may in some cases even impair fear extinction5.
A number of reports argue for the involvement of 5-HT and in particular serotonin 2c receptors (5-HT2CR) in anxiety. PTSD patients display a range of serotonergic abnormalities, including an exaggerated stress response to the anxiogenic 5-HT2CR agonist meta-chlorophenylpiperazine6 and typical traits of a serotonergic alteration including irritability, aggression, impulsivity, and suicidability7, which are themselves associated with upregulation of 5-HT2CR and altered 5-HT2CR mRNA splicing/editing8,9,10.
Animal models such as predator or aggressive conspecific exposure, or the single prolonged stress exposure, provided some understanding about the pathophysiology of PTSD11,12. These models create anxiety-like behaviors as well as alterations of brain-derived neurotrophic factor (BDNF)-TrkB and serotonergic receptors. Stresses triggering PTSD-like states increase the expression of brain 5-HT2CR. PTSD symptoms may be alleviated by antidepressant drugs with 5-HT2CR antagonist properties13,14,15 or by selective 5-HT2CR antagonists16,17,18. Notably agomelatine, an antidepressant with melatonergic agonist and 5-HT2C antagonist properties, is now considered as a possible compound for the treatment of anxiety disorders including PTSD as it alleviates anxiety symptoms in animal models19,20 and in humans21 while presenting a good tolerability profile in patients21.
The 5-HT2CR is among the most frequently pinpointed for its implications in anxiety, stress, and fear behaviors22,23,24,25. It is the only serotonergic receptor undergoing adenosine-to-inosine edition of its pre-mRNA. Maternal separation stress, generating PTSD-like predispositions, robustly increased 5-HT2CR editing26. We have shown that increasing 5-HT2CR editing level interferes with 5-HT2CR mRNA alternative splicing processes, leading to a large upregulation of the receptor at cell membrane24. Dysregulation of 5-HT2CR editing using mice expressing only the fully edited VGV isoform of the 5-HT2CR (VGV mice) enhanced anxiety, aggression, and innate fear behaviors24,27. We thus determined here if VGV mice could display additional features of PTSD in a conditioned fear paradigm.
Chronic PTSD is also associated with changes in biological markers, including BDNF and pro-inflammatory cytokines, the blood levels correlating with SSRI effectiveness28,29. 5-HT2CR blockade or deletion both alter the expression of BDNF30,31. This neurotrophin is a key regulator of synaptic plasticity and behaviors, while BDNF–serotonergic interactions appear to occur in anxio-depressive disorders32. BDNF is also known to regulate cortical, hippocampal, and amygdalar-dependent memories33, while the BDNF/TrkB pathway has been linked to fear conditioning processes34, fear extinction35,36, and fear generalization37. We thus focused on Bdnf and also the mRNA encoding tPA, which mediates the conversion of precursor proBDNF into mature BDNF. Furthermore, considering the important crosstalks between serotonin, BDNF, and inflammation38, as well as cytokine-5-HT2CR editing interactions39, we also examined brain levels of IL-6, IL-1β, and calcineurin in VGV mice. It has been proposed that a mutual feedback loop regulation involving the serotonin transporter and BDNF helps in maintaining the brain balance between serotonergic and neurotrophin signaling38. The cytokine-induced perturbations of brain serotonergic activity may also alter the BDNF/TrkB pathway38.
The objectives of this work were to define the consequence of the 5-HT2CR editing modification on fear behaviors, to pinpoint the involvement of serotonin and 5-HT2CR on these outcomes and on the downstream BDNF and inflammation pathways and finally to examine the effects of paroxetine treatments on behavioral and neurobiological changes found in VGV mice. We focused on paroxetine, the first-line antidepressant drug treatment for PTSD, which does not have affinity for 5-HT2CR, and which is known to desensitize 5-HT2CR after chronic treatment40.
Methods and materials
Animals
Ten-week-old male, either control C57BL/6J or expressing VGV 5-HT2CR, mice were used, unless detailed. Details are given in the Supplementary Materials. All procedures concerning animal care and treatment were carried out in accordance with protocols approved by French ethical committee #C2EA-05 Charles Darwin and licensed by Directorate General for Research and Innovation (French government), under protocol authorization #00966.02. The experimental groups were randomly designed.
Experimental design
For behavioral studies using chronic paroxetine, experimental designs are described in Fig. 2. For behavioral studies using acute paroxetine, experimental designs are described in Fig. 3. For Reverse transcription quantitative polymerase chain reaction (RT-qPCR) analyses, cerebral structures of interest were collected from dedicated groups after treatment.
Conditioned and innate fear procedures
To observe the consequences of the VGV genotype on innate and conditioned fear behaviors, we performed ultrasound-induced fear evaluation and fear conditioning experiments.
Apparatus and analysis are detailed in Supplementary Materials. Behaviors were monitored by a video camera, and freezing, defined as total lack of movement except respiration, was scored.
Fear conditioning: On Day 1, mice were placed in the conditioning chamber and after a 3-min baseline period, they received six times an auditory conditional stimulus (CS; 30 s, 2.5 kHz, 85 dB) immediately followed by the unconditioned stimulus (US; 2 s, 0.5 mA foot-shock, inter-trial intervals 2 min).
Cued extinction: On Day 2, for experimental designs 1 and 3, or Day 30, for experimental design 2 (Fig. 2a, f), mice received 20 exposures to the same tone (30 s, 2.5 kHz, 85 dB; inter-trial intervals 5 s) in a new context to assess CS-induced fear and its extinction. As shown in Fig. 3, the same procedure was repeated on Day 3, to determine the consolidation of the fear extinction memory.
The mice were tested for innate fear reactions to trains of ultrasonic stimuli (100 ms frequency sweeps of 17–20 kHz, 85 dB, alternately 2 s off and 2 s on) in their home cage during 1 min and after a 3-min baseline period, as previously described24. Data were monitored by a video camera. Innate fear corresponds to the immediate expression of reflex-like defensive behaviors, here freezing, generated by a brief stimulus not associated with a previous aversive event.
Barnes maze test
Tests were performed as previously described41. Learning and reversal learning were each performed during 4 days with three sessions per day. Errors and latencies before finding the escape box were measured. The reversal probe test was conducted immediately after the last reversal learning session.
Quantification of RNA levels by RT-qPCR
To determine the role of serotonin and the 5-HT2CR on the BDNF/TrkB and inflammation pathways, we quantified the mRNA expression of key molecules of these pathways.
Tissue samples were quickly removed and frozen in liquid nitrogen. Total mRNA was extracted using TRI Reagent (Ambion, Applied Biosystems, Courtaboeuf, France), following manufacturer’s instructions. Reverse-transcription was performed using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Courtaboeuf, France) and PCR amplifications were performed using a SYBR Green mix (KAPA SYBR Fast qPCR Master Mix, KAPA Biosystems, MA, USA). For detailed cycling protocols and primers’ sequences, see supplementary information.
Drug administrations
To assess the effect of SSRI on our different targets, we performed chronic and acute administrations of paroxetine, as well as acute administration of 5-HT2C antagonists.
