Abstract
Hyperconnectivity of the default-mode network (DMN) is one of the most widely replicated neuroimaging findings in major depressive disorder (MDD). Further, there is growing evidence for a central role of the lateral habenula (LHb) in the pathophysiology of MDD. There is preliminary neuroimaging evidence linking LHb and the DMN, but no causal relationship has been shown to date. We combined optogenetics and functional magnetic resonance imaging (fMRI), to establish a causal relationship, using an animal model of treatment-resistant depression, namely Negative Cognitive State rats. First, an inhibitory light-sensitive ion channel was introduced into the LHb by viral transduction. Subsequently, laser stimulation was performed during fMRI acquisition on a 9.4 Tesla animal scanner. Neural activity and connectivity were assessed, before, during and after laser stimulation. We observed a connectivity decrease in the DMN following laser-induced LHb perturbation. Our data indicate a causal link between LHb downregulation and reduction in DMN connectivity. These findings may advance our mechanistic understanding of LHb inhibition, which had previously been identified as a promising therapeutic principle, especially for treatment-resistant depression.
Similar content being viewed by others
Introduction
Converging evidence from human neuroimaging1,2 and post-mortem3 studies supports the notion that the lateral habenula (LHb) plays a crucial role in major depressive disorder (MDD). Data from patients2,4, as well as from rodent models of depression5,6 indicate that the LHb is generally overactive in depression, although other studies in both humans7 and animals8 have suggested a more complex relationship.
Physiologically, the LHb encodes negative reward prediction error, that is, the omission of an expected reward, or the occurrence of an unexpected punishment9. Through its efferents, the LHb modulates monoaminergic nuclei, particularly the dorsal raphe10 and ventral tegmental area9,11. This is a potentially important mechanistic link to depression pathophysiology in light of the monoamine hypothesis of mood disorders.
Furthermore, several lines of evidence indicate that LHb inhibition can be a therapeutic approach for depression: we reported on two patients with treatment-refractory depression in whom deep brain stimulation (DBS) of the LHb was applied successfully12. Corresponding to this, LHb DBS rescued electrophysiological abnormalities, as well as depressive-like behavior in a rat model of treatment-resistant depression, congenital learned helplessness (cLH)6. Similar behavioral effects were observed in this model following pharmacological inhibition13.
On the neural network level, default-mode network (DMN) hyperconnectivity is one of the most consistent findings in human depression14,15,16. It is the anterior DMN that is most reliably altered14,15,17,18,19,20,21 (for review, see Mulders et al.16), whereas hyperconnectivity of the posterior DMN has also been described16, but not unequivocally16,21,22,23,24. The DMN appears particularly promising for translation between humans and animal models as it has consistently been found across species—in rats, particularly, the DMN is remarkably homologous to the human DMN25,26,27, and the anterior DMN was delineated as a major subnetwork in a hypothesis-free analysis27. In light of this and the human depression literature cited above, our primary hypothesis concerned connectivity changes in the anterior DMN. As the medial parts of the posterior DMN (posterior cingulate cortex and precuneus in humans; retrosplenial cortex in rats) have also been widely implicated in human depression studies23,24,28,29,30,31, and since we previously reported on increased connectivity between anterior and posterior DMN regions (cingulate and retrosplenial cortices) in the same rat model of depression8, we also included the retrosplenial cortex in this hypothesis.
There is some limited evidence from human neuroimaging pointing to a connection between the DMN and the habenula: during rest, habenula blood oxygen level dependent (BOLD) activity correlated with several parts of the DMN1,32. Importantly, this correlation was associated with subclinical depression scores1.
However, no studies to date have investigated a potential mechanistic link between LHb and the DMN. Accordingly, the aim of the current study was to test a possible causal relationship between LHb inhibition and DMN connectivity. LHb inhibition, as detailed above, is a promising therapeutic principle for depressive disorders. DMN hyperconnectivity, on the other hand, is a highly consistent system-level phenotype of depression. To test a link between the two, we used the combination of optogenetics and functional magnetic resonance imaging (fMRI). Optogenetics refers to the transduction of neurons with a light-sensitive membrane protein33, leading to depolarization or hyperpolarization upon local laser illumination, and thus stimulation or inhibition, respectively. For the present study, we used archaerhodopsin, an inhibitory opsin. Translational resting-state fMRI has been developed to narrow the bridge between clinical and preclinical findings, thus enhancing drug development34.
Our primary hypothesis was that perturbation of LHb activity would lead to decreased DMN connectivity.
Materials and methods
Animals
We used the Negative Cognitive State (NC) rat model of depression35. The term NC has come to substitute the previous terminology of “cLH”. cLH rats had been bred for high learned helplessness36, that is, inescapable shock leading to failure to escape from escapable shock. In later generations, helpless behavior was found even without prior inescapable stress, thereby reducing animal harm36. Therefore, we have recently begun to use the term NC, which also reflects the depressive-like cognitive abnormalities present in this model37. cLH/NC is a valid model for MDD37,38, in particular treatment-resistant depression39.
In total, 19 male rats were used. Since this was an exploratory study, no formal power or sample-size estimation was performed. The group size was based on prior experience and literature reports on prior optogenetic-fMRI studies (e.g.,40,41,42).
All animal procedures were approved by the local Animal Welfare Committee (Regierungspräsidium Karlsruhe, Germany) and were in accordance with the regulations on animal research within the European Union (European Communities Council Directive 86/609/EEC) and the German Animal Welfare Act.
Optogenetics
For optogenetics, we used a rAAV-CaMKIIa-ArchT:GFP viral construct. rAAV denotes the recombinant adenoviral construct. The CaMKIIa promoter leads to specific expression in neurons, with a relative preference for glutamatergic neurons42. GFP is a fluorescent protein used for histological control of successful infection. ArchT(3.0) is an inhibitory opsin (archeorhodopsin), whose activation by yellow light leads to proton transport out of the cell, and thus hyperpolarization43. To describe this manipulation, we use the conservative term “perturbation” rather than “inhibition”, as the primary inhibition can be followed by rebound effects44,45, and prolonged illumination can lead to increased (rather than decreased) spontaneous synaptic activity, although the latter effect has only been observed for axon terminals44. In the present study, ArchT-expressing LHb neurons were illuminated predominantly at the cell body.
At 8 weeks of age, rats underwent isoflurane anesthesia and the virus was stereotactically injected bilaterally into the LHb (for details, see Supplementary Methods). Of the 13 rats injected with ArchT, 8 animals fulfilled inclusion criteria, namely bilateral robust GFP expression within, but no significant expression outside of, the LHb. Additionally, six animals (control group) were injected with a virus lacking ArchT. Thus, 14 animals (8 ArchT and 6 controls) were included in further analyses.
Three weeks after virus injection, animals were anesthetized again for bilateral stereotactic implantation of fiber-optic cannulae, which were fixed with dental cement (for details, see Supplementary Methods). Animals recovered at least three days before scanning (mean 4.0; range 3–5 days; matched between groups: p > 0.5).
Laser stimulation
Yellow light (593 nm, see Supplementary Methods) was applied during fMRI acquisition in the following sequence: four BOLD time series were acquired (see Fig. 1). First, a resting-state session of 10 min without any laser stimulation; second, a “block” sequence of 12 min with alternating “laser-on” and “laser-off” periods lasting 30 s each; third, a session of 10 min with continuous laser stimulation; fourth, another resting-state session of 10 min without any laser stimulation. (The high-resolution T2-weighted image was acquired between the second and third session, in order to reduce continuous energy deposition potentially resulting in heating artifacts.) Laser was applied with a frequency of 30 Hz of light pulses with a duration of 5 ms. (This applied both for the “laser-on” periods in the block session and during the session of continuous laser stimulation.) We decided to use a pulsed laser stimulation rather than continuous illumination, in order to minimize energy deposition and thus heating artifacts44.
Scanning procedures
MRI data were acquired with a Bruker Biospec MRI scanner (9.4T; Bruker, Ettlingen, Germany). Anesthesia was started with 4% isoflurane, which was then reduced to 2.5% for scanning preparations. Subsequently, a bolus of 0.08 mg/kg medetomidine was applied. Isoflurane was then tapered off within 10 min, after which a continuous dose of 0.3 mg/kg/h medetomidine was started. fMRI experiments commenced 20 min after isoflurane discontinuation.
Functional data were acquired using a T2*-weighted Echo Planar Imaging sequence with the following parameters: repetition time/echo time = 1.7 s/17.5 ms, 96 × 96 matrix, field of view = 35 × 35 mm², 29 slices (0.5 mm thickness, 0.2 mm gap). See Supplementary Materials for further details on scanning parameters.
Image data processing
All data were processed using FMRIB Software Library (FSL, version 4.1) (http://www.fmrib.ox.ac.uk/fsl), SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/), as well as our own Matlab (http://www.mathworks.com/products/matlab/) scripts. The brain was extracted from three-dimensional (3D) anatomical T2-weighted images. The functional images were corrected for geometrical distortions using the acquired B0-maps and realigned to the brain-extracted T2-weighted anatomical images. Subsequently, a correction for physiological noise was conducted using the Aztec46 software employing the RETROICOR47 method with the physiological data sampled during the measurements. Functional data were slice-time corrected and normalized to a Paxinos-space digital atlas48 using the normalization parameters of the 3D-structural image. For statistical parametric mapping (SPM) analyses, the normalized data were additionally smoothed with a 0.6 mm isotropic Gaussian kernel. For connectivity analyses, the cerebrospinal fluid signal was regressed out, and a bandpass filter (0.01–0.1 Hz) was applied.