For chronic treatment, paroxetine HCl (Sequoia Research Products, Pangbourne, UK) was administered in drinking water (~5.5 mg/kg/day, starting 4 weeks before the experiments), to avoid stress sensitizing VGV mice with the daily injection stress. Chronic treatment was not interrupted during behavioral studies. Treatment intake was closely monitored and the solution concentration was adjusted per animal, to ensure equivalent intake of paroxetine. Acute paroxetine HCl was injected intraperitoneally (i.p.) at 16 mg/kg (in 0.9% saline). For both paroxetine behavioral studies, experimental timeline is described in Figs. 2 and 3. SB242084 (Abcam Biochemicals, Cambridge, UK) was injected i.p. at 1 mg/kg (in 0.9% saline, 1% Tween 80). Agomelatine (Servier Laboratories, Suresnes, France) was injected i.p. at 50 mg/kg and dissolved in 1% hydroxyethylcellulose (Servier Laboratories).
Statistical analysis
The number of animals per experiment was based on a power analysis42. Data were analyzed using Prism (GraphPad, San Diego, USA). For two groups comparisons, an unpaired Student’s t-test was used, with Welch’s correction if needed. All remaining data were compared using a two-way analysis of variance (ANOVA), followed by the Bonferroni post-hoc test. See Supplementary section for details. Data are presented as mean ± s.e.m.
Results
The conditioned fear-related behaviors of VGV mice
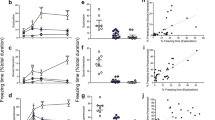
We first assessed the emotional neutrality of the auditory cue in transgenic mice. Mice were placed in the fear conditioning apparatus and submitted to the CS only. The 2.5 kHz tone did not a priori elicit any fear responses in VGV mice (Fig. 1a). Furthermore, on Day 1 of the fear conditioning procedure, similar to wild type (WT), VGV mice did not show freezing during baseline or in reaction to the first tone (Fig. 1b). Freezing progressively increased during the first five CS deliveries to reach ~70% in WT mice, but VGV mice reached this plateau at the third CS delivery.
a The sound used as CS (30 s, 85 dB, 2.5 kHz) for the conditioning is neutral and did not elicited fear responses in VGV mice when presented alone. b VGV mice acquired fear conditioning faster compared to WT mice. Mean ± s.e.m percent of time spent freezing during each CS presentation. Two-way ANOVA with repeated measures indicated a significant effect of Genotype [F (1, 16) = 28.42, p < 0.0001], Time [F (6, 96) = 107.9, p < 0.0001], and an interaction between those factors [F (6, 96) = 7.85, p < 0.0001]. c VGV mice displayed high fear generalization during the baseline period of the tone-testing session (t10.31 = 5.63, p = 0.0002). d Throughout the CS presentation, VGV mice presented very high freezing with a lack of fear extinction and a generalization of fear at t = 0. For the first extinction session, a two-way ANOVA with repeated measures indicated a significant effect of Genotype [F (1, 16) = 70.82, p < 0.0001], Time [F (12, 192) = 9.56, p < 0.0001], and an interaction between factors [F (12, 192) = 3.51, p = 0.0001]. e Twenty-four hours later, a second extinction session was conducted. Two-way ANOVA with repeated measures indicated a significant effect of Genotype [F (1, 16) = 117.8, p < 0.0001], Time [F (12, 192) = 6.21, p < 0.0001], and an interaction between factors [F (12, 192) = 4.51]. f WT mice displayed retention of the extinction memory, quantified by an extinction index defined as (Day 2 freezing at CS1) − (Day 3 freezing at CS1), while VGV did not (t15 = 2.45, p = 0.0271); *p < 0.05, ***p < 0.001, ****p < 0.0001 vs. WT
Extinction was induced by repeated CS exposure in a new context. At both Days 2 and 3, VGV mice exhibited a high baseline freezing, indicative of contextual fear generalization (freezing at Day 2: 54.6 ± 8.5%, Day 3: 60.2 ± 8.6%). On Day 2, the difference in baseline freezing in VGV mice compared to WT mice was as much as 50% (Freezing WT: 2.1 ± 1%, VGV: 54.6 ± 8.5%; Fig. 1c). Freezing decreased in WT mice with repeated CS, without shocks, to reach 20.4 ± 9.1%, while it remained maximal in VGV mice (Fig. 1d, e). Retention of extinction between the two days was quantified by analyzing an inter-session extinction index defined as (Day 2 freezing at CS1) − (Day 3 freezing at CS1), for each mouse, and indicated a significant reduction of extinction in VGV mice (Fig. 1f). Contrary to WT mice, there was no reduction in the total amount of freezing at Day 3 vs. Day 2 in VGV mice (WT = −15.3 ± 5.2% vs. VGV = −2.2 ± 2.9%; t15 = 2.12, p = 0.033). Considering that Htr2c is an X-linked gene43, we assessed whether gender differences existed, but there were none (Fig. S1).
Even 1 month after conditioning, deficits in fear extinction and fear generalization persisted (Fig. S2A, B). VGV mice also showed a deficit of context extinction (Fig. S2C) persisting even after 6 days of re-exposure (data not shown).
Spatial memory and cognitive flexibility in VGV mice
It was necessary to assess whether VGV mice have extended memory alterations beside those observed in fear conditioning. No difference was found in the Barnes maze (see supplementary data, Fig. S3). Furthermore, VGV mice could perform this test optimally as they did not present any locomotor impairment (Fig. S3C, S3F).
Assessment of paroxetine brain delivery
To validate the efficacy of oral chronic paroxetine treatment, corticotropin-releasing hormone (Crh) mRNA level was measured, as antidepressant treatments were found to reduce the Crh gene expression in rodents44. Crh mRNA was decreased to the same amplitude in the brain of WT and VGV mice (Fig. S4).
Effects of chronic paroxetine on conditioned and innate fear
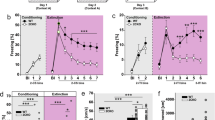
We first performed a chronic paroxetine treatment prior to fear conditioning, as this was shown to desensitize 5-HT2CR45 (see experimental design Fig. 2a). Paroxetine-treated VGV mice displayed reduced freezing during the acquisition phase of fear conditioning compared to vehicle-treated VGV mice (Fig. 2b). Note that freezing reactions during fear acquisition in paroxetine-treated VGV mice were not increased compared to WT mice (mean freezing calculated on the whole acquisition session for each group. Vehicle-treated WT: 27.3 ± 10%; vehicle-treated VGV: 59.7 ± 15.6%; paroxetine-treated VGV: 30.9 ± 6.7%; Fig. 2b). During extinction, a global decrease in freezing was observed in paroxetine vs. vehicle-treated VGV mice. However, the extinction deficit remained in paroxetine-treated VGV mice (Fig. 2c). Paroxetine reduced the total amount of freezing during repeated CS presentation from 80.8 ± 2.7% to 50 ± 4.8% and decreased from 54.6 ± 4.6% to 39.9 ± 4.7% the freezing during baseline (contextual generalization) in VGV mice (Fig. 2d, e; WT mice had no generalization).
For chronic paroxetine studies, groups underwent per os treatment (~5.5 mg/kg/day) for 28 days in drinking water and two different timelines of experiment were used. In each design, chronic treatment was not interrupted during the whole set of behavioral studies. For each experiment, only the 11 first CS are presented as, after a prolonged time, quiet immobility may be confounded with freezing. a In the first design, chronic treatment was given for 28 days prior to the fear conditioning, which started on the 29th day. b When administered before the acquisition session, chronic paroxetine decreased freezing in VGV mice during the CS presentation of the acquisition phase. In WT mice, two-way ANOVA indicated a significant effect of Time [F (6, 96) = 77.94, p < 0.0001] but no effect of treatment. In VGV mice, two-way ANOVA indicated a significant effect of Treatment [F (1, 20) = 33.85, p < 0.0001], Time [F (6, 120) = 63.73, p < 0.0001], and an interaction between factors [F (6, 120) = 11.32, p < 0.0001]. c Chronic paroxetine tended to impair extinction in WT mice. ANOVA indicated a nearly significant effect of Treatment [F (1, 31) = 3.64, p = 0.06], Time [F (11, 341) = 14.81, p < 0.0001], and an interaction between factors [F (11, 341) = 1.89, p = 0.03], while in contrast reducing overall freezing in VGV mice. ANOVA indicated a significant effect of Treatment [F (1, 19) = 25.98, p < 0.0001] and Time [F (11, 209) = 4.32, p < 0.0001] but no interaction. d When analyzing the mean total freezing during the extinction session, there was a significant effect of Genotype [F (1, 50) = 168, p < 0.0001], Treatment [F (1, 50) = 17.94, p < 0.0001], and an interaction between factors [F (1, 50) = 36.78, p < 0.0001]. e Fear generalization is reduced by chronic paroxetine in VGV mice (t19 = 2.21, p = 0.04). f In the second design, chronic treatment was started 24 h after the training day (Day 1) of the fear conditioning paradigm. The 28-day treatment was followed by the extinction session, which consequently took place on Day 30 of the timeline. g In the experimental design 2, no difference was detected in the acquisition of the fear conditioning among each genotype, prior to treatment. h When chronic paroxetine is administered between the acquisition and the extinction sessions, there was a significant effect of Genotype [F (1, 24) = 35.89, p < 0.0001], Treatment [F (1, 24) = 7.88, p = 0.0098], and an interaction between factors [F (1, 24) = 16.54, p = 0.0004] for the mean total freezing during the extinction session. i Fear generalization is reduced by chronic paroxetine in VGV mice (t17 = 2.28, p = 0.0357). j Chronic paroxetine reduced the overall freezing in VGV mice. In WT mice, ANOVA indicated no effect of Treatment [F (1, 8) = 3.46, p = 0.1], a significant effect of Time [F (11, 88) = 2.35, p = 0.0138], and no interaction between factors [F (11, 88) = 1.01, p = 0.44]. In VGV mice, ANOVA indicated a significant effect of Treatment [F (1, 16) = 23.08, p = 0.0002] and Time [F (11, 176) = 2.44, p = 0.0074] but no interaction. ***p < 0.001, ****p < 0.0001 vs. WT Vehicle; #p < 0.05, ####p < 0.0001 vs. VGV Vehicle
Using experimental design 2 (Fig. 2f), we determined the effect of chronic paroxetine on extinction once the training phase was completed, to mimic post-trauma paroxetine treatment in patients. First, we verified that groups from the same genotypes were not different before treatment during the acquisition session (Fig. 2g). Again, when measured 24 h after the end of paroxetine treatment, a global decrease in freezing (from 79.4 ± 5.7% to 35.5 ± 6.9%) and in fear generalization (Fig. 2j) occurred in paroxetine vs. vehicle-treated VGV mice while their extinction deficit remained (Fig. 2h, i).
We had previously shown that VGV mice display higher freezing to an innately aversive ultrasound delivered in their home cage24. VGV mice exhibited high freezing (43.9%) during the 1-min post-stimulus period, while WT mice displayed very little freezing during this period (Fig. S5). Chronic paroxetine effectively reduced ultrasound-induced freezing in VGV mice (−19.4 ± 8.7%; Fig. S5).
All these data suggest that chronic paroxetine induced an anxiolytic-like effect without restoring fear extinction in VGV mice.
Behavioral effects of acute paroxetine
Because paroxetine desensitizes autoreceptors during chronic treatments, and somehow delayed here fear extinction in WT mice (Fig. 2c, h), we decided to assess the effect of an acute treatment (Fig. 3a) in conditions similar to antidepressant behavioral-screening assays (16 mg/kg, i.p., 30 min before test). Acute paroxetine decreased the expression of freezing during the extinction in both WT and VGV mice (Fig. 3b). In addition, it induced a significant progressive decrease of freezing within session in VGV mice (Fig. 3b, Fig. S6), as quantified by significant differences in the intra-session extinction index (Fig. S6A). After a 24-h period, a second extinction session was conducted (without paroxetine injection; Fig. 3a). Extinction was still observed within the session in VGV mice previously administered with paroxetine (Fig. 3c and Fig. S6B). However, fear extinction was not consolidated between Days 2 and 3 in VGV mice, as opposed to the WT vehicle group (inter-session extinction index, Fig. S6C). Nevertheless, acute paroxetine at D2 exerted also a decrement of fear generalization, and this decrement appeared consolidated at D3 (D2: t22 = 4.32, p = 0.0003; D3: t22 = 3.62, p = 0.0015; Fig. 3b, c at CS 0).
a We assessed the effect of an acute injection of paroxetine on the extinction process. Paroxetine was injected intraperitoneally 24 h after the conditioned fear acquisition session and 30 min before the first extinction session. A drug-free extinction session was also performed 24 h later. b Acute paroxetine (16 mg/kg, i.p., 30 min before the first extinction session) strongly decreased freezing in WT mice (effect of Treatment [F (1, 20) = 21.44, p = 0.0002], effect of Time [F (11, 220) = 6.26, p < 0.0001], interaction between factors [F (11, 220) = 2.75, p = 0.002]) and decreased freezing during extinction in VGV mice (effect of Treatment [F (1, 23) = 135.0, p < 0.0001], effect of Time [F (11, 253) = 3.79, p < 0.0001], interaction between both factors [F (11, 253) = 3.96, p < 0.0001]). In addition, in the paroxetine-injected VGV mice, CS-induced freezing was similar to that of vehicle-treated WT mice (no effect of treatment, F (1, 22) = 2.59, p = 0.12). c After a 24-h period, VGV mice treated on the previous day with paroxetine presented extinction (effect of Treatment [F (1, 22) = 99.52, p < 0.0001], effect of Time [F (11, 242) = 3.02, p = 0.0008], interaction between factors [F (11, 242) = 2.84, p = 0.002]) while paroxetine-treated WT mice presented reduced freezing (effect of Treatment [F (1, 20) = 10.88, p = 0.0036], effect of Time [F (11, 220) = 9.89, p < 0.0001], but no interaction). Moreover, there was no difference in freezing between VGV mice treated on the previous day with paroxetine and vehicle-treated WT mice (no effect of treatment, F (1, 22) = 0.93, p = 0.34). Finally, the extinction index was significantly increased in paroxetine-treated VGV mice (F (3, 40) = 4.67, p = 0.0069, with Bonferroni post-hoc test indicating a significant difference between WT Vehicle and VGV Vehicle groups as well as between VGV Vehicle and VGV Paroxetine groups); ****p < 0.0001 vs. Vehicle
Behavioral effects of 5-HT2CR antagonists
Administration of the selective and potent 5-HT2CR antagonist SB242084 (1 mg/kg, i.p.) also strongly inhibited freezing in VGV mice during the extinction process (Fig. S7A). However, SB242084 produced great hyperactivity in VGV mice, as might have been expected from the literature on the effect of SB242084 in WT mice46. To avoid this confounding effect, a less potent 5-HT2CR antagonist, the antidepressant compound agomelatine (50 mg/kg, i.p.), was used. Acute administration of agomelatine in VGV mice only tended to decrease both the total amount of freezing during the extinction session (Fig. S7B) and fear generalization (Fig. S7C) and did not favor any extinction in VGV mice (data not shown).