Statistical analysis
BOLD activity analysis
As a hypothesis-free analysis, we tested whether LHb perturbation would lead to acute changes in BOLD level. To this end, the block-design imaging sequence was analyzed with a standard general linear model approach. Briefly, the “laser-on” condition was used as the independent variable for a first-level analysis in each animal. For group statistics (ArchT vs. control virus), the resulting beta images were then analyzed in a second-level analysis.
Network connectivity analyses
We chose a network approach for analysis of DMN connectivity changes following LHb perturbation. To define the nodes of the network, we used the rat brain MRI atlas to which the imaging data had been normalized48. According to previous work on the DMN in rats25,26 and its subnetworks27, we selected the following atlas regions, separately for each hemisphere: prelimbic cortex, orbitofrontal cortex, cingulate cortex 1 and 2 (dorsal and ventral part, respectively) and retrosplenial cortex. This yielded a network of 10 nodes; see Fig. 2 for anatomical illustration. Mean BOLD time courses were extracted from these regions, separately for each hemisphere. Then, Pearson’s correlation coefficient was computed pairwise between regional time courses, for each animal. For further analyses, we used Fisher-Z-transformed correlation matrices.
For assessment of acute connectivity changes during the block design, we used network-wide psychophysiological interaction (PPI) analysis49. PPI tests whether the temporal correlation between two regional BOLD time courses (“physio”) changes depending on a paradigm (“psycho”, in this case, the laser block sequence). Group comparison of the PPI matrices was then done using the Network-based statistic (NBS) approach50, see below for details.
Second, we subtracted the correlation matrices of the third BOLD sequence (continuous laser stimulation) minus the matrices from the first BOLD sequence (resting-state; see Fig. 1). The subtraction matrices were then compared between ArchT and control groups, again using NBS.
Third, to test for connectivity changes that are delayed or maintained, we did an analogous subtraction, but this time between the fourth and the first BOLD sequence.
Briefly, NBS is a non-parametric method for network-wide hypothesis testing. It employs a cluster-based permutation test to control for multiple comparisons. First, for each single edge, a two-sample t-test is performed comparing the ArchT and control groups. At this point, no statistical inference is done; only the t-value is stored. Next, edges with t-values below a certain threshold are discarded. (This threshold was set to t = 1.8, corresponding to p < 0.05 with 13 degrees of freedom; note that the selection of this threshold still leads to a conservative treatment of the multiple comparison problem since a permutation test is subsequently performed.) Contiguous threshold-surviving edges are defined as a cluster. The intensity of the resulting cluster is noted, that is, the number of contiguous threshold-surviving edges weighted by the t-values. Finally, a permutation test compares this intensity with the maximum intensities of 5000 random permutations. As a one-sided test is computed within the NBS framework, we set the alpha-level for the individual permutation test to p = 0.025.
In addition, we employed standard seed-based connectivity analyses as previously described51, using the DMN atlas regions as seeds and a mask comprising all DMN regions for second-level analysis. For details, see Supplementary Information.
Behavioral testing and histology
Five days after scanning, rats underwent behavioral testing using an escapable foot shock paradigm38. Afterward, animals were killed for verification of virus expression and measurement of c-fos in the LHb as a marker of neuronal activity. See supplementary material for detailed methods and results.
Results
BOLD activity analysis
There was no significant group difference regarding BOLD activation during the laser block paradigm.
Connectivity analyses
PPI did not reveal acute connectivity changes during the block laser sequence. Similarly, comparing the third and the first BOLD sequence (see Fig. 1), NBS yielded no significant group difference that would have indicated connectivity changes during continuous laser stimulation.
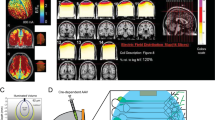
Testing for delayed effects (by subtracting the first from the fourth sequence), we found a subnetwork showing a stronger connectivity decrease in the ArchT group (p = 0.009, all p-values refer to NBS permutation tests, one-sided, significance threshold p = 0.025). There was no opposite effect (i.e., no evidence for a stronger increase in ArchT group: p = 1.0). The significant network comprised eleven edges and nine nodes (right orbitofrontal cortex, bilateral prelimbic cortex, bilateral cingulate cortices 1 and 2 and bilateral retrosplenial cortex; see Figs. 3a, b). Post-hoc, a paired T-test within the ArchT group (post-laser vs. pre-laser) confirmed a significant decrease in connectivity (p = 0.014). In the control group, there was no significant change; if anything, the data showed a trend-level increase in connectivity (p = 0.04; significance threshold was p = 0.025 as NBS permutation test is one sided).