Alterations of Bdnf mRNA expression in VGV mice
Basal expression of Bdnf mRNA was explored in several brain areas. The Bdnf gene is formed of nine exons, with the coding region located in exon IX corresponding to total Bdnf. Bdnf transcription occurs with various patterns of exons but we focused our analysis on exons I and IV because they are the main exons reported to be modulated in response to stress47, fear48,49, and neuronal activity50. Bdnf was decreased in the hippocampus (Fig. 4a, left), and there was also a tendency for a decrease in the frontal cortex (t14 = 1.86, p = 0.08; Fig. 4a, middle) of VGV mice. In contrast, Bdnf was increased in the amygdala of VGV mice (Fig. 4a, right). Bdnf exon IV was decreased in the hippocampus (Fig. 4b, left) and the frontal cortex (Fig. 4b, middle), and Bdnf exon I was increased in the amygdala of VGV mice (Fig. 4c, right).
a, b Total Bdnf and exon IV mRNA levels were significantly decreased in the hippocampus of VGV mice (Total Bdnf; t14 = 3.04, p = 0.009; Exon IV: t14 = 6.17, p < 0.0001). No difference was detected in exon I. b There were no significant changes in the frontal cortex in total Bdnf and exon I mRNA expression, although Bdnf exon IV mRNA expression was significantly decreased in VGV mice (t14 = 5.55, p < 0.0001). a, c Total Bdnf and exon I mRNA levels were significantly increased in the amygdala of VGV mice (Total Bdnf; t15 = 4.59, p = 0.0004; Exon I: t9.69 = 3.39, p = 0.007)
Effects of chronic paroxetine on trophic and inflammation factors in VGV mice
We examined the effect of chronic paroxetine in areas where total Bdnf was altered in VGV mice. In water-treated VGV mice, Bdnf mRNA expression was found decreased in the hippocampus (t16 = 3.92, p = 0.0012) and increased in the amygdala (t13 = 2.85, p = 0.0136; Table 1A). After chronic paroxetine, no difference in total Bdnf mRNA expression was detected between WT and VGV mice (Table 1A). In a separate analysis, we observed that chronic paroxetine normalized Bdnf mRNA in paroxetine-treated VGV mice compared to vehicle-treated mice, as it decreased amygdalar Bdnf mRNA and tended to increase hippocampal Bdnf mRNA (t14 = 2.55, p = 0.02 and t12 = 1.93, p = 0.08, respectively; data not shown).
Chronic paroxetine had distinct effects on Bdnf exon IV depending on areas: in water-treated VGV mice, exon IV mRNA was found again decreased in the hippocampus, not in the amygdala (Table 1A). However, in the amygdala, paroxetine-treated VGV mice displayed lower Bdnf exon IV mRNA levels compared to paroxetine-treated WT mice (Table 1A). The treatment did not change Bdnf exons I or IV in the hippocampus (Table 1A). The higher amygdalar Bdnf exon I mRNA expression observed in water-treated VGV mice was not observed after chronic paroxetine (Table 1A).
The conversion of proBDNF into the mature BDNF form is mediated by tPA. In water-treated animals, tPA mRNA expression was significantly reduced in the hippocampus of VGV mice compared to WT mice, but significantly increased in the amygdala (Table 1A). After chronic paroxetine, no difference was detected between WT and VGV mice in the hippocampus, but tPA mRNA level remained significantly higher in the amygdala (Table 1A). The alteration of tPA in the hippocampus was linked to the effect of treatment (paroxetine-treated VGV mice vs. vehicle-treated mice; t15 = 2.31, p = 0.0358; data not shown).
Because 5-HT2C receptor editing appears to alter neuro-inflammation39, classical cytokines (IL-6, IL-1β) mRNA expression levels were determined in the same brain areas of VGV mice. We also examined calcineurin, a cytokine regulated by fear extinction51. Amygdalar IL-1β, IL-6 mRNA levels and hippocampal IL-1β mRNA level were increased in VGV mice, but these differences persisted after paroxetine treatment (Table 1B). Calcineurin mRNA levels was increased in the amygdala of vehicle-treated VGV mice. In contrast, this difference did not exist in paroxetine-treated VGV mice (Table 1B).
Discussion
Increased 5-HT2CR transmission has long been involved in anxiety, and ADAR1, a 5-HT2CR editing enzyme, is increased by stress in animals and associated with suicide in patients17,52,53. We had previously studied VGV mice expressing only the fully edited 5-HT2CR VGV isoform, which, as a result of altered splicing event, massively express 5-HT2CR in limbic areas24. Because these mice display anxiety, aggressive behaviors, and strong freezing to an innately aversive stimulus24, we determined if they had additional features relevant to PTSD. We demonstrated that VGV mice exhibit faster fear acquisition during conditioning, extensive fear extinction deficits and fear generalization, together with alterations in brain BDNF and neuroinflammation. Our data thus suggest that VGV mice could be used as a genetic model of PTSD vulnerability as, when exposed to an important stress stimulus, they display PTSD-like behavioral and neurobiological features, some of which could be prevented by chronic paroxetine, a first line treatment of PTSD. The high freezing profile of VGV mice to an innate fear stimulus is characteristic of a state of stress sensitization54, similar to stress-induced 5-HT2CR activation in the amygdala triggered by behavioral stress procedures17,55. It was hypothesized that 5-HT2CR hyperactivity in the amygdala is central to anxiety symptoms in PTSD. Consequently, VGV mice, with their high expression of 5-HT2CR, may constitutively mimic a history of stress sensitization.
Under stress conditions, the 5-HT2CR was shown to exert a control over 5-HT neurotransmission45. In parallel, stress disturbs BDNF gene expression and this effect seems to be mediated at least in part, through perturbations in the serotonin signaling32. Inducing a reduction in BDNF also appears to intensify the anxiety-like behaviors and the stress signaling responses in mice deficient for the serotonin transporter56, suggesting that, in addition to their reciprocal regulatory feedback mechanism, serotonin and BDNF interact in the modulation of anxiety and stress. The BDNF Val66Met polymorphism, impacting activity-dependent secretion of BDNF, has been repeatedly described as a predisposition factor, associated in both human and animals with anxiety57, impaired fear extinction58,59, and fear generalization37, suggesting its involvement in PTSD60. VGV mice had a marked fear extinction deficit, which is consistent with the results observed after hippocampus-specific BDNF modulations35,36. The fear generalization observed in VGV mice is also likely an inability to properly use contextual cues to modulate fear responses via the hippocampus37. The increase in Bdnf mRNA expression in the amygdala of VGV mice is also interesting as amygdalar BDNF plays a central role for acquisition and consolidation of conditioned fear34,49 correlating with cue conditioned responses61. As already mentioned, we studied exons I and IV because they are the mains exons affected in response to stress47, fear48,49, and neuronal activity50. It would also be interesting to study the expression of other Bdnf exons. It has been shown that proBDNF, on the other hand, could disturb learning and memory62 and that the proteolysis of proBDNF by tPA is involved in memory formation63. Low levels of tPA create a deficit in long term potentiation (LTP)64 that has been linked to impairment in contextual and fear memory65,66. Moreover, stress upregulates tPA in the amygdala and this increase is linked to higher anxiety-like behaviors67, which is consistent with the stress sensitization-like profile of VGV mice.
Inflammation, which has important crosstalks with BDNF and serotonin38, has been involved in PTSD, as increased pro-inflammatory cytokines levels were detected in PTSD patients68,69. Extensive data indicate that inflammation affects the activity of the serotonergic system, notably the activity of the serotonin transporter (for review, see ref. 38). More interestingly, inflammatory cytokines were shown to induce an increase in 5-HT2CR editing levels39. Aside from the perturbations that an inflammatory state could induce in the mutually regulating brain balance between serotonergic and BDNF signaling, direct links between inflammation, memory, or neuroplasticity involving BDNF were also found70,71,72 as well as between inflammation and memory relevant to fear processing73,74. Calcineurin was also involved in fear memory75,76 and an enhanced calcineurin activity in the amygdala was linked to fear extinction deficits51. Finally, neuro-inflammation also impairs contextual discrimination, without impacting other hippocampal-dependent tasks such as spatial memory77,78. VGV mice seem to constitutively present a pro-inflammatory status, indicated by the overexpression of IL-1β, IL-6, and calcineurin mRNA. We could hypothesize that the augmented expression of 5-HT2CR in VGV mice may trigger, through Gq-protein-mediated PLC activity, an increased Ca2+ mobilization in affected neurons and a high intracellular Ca2+ level is known to induce neuroinflammation by activating caspase-1, an enzyme responsible for the maturation of IL-1β79. Additionally, the 5-HT2CRs via Gq-protein induce PLA2 activity is also well-known to mediate inflammatory responses80. The neuroinflammation observed here in VGV mice is therefore consistent with the literature.
Since chronic paroxetine is known to desensitize 5-HT2CRs40, we first used this treatment to reduce the mRNA editing-mediated 5-HT2CRs overexpression phenotype of VGV mice and examine its effects on the behavioral and neurobiological changes found in VGV mice. Chronic paroxetine reduced generalization, maybe by normalizing hippocampal BDNF and neuronal excitability81. The increased Bdnf in the amygdala of VGV mice was successfully prevented by chronic paroxetine, consistently with its anxiolytic effect82, with data about 5-HT2CR desensitization occurring, at the behavioral, neurochemical, and cell-signaling levels, and after 5-HT reuptake carrier inactivation40,45. The paroxetine-induced 5-HT2CR desensitization led to reduced freezing in VGV mice during both conditioning and tone testing via an attenuation of non-associative fear sensitization. However, trauma-associated fear memory can be subdivided in two forms of learning: associative memories, directly resulting from CS–US pairing during the conditioning process, and non-associative memories, involving anxiety-like fear sensitization83,84. Both of these memory components (present in VGV mice; Fig. 5) should be inhibited to allow successfully overcoming a traumatic event and preventing later fear reinstatement. Additionally, except for amygdalar calcineurin mRNA levels, neuroinflammation was not prevented by chronic paroxetine. Note that calcineurin can directly interact with the serotonin transporter85, a mechanism which may underlie the positive effect of paroxetine on calcineurin in VGV mice. In a previous study, a tricyclic antidepressant drug was better than paroxetine to decrease brain inflammation factors86. The present murine model could thus be used to decipher how to best treat anxiety-associated inflammation. It has been argued that SSRI’s anti-inflammatory effect is not sufficient, justifying the necessity of investigating alternative agents with clear anti-inflammatory properties, such as glucocorticoids that additionally have a therapeutic effect on other PTSD symptoms87.
Assessment of current data in view of a PTSD validity criteria checklist93. a Face validity: both associative and non-associative memories are part of the traumatic fear state, and these two components need to be inhibited to treat PTSD. VGV mice displayed both abnormal associative memories (impaired fear extinction) (Fig. 1d, e) and non-associative memories (fear sensitization) (Fig. 1c). Human PTSD is most often triggered by a brief event and the PTSD-like behavioral state of VGV mice can be induced by a relatively brief aversive stressor. Another criterion is symptom perseverance: VGV mice’s fear-related dysregulations persisted weeks after conditioning and did not spontaneously go into extinction, even after numerous context or cue presentations (Fig. S2A). VGV mice also presented exaggerated fear responses, that is hypervigilance, to trauma-related cues (CS) (Fig. 1b) as well as to innately aversive stimuli (Fig. S5)24,27. b Construct validity: Bdnf mRNA exons and tPA mRNA dysregulations were found in the hippocampus, the amygdala, and the frontal cortex of VGV mice (Fig. 4 and Table 1A). Such dysregulations had previously been suggested to account for memory processes, synaptic plasticity, and neuronal activity impairments in patients. Deficit in fear extinction correlates with lower hippocampal activation97 and hyperactivation of the amygdala98. An increase in pro-inflammatory cytokines IL-1β and IL-6 mRNA levels was also found in the hippocampus and the amygdala of VGV mice (Table 1B). Inflammation appears to be involved in both the pathophysiology of PTSD95,99 and in PTSD vulnerability96. 5-HT2CR expression is upregulated in the VGV mice and these receptors are known to be associated with vulnerability to mood disorders in human100,101,102. One limitation of the present genetic model is the extent of 5-HT2CR upregulation in these mice. c Predictive validity: paroxetine is the first-line pharmacological treatment of PTSD. Chronic paroxetine produces anxiolytic effects in human PTSD as well as in VGV mice. Chronic paroxetine is given here either before the fear conditioning or as a post-traumatic treatment, with similar results (Fig. 2). The putative improved therapeutic effect of a paroxetine treatment starting prior to exposure therapy still needs to be assessed in human
Here, our two different experimental timelines for the chronic paroxetine treatment produced the same behavioral outcomes in VGV mice. However, one has to keep in mind that the rationale for the first chronic treatment prior to shock exposure was to determine the effects of 5-HT2CR desensitization, not to mimic a clinical use of paroxetine as a prophylactic treatment. Indeed, as previously observed in rats5, we observed that chronic paroxetine tends to impair fear extinction in WT mice, which argues against using paroxetine as a prophylactic treatment.
Chronic paroxetine was ineffective in restoring the extinction process in VGV mice. We thus decided to assess whether blocking 5-HT2CR would have an effect. Agomelatine, which is a relatively weak and non-selective 5-HT2CR antagonist, did not produce a significant effect, while the hyperactivity produced by the potent and selective 5-HT2CR antagonist SB24208446, precluded any conclusion. Interestingly, it has been demonstrated that the binding profile of agomelatine is not modified by the level of edition of the 5-HT2CR isoforms88, which is a factor to consider when targeting directly the 5-HT2CR. In turn, acute paroxetine did trigger within session fear extinction in VGV mice. Since the half-life of paroxetine in the mice brain was estimated at 2 h89 and its metabolites are inactive90, this strongly suggests that the effects observed at Day 3 are not the effect of some residual paroxetine molecules administered on the previous day, but rather are long-term consequences of some processes initiated on Day 2. The injection at Day 2 might have produced therapeutic-like effects by producing a surge in extracellular 5-HT, triggering somatodendritic 5-HT1A and terminal 5-HT1B autoreceptors activation, thereby decreasing neuronal firing and intrasynaptic 5-HT availability. Alternatively, acute paroxetine-induced surge in extracellular 5-HT might have restored fear extinction by activating post-synaptic 5-HT receptors, such as the 5-HT2AR subtype91.