a Optogenetically reduced connectivity within the default-mode network in ArchT vs. control rats. Network-based statistics50 was used to test whether the longitudinal change (pre- vs. post-laser resting state) was different between ArchT and control group (p = 0.009). View is from left-anterior-superior. Colored spheres represent the DMN regions, and color-coding of the regions is equivalent to Fig. 2. Edges that are part of the significant network are indicated by orange/red lines, with the line color representing the T-value (see color bar on the right). b Quantitative representation of the connectivity changes in the DMN. Correlation coefficients were averaged over the network in order to display the group-by-time interaction effect. In the ArchT group, DMN connectivity significantly decreased following laser stimulation. Mean and standard deviation are shown, whereas individual correlation values are indicated in gray/pale red. For details, see main text. c, d Analogous analyses of an equally sized set of cortical regions c or the whole-brain network d did not reveal any group differences, suggesting the specificity of the DMN finding
Next, to assess the specificity of our subnetwork and to exclude the possibility that the observed DMN connectivity decrease in the ArchT group was due to an unspecific global reduction in connectivity, we conducted the following additional analyses:
First, selecting a comparably sized network outside the DMN did not show analogous connectivity differences. Specifically, we performed an NBS analysis on a frontoparietal network, which—like the DMN network—consisted of five anatomically adjacent cortical regions per hemisphere (primary and secondary motor cortices, primary and secondary sensory cortices, parietal association cortex; p = .3, NBS permutation test; see Fig. 3c).
Second, an NBS analysis including all atlas regions (n = 92) did not reveal any significant or trend-level connectivity decrease (nor increase) on the whole-network level (p > 0.5, NBS permutation test). Correspondingly, we averaged correlation coefficients over the whole-brain correlation matrix (92 × 92), comparing them between groups (control vs. ArchT) and time (pre vs. post session). Confirming the specificity of our results, we could not detect neither a main effect of time or group, nor a group-by-time interaction effect (all p-values > 0.5)—see Fig. 3d.
Additional seed-connectivity analyses revealed altered connectivity between prefrontal and cingulate cortices (family-wise error corrected), both comparing pre vs. post-laser resting-state sessions, as well as pre vs. continuous laser stimulation. For details, see Supplementary Information.
Cardiac frequency and head movement
In order to test whether anesthesia yielded stable physiological conditions, we compared heart rate between groups and sessions (and whether longitudinal change in heart rate might be different between the two groups)—this did not yield any differences. The same was true for head motion. See supplementary materials for details.
Discussion
This is—to our knowledge—the first study to demonstrate a causal link between habenula modulation and DMN connectivity. We achieved this by using og-fMRI. Specifically, we detected a reduction in DMN connectivity following LHb perturbation with an inhibitory opsin in a rat model of treatment-resistant depression.
In human-depressive disorders, hyperconnectivity of the (especially anterior) DMN is one the most consistent neuroimaging findings14,15,16. Conversely, antidepressant administration leads to reduction in DMN connectivity, both in healthy volunteers52 and patients53,54. Thus, there is preliminary evidence that reduction of DMN connectivity is a neuroimaging correlate of antidepressant therapy.
On the other hand, downregulation of LHb activity appears as a promising therapeutic principle for treatment-resistant depression, based on both preclinical6,55 and preliminary clinical12 evidence. Our data suggest a connection between these two aspects of depression pathophysiology and treatment, although without demonstrating a specificity to depressive states, for lack of an analogous control experiment in wild-type animals.
According to a recent review16, it is the anterior DMN (medial prefrontal cortex, anterior cingulate cortex) that most consistently shows hyperconnectivity (in the sense of increased temporal correlation among the constituent regions) in resting-state studies of human depression17,18,19,20,21. Two recent meta-analyses also found DMN hyperconnectivity especially with the medial prefrontal cortex14,15, whereas hyperconnectivity with other regions (such as temporal or parietal regions) did not show consistent results between these two meta-analyses, probably due to differing inclusion criteria. Interestingly, hyperconnectivity within the anterior DMN was found to correlate with rumination21 and depression refractoriness17.
Hyperconnectivity within the posterior DMN has also been reported17,19,20, but less consistently21. Changes in connectivity between anterior and posterior DMN regions have been widely replicated (for review, see Mulders et al.16), although the direction of these changes probably differs between subregions: some studies showed increased connectivity in depression28,29,31, whereas others saw decreased connectivity22,23,24 or bidirectional changes30. When altered connectivity involving the posterior DMN was found, it concerned almost exclusively its medial portions, namely posterior cingulate cortex and adjacent precuneus23,24,28,29,30,31. The posterior cingulate cortex is considered homologous to the rat retrosplenial cortex25,56, which in turn is one of the central nodes in the rat DMN25.
Of note, we did not observe an acute effect on BOLD activity (as assessed during the 30-s block stimulation). This may be due to the inhibitory nature of the LHb modulation, which does not lead to immediate neuronal activation. Perhaps in correspondence to this, NBS did not reveal acute connectivity changes immediately during the laser stimulation (although such effects, spatially quite limited, were detected with the seed-based approach). Rather, the effects were delayed, such that connectivity alterations were detected during a second resting-state session after the laser stimulation. Speculatively, this suggests effects on brain plasticity, and is compatible with the functional neuroanatomy of the LHb: there is no direct connection between LHb and cortical areas. Rather, the LHb sends its main efferents to the monoaminergic nuclei (ventral tegmental area (VTA) and dorsal raphe nucleus), with mostly inhibitory effects9,10,11. The monoaminergic nuclei, in turn, have wide-ranging, modulatory efferents to subcortical and cortical areas. Rodent studies have begun to investigate the cortical downstream effects of the LHb-monoamine pathway: LHb neurons synapse primarily onto those VTA neurons that project to the mPFC57, and LHb inactivation leads to increased prefrontal dopamine release58. This pathway appears functionally relevant for avoidance behaviors57. In non-human primates, LHb and VTA show strongly opposite behavior in response to reward or punishment, with LHb inhibiting VTA9. The anterior cingulate cortex (part of the DMN) is also involved in monitoring negative reward prediction error and, speculatively, receives this information from the LHb59, perhaps via VTA. Finally, human neuroimaging data have suggested a modulation of DMN connectivity by both dopamine60,61 and serotonin62,63. This is in line with antidepressant drugs reducing DMN connectivity, as mentioned above52,53,54.
A better understanding of therapeutic mechanisms is urgently needed. In contrast to the case reports of LHb DBS cited above12, as well as some successful open DBS trials targeting other brain regions (reviewed in Morishita et al.64), the only two double-blind sham-controlled DBS studies yielded negative results65,66. This underscores the necessity to investigate the circuit dysfunctions in treatment-resistant depression, and how they are modified by treatment. Such an understanding would allow patient selection, monitoring of therapeutic response and refinement of treatment protocols67.
FMRI, unlike many other methods used in basic neuroscience, is a translational tool applicable in rodent models, non-human primates as well as in patients68. As mentioned above, recent high-resolution fMRI studies in humans have shown that the LHb is functionally connected with DMN regions1,32, and these connectivities were associated with depression scores1. This supports the potential clinical relevance of our findings. Naturally, these human data do not inform about causality, unlike the findings presented herein.
We also probed in an exploratory behavioral experiment whether a brief perturbation of a fraction of LHb neurons through ArchT inhibition would suffice to exert a relevant influence on behavior several days later, as predicted by pharmacologic LHb inhibition, where delayed, but not acute, effects on helplessness were observed22 (see Supplementary Methods). Although we observed a correlation between helpless behavior and LHb c-fos levels (as a marker of neuronal activity) within the ArchT group, we did not detect a group effect in our behavioral testing. One possible explanation might be that previous pharmacological inhibition of LHb13 had a longer duration of action. Future studies may need to probe different and in particular longer stimulation paradigms to identify an effective perturbation to influence behavior.
A further important consideration pertains to the fact that our study did not include analogous experiments assessing the effect of LHb perturbation in a non-depressed rat strain or other animal models, which means that we cannot show whether the mechanism demonstrated in this article operates outside the context of the animal model for depression we studied. Further studies of this kind are needed to test whether this effect is specific to certain animal models. Moreover, our data do not prove the depressive-like state of our sample, considering that stress exposure is needed in this animal model to cause a full-blown depressive phenotype69. We had chosen not to introduce further stress or perform behavioral testing before the MRI measurements, in order to minimize potential confounds, and considering that the animals had already been exposed to substantial stressors (e.g., repeated surgeries, single housing).
Additionally, apart from the escapable foot shock, other behavioral measures were not assessed, which might have revealed an effect of LHb perturbation. Also, we did not test behavior under concurrent laser stimulation. Future studies should include additional behavioral tests and assess acute vs. chronic effects of the optogenetic intervention on behavior.
Finally, a limitation of the NBS approach is the anatomical non-specificity of the findings, as the inference pertains to the cluster of significantly changing connections as a whole, rather than to individual region-of-interest (ROI) pairs. Our additional seed-based analysis yielded spatially much more restricted effects. However, the seed analysis also detected an immediate effect during the continuous laser stimulation, which was missed by NBS. This reflects the complementary strengths and weaknesses of seed-based vs. NBS analyses: whereas NBS is more sensitive to larger clusters of altered connectivity, seed-based approaches are more powerful for detecting localized changes.