Currently, neither pharmacological nor behavioral approaches are completely effective as there are non-responders to either approach. Data reporting the effects of combining one of the approved SSRI with an exposure therapy are rare and rather controversial. In a clinically relevant perspective (Fig. 5), the latter data suggest that combining these two types of therapies could present beneficial outcomes, but with a precise timeline of SSRI administration. More precisely, it suggests that the best period to initiate a SSRI treatment in PTSD patients is at the very beginning of an exposure therapy, as it could initially facilitate fear extinction memory acquisition, while prolongation of treatment up to chronicity can provide additional anxiolytic effects. However, caution has to be taken as it has been extensively described that acute SSRI treatments can trigger anxiogenic effects. In any case, chronic treatment has advantages compared to acute treatment, as acute SSRI treatment presents several well-described adverse effects (sexual and gastro-intestinal dysregulations, perturbations of appetite and weight, increased anxiety, among others) that nevertheless tend to disappear with chronic treatment. The idea that a specific timeline of treatment administration could provide beneficial effects was also recently studied with agomelatine. A single dose of this drug administered rapidly in the aftermath of a traumatic event seems to reduce the development of PTSD-like behavioral responses and the hippocampal stress-induced damages92.
The putative “face validity” of the present model should regroup pre-trauma cognitive vulnerability factors and PTSD-like symptoms in line with the “dual-branch hypothesis of PTSD”93. Accordingly, a PTSD model needs to combine both the memory- and stress-related processes. The characteristics of the PTSD-like symptoms of VGV model are in line with this criterion, as detailed in the Fig. 5. We hypothesized earlier that VGV mice could constitutively model a state of stress sensitization. This characteristic could mimic a reported vulnerability factor, the looming cognitive style94. The increase responses of VGV mice’s response to innate fear stimuli (initial reactions to foot-shocks and ultrasonic stimulus) could thus represent a behavioral manifestation of a fear sensitization in these animals. Regarding the “construct validity” criterion (Fig. 5), while it is not yet known why certain individuals develop PTSD after a traumatic event while others do not, it remains worth assessing this validity criterion around some of the most accepted hypotheses concerning PTSD pathophysiology, and numerous authors suggest relations between BDNF, inflammation, and PTSD predisposition95,96.
Overall, this study shows that VGV mice may constitute an interesting model of PTSD predisposition. These mice present important enhancements of both innate and conditioned fear. The present model has, nevertheless, limitations common to most genetic models, since the major alterations in serotonergic transmission, predisposing to PTSD-like behaviors, are triggered during development. Further studies should also investigate whether the PTSD-like profile of adult VGV mice can be reversed by vector-induced reduction of 5-HT2CR in the amygdala or by modulating amygdalar and hippocampal BDNF–TrkB pathway. Moreover, the genetic VGV model provides opportunities to further understand the role of the serotonin signaling on both the psychophysiological and biological correlates of PTSD. Finally, because it readily mimics an intense state of stress-sensitization and neuroinflammation, it offers new perspectives to quickly and effectively screen innovative drugs for PTSD.
References
Blechert, J., Michael, T., Vriends, N., Margraf, J. & Wilhelm, F. H. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav. Res. Ther. 45, 2019–2033 (2007).
Lopresto, D., Schipper, P. & Homberg, J. R. Neural circuits and mechanisms involved in fear generalization: implications for the pathophysiology and treatment of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 60, 31–42 (2016).
Simon, N. M. et al. Paroxetine CR augmentation for posttraumatic stress disorder refractory to prolonged exposure therapy. J. Clin. Psychiatry 69, 400–405 (2008).
Hetrick, S. E., Purcell, R., Garner, B. & Parslow, R. Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD). Cochrane Database Syst. Rev. 7, 1465–1858 (2010).
Burghardt, N. S., Sigurdsson, T., Gorman, J. M., McEwen, B. S. & LeDoux, J. E. Chronic antidepressant treatment impairs the acquisition of fear extinction. Biol. Psychiatry 73, 1078–1086 (2013).
Southwick, S. M. et al. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch. Gen. Psychiatry 54, 749–758 (1997).
Panagioti, M., Gooding, P. A., Triantafyllou, K. & Tarrier, N. Suicidality and posttraumatic stress disorder (PTSD) in adolescents: a systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 50, 525–537 (2015).
Niswender, C. M. et al. RNA editing of the human serotonin 5-HT2C receptor. alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology 24, 478–491 (2001).
Dracheva, S., Chin, B. & Haroutunian, V. Altered serotonin 2C receptor RNA splicing in suicide: association with editing. Neuroreport 19, 379–382 (2008).
Di Narzo, A. F. et al. A unique gene expression signature associated with serotonin 2C receptor RNA editing in the prefrontal cortex and altered in suicide. Hum. Mol. Genet. 23, 4801–4813 (2014).
Yamamoto, S. et al. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety 26, 1110–1117 (2009).
Pitman, R. K. et al. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787 (2012).
Alderman, C. P., Condon, J. T. & Gilbert, A. L. An open-label study of mirtazapine as treatment for combat-related PTSD. Ann. Pharmacother. 43, 1220–1226 (2009).
De Berardis, D. et al. Agomelatine for the treatment of posttraumatic stress disorder: a case report. Ann. Clin. Psychiatry 24, 241–242 (2012).
Hidalgo, R. et al. Nefazodone in post-traumatic stress disorder: results from six open-label trials. Int. Clin. Psychopharmacol. 14, 61–68 (1999).
Harada, K., Yamaji, T. & Matsuoka, N. Activation of the serotonin 5-HT2C receptor is involved in the enhanced anxiety in rats after single-prolonged stress. Pharmacol. Biochem. Behav. 89, 11–16 (2008).
Baratta, M. V. et al. Stress enables reinforcement-elicited serotonergic consolidation of fear memory. Biol. Psychiatry 79, 814–822 (2016).
Foilb, A. R. & Christianson, J. P. Serotonin 2C receptor antagonist improves fear discrimination and subsequent safety signal recall. Prog. Neuropsychopharmacol. Biol. Psychiatry 65, 78–84 (2016).
Millan, M. J., Brocco, M., Gobert, A. & Dekeyne, A. Anxiolytic properties of agomelatine, an antidepressant with melatoninergic and serotonergic properties: role of 5-HT2C receptor blockade. Psychopharmacology 177, 448–458 (2005).
Papp, M., Litwa, E., Gruca, P. & Mocaer, E. Anxiolytic-like activity of agomelatine and melatonin in three animal models of anxiety. Behav. Pharmacol. 17, 9–18 (2006).
De Berardis, D. et al. Is there a role for agomelatine in the treatment of anxiety disorders? A review of published data. Int. J. Immunopathol. Pharmacol. 26, 299–304 (2013).
Burghardt, N. S., Bush, D. E. A., McEwen, B. S. & LeDoux, J. E. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT2C receptor antagonist. Biol. Psychiatry 62, 1111–1118 (2007).
Heisler, L. K., Zhou, L., Bajwa, P., Hsu, J. & Tecott, L. H. Serotonin 5-HT2C receptors regulate anxiety-like behavior. Genes Brain Behav. 6, 491–496 (2007).