Some further methodological aspects of our study deserve consideration: as we used a comparison group that underwent the same experimental protocol, our findings cannot be explained by laser artifacts, anesthesia duration, physiological changes or scanning-related stress. In particular, anesthesia is a highly relevant confounder, which needs to be adequately controlled for in functional connectivity studies. Medetomidine, which was used in our study, is associated with reduced cortical connectivity70. Importantly, we did not observe any group differences or longitudinal trends in potential confounds such as cardiac frequency (as a marker of arousal/anesthesia depth) or head motion, supporting the robustness of our findings.
It should be noted that only a part of all glutamatergic cells in the LHb were successfully transduced with the virus, thus limiting LHb perturbation to this sub-population. We did, however, not intend to completely abolish LHb function but rather reduce its activity, in order to model clinical interventions in humans. This is in accordance with recent data in the same animal model, where knock-down of calcium/calmodulin-dependent protein kinase type II (betaCaMKII, a synaptic scaffolding protein) reduced depressive-like behaviors, even at an infection rate of only around 20%55. The authors concluded that there was probably little redundancy in the LHb network, such that inhibition of a sub-population already has functional consequences.
Finally, it should be noted that in our study, we did not intend to assess functional connectivity of the LHb itself—such an analysis would have been unreliable due to fMRI artifacts around the tip of the optic fiber.
In summary, we employed og-fMRI as a translational tool, linking cellular neuroscience to neuroimaging phenotypes. Our study is the first to provide evidence for a causal relationship between LHb modulation and connectivity alterations in the DMN—two important factors of depression pathophysiology and, potentially, therapy.
References
Ely, B. A. et al. Resting-state functional connectivity of the human habenula in healthy individuals: associations with subclinical depression. Hum. Brain Mapp. 37, 2369–2384 (2016).
Roiser, J. P. et al. The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biol. Psychiatry 66, 441–450 (2009).
Ranft, K. et al. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol. Med. 40, 557–567 (2010).
Morris, J. S., Smith, K. A., Cowen, P. J., Friston, K. J. & Dolan, R. J. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage 10, 163–172 (1999).
Lecca, S. et al. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat. Med. 22, 254–261 (2016).
Li, B. et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470, 535–539 (2011).
Lawson, R. P. et al. Disrupted habenula function in major depression. Mol. Psychiatry 22, 202–208 (2016).
Gass, N. et al. Functionally altered neurocircuits in a rat model of treatment-resistant depression show prominent role of the habenula. Eur. Neuropsychopharmacol. 24, 381–390 (2014).
Matsumoto, M. & Hikosaka, O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447, 1111–1115 (2007).
Amat, J. et al. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 917, 118–126 (2001).
Ji, H. & Shepard, P. D. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J. Neurosci. 27, 6923–6930 (2007).
Kiening, K. & Sartorius, A. A new translational target for deep brain stimulation to treat depression. EMBO Mol. Med. 5, 1151–1153 (2013).
Winter, C., Vollmayr, B., Djodari-Irani, A., Klein, J. & Sartorius, A. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behav. Brain Res. 216, 463–465 (2011).
Hamilton, J. P., Farmer, M., Fogelman, P. & Gotlib, I. H. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry 78, 224–230 (2015).
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D. & Pizzagalli, D. A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611 (2015).
Mulders, P. C., van Eijndhoven, P. F., Schene, A. H., Beckmann, C. F. & Tendolkar, I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci. Biobehav Rev. 56, 330–344 (2015).
Greicius, M. D. et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 62, 429–437 (2007).
Guo, W. et al. Abnormal default-mode network homogeneity in first-episode, drug-naive major depressive disorder. PLoS ONE 9, e91102 (2014).
Li, B. et al. A treatment-resistant default mode subnetwork in major depression. Biol. Psychiatry 74, 48–54 (2013).
Manoliu, A. et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Human. Neurosci. 7, 930 (2013).
Zhu, X. et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol. Psychiatry 71, 611–617 (2012).
Abbott, C. C. et al. Electroconvulsive therapy response in major depressive disorder: a pilot functional network connectivity resting state FMRI investigation. Front. Psychiatry 4, 10 (2013).
Andreescu, C. et al. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 214, 313–321 (2013).
van Tol, M. J. et al. Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol. Med. 44, 2053–2065 (2014).
Lu, H. et al. Rat brains also have a default mode network. Proc. Natl. Acad. Sci. USA. 109, 3979–3984 (2012).
Schwarz, A. J. et al. Anti-correlated cortical networks of intrinsic connectivity in the rat brain. Brain Connect. 3, 503–511 (2013).
Hsu, L. M. et al. Constituents and functional implications of the rat default mode network. Proc. Natl. Acad. Sci. USA. 113, E4541–E4547 (2016).
Alexopoulos, G. S. et al. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord. 139, 56–65 (2012).
Berman, M. G. et al. Depression, rumination and the default network. Social. Cogn. Affect. Neurosci. 6, 548–555 (2011).
Wu, Q. Z. et al. Abnormal regional spontaneous neural activity in treatment-refractory depression revealed by resting-state fMRI. Hum. Brain Mapp. 32, 1290–1299 (2011).
Zhou, Y. et al. Increased neural resources recruitment in the intrinsic organization in major depression. J. Affect. Disord. 121, 220–230 (2010).
Torrisi, S. et al. Resting state connectivity of the human habenula at ultra-high field. Neuroimage 147, 872–879 (2016).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Artigas, F. et al. Defining the brain circuits involved in psychiatric disorders: IMI-NEWMEDS. Nat. Rev. Drug Discov. 16, 1–2 (2017).
Gass, N. et al. Brain network reorganization differs in response to stress in rats genetically predisposed to depression and stress-resilient rats. Transl. Psychiatry 6, e970 (2016).
Henn, F. A. & Vollmayr, B. Stress models of depression: forming genetically vulnerable strains. Neurosci. Biobehav Rev. 29, 799–804 (2005).
Enkel, T. et al. Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacology 35, 1008–1015 (2010).
Vollmayr, B. & Gass, P. Learned helplessness: unique features and translational value of a cognitive depression model. Cell Tissue Res. 354, 171–178 (2013).
Sartorius, A., Mahlstedt, M. M., Vollmayr, B., Henn, F. A. & Ende, G. Elevated spectroscopic glutamate/gamma-amino butyric acid in rats bred for learned helplessness. Neuroreport 18, 1469–1473 (2007).
Abe, Y. et al. Opto-fMRI analysis for exploring the neuronal connectivity of the hippocampal formation in rats. Neurosci. Res. 74, 248–255 (2012).
Kahn, I. et al. Optogenetic drive of neocortical pyramidal neurons generates fMRI signals that are correlated with spiking activity. Brain Res. 1511, 33–45 (2013).
Lee, J. H. et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792 (2010).
Han, X. et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front. Syst. Neurosci. 5, 18 (2011).
Mahn, M., Prigge, M., Ron, S., Levy, R. & Yizhar, O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat. Neurosci. 19, 554–556 (2016).
Madisen, L. et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012).
van Buuren, M. et al. Cardiorespiratory effects on default‐mode network activity as measured with fMRI. Human. brain Mapp. 30, 3031–3042 (2009).
Glover, G. H., Li, T. Q. & Ress, D. Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 44, 162–167 (2000).
Schwarz, A. J. et al. A stereotaxic MRI template set for the rat brain with tissue class distribution maps and co-registered anatomical atlas: application to pharmacological MRI. Neuroimage 32, 538–550 (2006).
Gerchen, M. F., Bernal-Casas, D. & Kirsch, P. Analyzing task-dependent brain network changes by whole-brain psychophysiological interactions: a comparison to conventional analysis. Hum. Brain Mapp. 35, 5071–5082 (2014).
Zalesky, A., Fornito, A. & Bullmore, E. T. Network-based statistic: identifying differences in brain networks. Neuroimage 53, 1197–1207 (2010).
Gass, N. Sub-anesthetic ketamine modulates intrinsic BOLD connectivity within the hippocampal-prefrontal circuit in the rat. Neuropsychopharmacology 39, 895–906 (2013).
van de Ven, V., Wingen, M., Kuypers, K. P., Ramaekers, J. G. & Formisano, E. Escitalopram decreases cross-regional functional connectivity within the default-mode network. PLoS ONE 8, e68355 (2013).
Posner, J. et al. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry 70, 373–382 (2013).
Karim, H. T. et al. Intrinsic functional connectivity in late-life depression: trajectories over the course of pharmacotherapy in remitters and non-remitters. Mol. Psychiatry 22, 450–457 (2016).
Li, K. et al. betaCaMKII in lateral habenula mediates core symptoms of depression. Science 341, 1016–1020 (2013).
Vogt, B. A. & Peters, A. Form and distribution of neurons in rat cingulate cortex: areas 32, 24, and 29. J. Comp. Neurol. 195, 603–625 (1981).
Lammel, S. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012).
Lecourtier, L., Defrancesco, A. & Moghaddam, B. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur. J. Neurosci. 27, 1755–1762 (2008).