Martin, C. B. P. et al. RNA splicing and editing modulation of 5-HT2C receptor function: relevance to anxiety and aggression in VGV mice. Mol. Psychiatry 18, 656–665 (2013).
Marcinkiewcz, C. A. et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537, 97–101 (2016).
Bhansali, P., Dunning, J., Singer, S. E., David, L. & Schmauss, C. Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of the heterotrimeric G-protein G q. J. Neurosci. 27, 1467–1473 (2007).
Mombereau, C., Kawahara, Y., Gundersen, B. B., Nishikura, K. & Blendy, J. A. Functional relevance of serotonin 2C receptor mRNA editing in antidepressant- and anxiety-like behaviors. Neuropharmacology 59, 468–473 (2010).
Tucker, P. et al. Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol. Psychiatry 56, 121–128 (2004).
Berger, W. et al. Serum brain-derived neurotrophic factor predicts responses to escitalopram in chronic posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 1279–1284 (2010).
Hill, R. A. et al. Brain-derived neurotrophic factor expression is increased in the hippocampus of 5-HT(2C) receptor knockout mice. Hippocampus 21, 434–445 (2011).
Ring, R. M. & Regan, C. M. Captodiamine, a putative antidepressant, enhances hypothalamic BDNF expression in vivo by synergistic 5-HT2c receptor antagonism and sigma-1 receptor agonism. J. Psychopharmacol. 27, 930–939 (2013).
Martinowich, K. & Lu, B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology 33, 73–83 (2008).
Bekinschtein, P., Cammarota, M. & Medina, J. H. BDNF and memory processing. Neuropharmacology 76, 677–683 (2014).
Rattiner, L. M., Davis, M., French, C. T. & Ressler, K. J. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J. Neurosci. 24, 4796–4806 (2004).
Heldt, S. A., Stanek, L., Chhatwal, J. P. & Ressler, K. J. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 12, 656–670 (2007).
Rosas-Vidal, L. E., Do-Monte, F. H., Sotres-Bayon, F. & Quirk, G. J. Hippocampal–prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology 39, 2161–2169 (2014).
Mühlberger, A. et al. The BDNF Val66Met polymorphism modulates the generalization of cued fear responses to a novel context. Neuropsychopharmacology 39, 1187–1195 (2014).
Haase, J. & Brown, E. Integrating the monoamine, neurotrophin and cytokine hypotheses of depression–a central role for the serotonin transporter? Pharmacol. Ther. 147, 1–11 (2015).
Yang, W., Wang, Q., Kanes, S. J., Murray, J. M. & Nishikura, K. Altered RNA editing of serotonin 5-HT2C receptor induced by interferon: implications for depression associated with cytokine therapy. Brain Res. Mol. Brain Res. 124, 70–78 (2004).
Martin, C. B. et al. 5-HT2C receptor desensitization moderates anxiety in 5-HTT deficient mice: from behavioral to cellular evidence. Int. J. Neuropsychopharmacol. 18, pyu056 (2014).
Stragier, E. et al. Brain plasticity and cognitive functions after ethanol consumption in C57BL/6J mice. Transl. Psychiatry 5, e696 (2015).
Charan, J. & Kantharia, N. D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 4, 303–306 (2013).
Drago, A. & Serretti, A. Focus on HTR2C: a possible suggestion for genetic studies of complex disorders. Am. J. Med. Genet. Part B 150B, 601–637 (2009).
Brady, L. S., Gold, P. W., Herkenham, M., Lynn, A. B. & Whitfield, H. J. Jr. The antidepressants fluoxetine, idazoxan and phenelzine alter corticotropin-releasing hormone and tyrosine hydroxylase mRNA levels in rat brain: therapeutic implications. Brain Res. 572, 117–125 (1992).
Mongeau, R. et al. 5-HT2C receptor activation prevents stress-induced enhancement of brain 5-HT turnover and extracellular levels in the mouse brain: modulation by chronic paroxetine treatment. J. Neurochem. 115, 438–449 (2010).
Fletcher, P. J. et al. Characterizing the effects of 5-HT(2C) receptor ligands on motor activity and feeding behaviour in 5-HT(2C) receptor knockout mice. Neuropharmacology 57, 259–267 (2009).
Tsankova, N. M. et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 9, 519–525 (2006).
Lubin, F. D., Roth, T. L. & Sweatt, J. D. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J. Neurosci. 28, 10576–10586 (2008).
Ou, L. C. & Gean, P. W. Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory. Mol. Pharmacol. 72, 350–358 (2007).
Zheng, F. et al. Regulation of brain-derived neurotrophic factor exon IV transcription through calcium responsive elements in cortical neurons. PLoS ONE 6, e28441 (2011).
Hollis, F., Sevelinges, Y., Grosse, J., Zanoletti, O. & Sandi, C. Involvement of CRFR1 in the basolateral amygdala in the immediate fear extinction deficit. eNeuro 3, pii: ENEURO.0084–16 (2016).
Martin, C. B., Hamon, M., Lanfumey, L. & Mongeau, R. Controversies on the role of 5-HT(2C) receptors in the mechanisms of action of antidepressant drugs. Neurosci. Biobehav. Rev. 42, 208–223 (2014).
Simmons, M., Meador-Woodruff, J. H. & Sodhi, M. S. Increased cortical expression of an RNA editing enzyme occurs in major depressive suicide victims. Neuroreport 21, 993–997 (2010).
Mongeau, R., Miller, G. A., Chiang, E. & Anderson, D. J. Neural correlates of competing fear behaviors evoked by an innately aversive stimulus. J. Neurosci. 23, 3855–3868 (2003).
Christianson, J. P. et al. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol. Psychiatry 67, 339–345 (2010).
Ren-Patterson, R. F. et al. Loss of brain-derived neurotrophic factor gene allele exacerbates brain monoamine deficiencies and increases stress abnormalities of serotonin transporter knockout mice. J. Neurosci. Res. 79, 756–771 (2005).
Chen, Z.-Y. et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 314, 140–143 (2006).
Yu, H. et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J. Neurosci. 29, 4056–4064 (2009).
Soliman, F. et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 327, 863–866 (2010).
Rakofsky, J. J., Ressler, K. J. & Dunlop, B. W. BDNF function as a potential mediator of bipolar disorder and post-traumatic stress disorder comorbidity. Mol. Psychiatry 17, 22–35 (2012).
Chou, D., Huang, C. C. & Hsu, K. S. Brain-derived neurotrophic factor in the amygdala mediates susceptibility to fear conditioning. Exp. Neurol. 255, 19–29 (2014).
Chen, J. et al. proBDNF attenuates hippocampal neurogenesis and induces learning and memory deficits in aged mice. Neurotox. Res. 29, 47–53 (2016).
Barnes, P. & Thomas, K. L. Proteolysis of proBDNF is a key regulator in the formation of memory. PLoS ONE 3, e3248 (2008).
Huang, Y. Y. et al. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc. Natl Acad. Sci. USA 93, 8699–8704 (1996).
Calabresi, P. et al. Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur. J. Neurosci. 12, 1002–1012 (2000).
Pawlak, R. et al. Rapid, specific and active site-catalyzed effect of tissue-plasminogen activator on hippocampus-dependent learning in mice. Neuroscience 113, 995–1001 (2002).
Pawlak, R., Magarinos, A. M., Melchor, J., McEwen, B. & Strickland, S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat. Neurosci. 6, 168–174 (2003).