Baker, P. M. et al. The lateral habenula circuitry: reward processing and cognitive control. J. Neurosci. 36, 11482–11488 (2016).
Meyer, B. M. et al. Oppositional COMT Val158Met effects on resting state functional connectivity in adolescents and adults. Brain Struct. Funct. 221, 103–114 (2016).
Schrantee, A. et al. Effects of dexamphetamine-induced dopamine release on resting-state network connectivity in recreational amphetamine users and healthy controls. Brain Imaging Behav. 10, 548–558 (2016).
Hahn, A. et al. Differential modulation of the default mode network via serotonin-1A receptors. Proc. Natl. Acad. Sci. USA. 109, 2619–2624 (2012).
Kunisato, Y. et al. Modulation of default-mode network activity by acute tryptophan depletion is associated with mood change: a resting state functional magnetic resonance imaging study. Neurosci. Res. 69, 129–134 (2011).
Morishita, T., Fayad, S. M., Higuchi, M. A., Nestor, K. A. & Foote, K. D. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. NeuroTherapeutics 11, 475–484 (2014).
Cavuoto J. Depressing innovation. 2013. Available from: http://www.neurotechreports.com/pages/publishersletterDec13.html.
Dougherty, D. D. et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol. Psychiatry 78, 240–248 (2015).
Widge, A. S., Deckersbach, T., Eskandar, E. N. & Dougherty, D. D. Deep brain stimulation for treatment-resistant psychiatric illnesses: what has gone wrong and what should we do next? Biol. Psychiatry 79, e9–e10 (2016).
Hoyer, C., Gass, N., Weber-Fahr, W. & Sartorius, A. Advantages and challenges of small animal magnetic resonance imaging as a translational tool. Neuropsychobiology 69, 187–201 (2014).
Enkel, T., Spanagel, R., Vollmayr, B. & Schneider, M. Stress triggers anhedonia in rats bred for learned helplessness. Behav. Brain Res. 209, 183–186 (2010).
Nasrallah, F. A., Lew, S. K., Low, A. S. & Chuang, K. H. Neural correlate of resting-state functional connectivity under alpha2 adrenergic receptor agonist, medetomidine. Neuroimage 84, 27–34 (2014).
Acknowledgements
The research leading to these results was supported by the German Research Foundation SFB1158 to A.S. and W.W.-F., DFG KE1661/1–2 to W.K. and DFG GA 2109/2–1 to N.G. Further support was provided by the DFG SA 1869/11–2 awarded to A.S. A.M.-L. acknowledges funding from the European Union Seventh Framework Programme under the grant agreements no. 115008 (EU-NEWMEDS), no. 115300 (EU-AIMS), no. 602805 (EU-Aggressotype) and from the German Federal Ministry of Education and Research (BMBF) through the Integrated Network IntegraMent (Integrated Understanding of Causes and Mechanisms in Mental Disorders) under the auspices of the e:Med Programme (grant no. 01ZX1314G). A.M.-L. received consultancy fees from Astra Zeneca, Elsevier, F. Hoffmann-La Roche, Gerson Lehrman Group, Lundbeck Foundation, Outcome Europe Sárl, Outcome Sciences, Roche Pharma, Servier International and Thieme Verlag, and lecture fees—including the travel fees—from Abbott, Astra Zeneca, Aula Médica Congresos, BASF, Groupo Ferrer International, Janssen-Cilag, Lilly Deutschland, LVR Klinikum Düsseldorf, Servier Deutschland, Otsuka Pharmaceuticals. Part of this work was presented in poster format at the DGPPN congress (Berlin, Germany) in November 2016 (C.C.v.H.) and as an invited mini panel talk at the ACNP meeting 2015 in Miami (A.S.). The authors thank Felix Hörner, Claudia Falfan-Melgoza, Cathrin Huber and Katja Lankisch and for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Clemm von Hohenberg, C., Weber-Fahr, W., Lebhardt, P. et al. Lateral habenula perturbation reduces default-mode network connectivity in a rat model of depression. Transl Psychiatry 8, 68 (2018). https://doi.org/10.1038/s41398-018-0121-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-018-0121-y
This article is cited by
-
Social defeat drives hyperexcitation of the piriform cortex to induce learning and memory impairment but not mood-related disorders in mice
Translational Psychiatry (2022)
-
Striatal hub of dynamic and stabilized prediction coding in forebrain networks for olfactory reinforcement learning
Nature Communications (2022)
-
Alterations to cognitive abilities and functional networks in rats post broad-band intense noise exposure
Brain Imaging and Behavior (2022)
-
miR-323a regulates ERBB4 and is involved in depression
Molecular Psychiatry (2021)
-
Altered directed functional connectivity of the right amygdala in depression: high-density EEG study
Scientific Reports (2020)