Newton, T. L., Fernandez-Botran, R., Miller, J. J. & Burns, V. E. Interleukin-6 and soluble interleukin-6 receptor levels in posttraumatic stress disorder: associations with lifetime diagnostic status and psychological context. Biol. Psychol. 99, 150–159 (2014).
Gola, H. et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry 13, 40 (2013).
Calabrese, F. et al. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front. Cell. Neurosci. 8, 430 (2014).
Tong, L., Balazs, R., Soiampornkul, R., Thangnipon, W. & Cotman, C. W. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol. Aging 29, 1380–1393 (2008).
Goshen, I. et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology 32, 1106–1115 (2007).
Jones, M. E., Lebonville, C. L., Barrus, D. & Lysle, D. T. The role of brain interleukin-1 in stress-enhanced fear learning. Neuropsychopharmacology 40, 1289–1296 (2015).
Hao, Y. et al. Intra-amygdala microinfusion of IL-6 impairs the auditory fear conditioning of rats via JAK/STAT activation. Behav. Brain Res. 275, 88–95 (2014).
Lin, C. H. et al. Identification of calcineurin as a key signal in the extinction of fear memory. J. Neurosci. 23, 1574–1579 (2003).
Baumgartel, K. et al. Control of the establishment of aversive memory by calcineurin and Zif268. Nat. Neurosci. 11, 572–578 (2008).
Czerniawski, J. & Guzowski, J. F. Acute neuroinflammation impairs context discrimination memory and disrupts pattern separation processes in hippocampus. J. Neurosci. 34, 12470–12480 (2014).
Czerniawski, J., Miyashita, T., Lewandowski, G. & Guzowski, J. F. Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial, memory: evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain Behav. Immun. 44, 159–166 (2015).
Abdul-Muneer, P. M., Long, M., Conte, A. A., Santhakumar, V. & Pfister, B. J. High Ca(2+) influx during traumatic brain injury leads to caspase-1-dependent neuroinflammation and cell death. Mol. Neurobiol. 54, 3964–3975 (2017).
Farooqui, A. A. & Horrocks, L. A. Brain phospholipases A2: a perspective on the history. Prostaglandins Leukot. Essent. Fatty Acids 71, 161–169 (2004).
Philbert, J., Belzung, C. & Griebel, G. The CRF1 receptor antagonist SSR125543 prevents stress-induced cognitive deficit associated with hippocampal dysfunction: comparison with paroxetine and d-cycloserine. Psychopharmacology 228, 97–107 (2013).
Sánchez, C. & Meier, E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology 129, 197–205 (1997).
Lissek, S. & van Meurs, B. Learning models of PTSD: theoretical accounts and psychobiological evidence. Int. J. Psychophysiol. 98(3, Part 2), 594–605 (2015).
Kamprath, K. & Wotjak, C. T. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn. Mem. 11, 770–786 (2004).
Seimandi, M. et al. Calcineurin interacts with the serotonin transporter C-terminus to modulate its plasma membrane expression and serotonin uptake. J. Neurosci. 33, 16189–16199 (2013).
Shen, Y., Connor, T. J., Nolan, Y., Kelly, J. P. & Leonard, B. E. Differential effect of chronic antidepressant treatments on lipopolysaccharide-induced depressive-like behavioural symptoms in the rat. Life Sci. 65, 1773–1786 (1999).
Hori, H. & Kim, Y. Inflammation and posttraumatic stress disorder. Psychiatry Clin. Neurosci. (2019) [Epub ahead of print].
Millan, M. J. et al. The melatonergic agonist and clinically active antidepressant, agomelatine, is a neutral antagonist at 5-HT(2C) receptors. Int. J. Neuropsychopharmacol. 14, 768–783 (2011).
Kreilgaard, M., Smith, D. G., Brennum, L. T. & Sanchez, C. Prediction of clinical response based on pharmacokinetic/pharmacodynamic models of 5-hydroxytryptamine reuptake inhibitors in mice. Br. J. Pharmacol. 155, 276–284 (2008).
Haddock, R. E. et al. Metabolic pathway of paroxetine in animals and man and the comparative pharmacological properties of its metabolites. Acta Psychiatr. Scand. Suppl. 350, 24–26 (1989).
Zhang, G. et al. Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology 64, 403–413 (2013).
Cohen, H., Zohar, J. & Carmi, L. Effects of agomelatine on behaviour, circadian expression of period 1 and period 2 clock genes and neuroplastic markers in the predator scent stress rat model of PTSD. World J. Biol. Psychiatry 1–19 (2018) [Epub ahead of print].
Siegmund, A. & Wotjak, C. T. Toward an animal model of posttraumatic stress disorder. Ann. N Y Acad. Sci. 1071, 324–334 (2006).
Bomyea, J., Risbrough, V. & Lang, A. J. A consideration of select pre-trauma factors as key vulnerabilities in PTSD. Clin. Psychol. Rev. 32, 630–641 (2012).
Michopoulos, V., Norrholm, S. D. & Jovanovic, T. Diagnostic biomarkers for posttraumatic stress disorder: promising horizons from translational neuroscience research. Biol. Psychiatry 78, 344–353 (2015).
Eraly, S. A. et al. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry 71, 423–431 (2014).
Rougemont-Bücking, A. et al. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci. Ther. 17, 227–236 (2011).
Hayes, J. P., Hayes, S. M. & Mikedis, A. M. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood Anxiety Disord. 2, 9 (2012).
Wang, Z. & Young, M. R. PTSD, a disorder with an immunological component. Front. Immunol. 7, 219 (2016).
Lerer, B. et al. Variability of 5-HT2C receptor cys23ser polymorphism among European populations and vulnerability to affective disorder. Mol. Psychiatry 6, 579–585 (2001).
Videtic, A., Peternelj, T. T., Zupanc, T., Balazic, J. & Komel, R. Promoter and functional polymorphisms of HTR2C and suicide victims. Genes Brain Behav. 8, 541–545 (2009).
Brummett, B. H. et al. A putatively functional polymorphism in the HTR2C gene is associated with depressive symptoms in white females reporting significant life stress. PLoS ONE 9, e114451 (2014).
Acknowledgements
We thank Dr. K. Nishikura and The Wistar Institute (PA) for providing the VGV mice. This research was supported by INSERM. M.R. was recipient of a fellowship from the French Ministère de lʼEnseignement supérieur, de la Recherche et de lʼInnovation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Règue, M., Poilbout, C., Martin, V. et al. Increased 5-HT2C receptor editing predisposes to PTSD-like behaviors and alters BDNF and cytokines signaling. Transl Psychiatry 9, 100 (2019). https://doi.org/10.1038/s41398-019-0431-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-019-0431-8
This article is cited by
-
Emphasizing the Crosstalk Between Inflammatory and Neural Signaling in Post-traumatic Stress Disorder (PTSD)
Journal of Neuroimmune Pharmacology (2023)
-
The effect of SSRIs on fear learning: a systematic review and meta-analysis
Psychopharmacology (2023)
-
Constitutive 5-HT2C receptor knock-out facilitates fear extinction through altered activity of a dorsal raphe-bed nucleus of the stria terminalis pathway
Translational Psychiatry (2022)
-
RNA editing of the 5-HT2C receptor in the central nucleus of the amygdala is involved in resilience behavior
Translational Psychiatry (2021)