Abstract
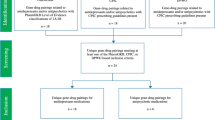
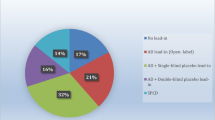
The study aimed to conduct a meta-analysis of studies comparing pharmacogenetically guided dosing of antidepressants with empiric standard of care. Publications referring to genotype-guided antidepressant therapy were identified via PubMed, Google Scholar, Scopus, Web of Science, Embase, and Cochrane databases from the inception of the databases to 2021. In addition, bibliographies of all articles were manually searched for additional references not identified in primary searches. Studies comparing clinical outcomes between two groups of patients who received antidepressant treatment were included in meta-analysis. Analysis of the data revealed statistically significant differences between the experimental group receiving pharmacogenetically guided dosing and the empirically treated controls. Specifically, genotype-guided treatment significantly improved response and remission of patients after both eight and twelve weeks of therapy, whereas no effect on the development of adverse drug reactions was observed. This meta-analysis indicates that the use of preemptive genotyping to guide dosing of antidepressants might increase treatment efficacy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the preparation of the current meta-analysis, are available from the corresponding author on reasonable request.
References
Vilches S, Tuson M, Vieta E, Álvarez E, Espadaler J. Effectiveness of a pharmacogenetic tool at improving treatment efficacy in major depressive disorder: a meta-analysis of three clinical studies. Pharmaceutics. 2019;11:453. https://doi.org/10.3390/pharmaceutics11090453.
van Schaik RHN, Müller DJ, Serretti A, Ingelman-Sundberg M. Pharmacogenetics in psychiatry: an update on clinical usability. Front Pharm. 2020;11:575540. https://doi.org/10.3389/fphar.2020.575540.
Brown L, Vranjkovic O, Li J, Yu K, Al Habbab T, Johnson H, et al. The clinical utility of combinatorial pharmacogenomic testing for patients with depression: a meta-analysis. Pharmacogenomics. 2020;21:559–69. https://doi.org/10.2217/pgs-2019-0157.
Karamperis K, Koromina M, Papantoniou P, Skokou M, Kanellakis F, Mitropoulos K, et al. Economic evaluation in psychiatric pharmacogenomics: a systematic review. Pharmacogenomics J. 2021;21:533–41. https://doi.org/10.1038/s41397-021-00249-1.
Solomon HV, Cates KW, Li KJ. Does obtaining CYP2D6 and CYP2C19 pharmacogenetic testing predict antidepressant response or adverse drug reactions? Psychiatry Res. 2019;271:604–13. https://doi.org/10.1016/j.psychres.2018.12.053.
Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med 2020;3:17. https://doi.org/10.1038/s41746-020-0221-y.
Malsagova KA, Butkova TV, Kopylov AT, Izotov AA, Potoldykova NV, Enikeev DV, et al. Pharmacogenetic testing: A tool for personalized drug therapy optimization. Pharmaceutics. 2020;12:1240. https://doi.org/10.3390/pharmaceutics12121240.
Khairat S, Marc D, Crosby W, Al Sanousi A. Reasons for physicians not adopting clinical decision support systems: Critical analysis. JMIR Med Inf. 2018;6:e24. https://doi.org/10.2196/medinform.8912.
Bousman CA, Hopwood M. Commercial pharmacogenetic-based decision-support tools in psychiatry. Lancet Psychiatry. 2016;3:585–90. https://doi.org/10.1016/S2215-0366(16)00017-1.
Mueller D, Tiwari A, Zai C, Kennedy J. S222. Clinical utility of pharmacogenetic testing in schizophrenia treatment. Schizophr Bull 2018;44:S412. https://doi.org/10.1093/schbul/sby018.1009.
Kibitov A, Kiryanova E, Salnikova L, Zmeyeva E, Shmukler A, Kibitov A. The DRD2/ANKK1 Taq1A polymorphism in CYP2D6 extensive metabolizers is associated with the severity of extrapyramidal side effects of haloperidol treatment in schizophrenia spectrum disorders patients. Eur Psychiatry 2021;64:S122–S123. https://doi.org/10.1192/j.eurpsy.2021.346.
Routhieaux M, Keels J, Tillery EE. The use of pharmacogenetic testing in patients with schizophrenia or bipolar disorder: A systematic review. Ment Health Clin [Internet]. 2018;8:294–302. https://doi.org/10.9740/mhc.2018.11.294.
Liberati EG, Ruggiero F, Galuppo L, Gorli M, González-Lorenzo M, Maraldi M, et al. What hinders the uptake of computerized decision support systems in hospitals? A qualitative study and framework for implementation. Implement Sci. 2017;12:113. https://doi.org/10.1186/s13012-017-0644-2.
Roosan D, Hwang A, Law AV, Chok J, Roosan MR. The inclusion of health data standards in the implementation of pharmacogenomics systems: a scoping review. Pharmacogenomics. 2020;21:1191–202. https://doi.org/10.2217/pgs-2020-0066.
Relling MV, Klein TE, Gammal RS, Whirl-Carrillo M, Hoffman JM, Caudle KE. The clinical pharmacogenetics implementation consortium: 10 years later. Clin Pharm Ther. 2020;107:171–5. https://doi.org/10.1002/cpt.1651.
Caudle KE, Klein TE, Hoffman JM, Muller DJ, Whirl-Carrillo M, Gong L, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15:209–17. https://doi.org/10.2174/1389200215666140130124910.
Klein ME, Parvez MM, Shin JG. Clinical implementation of pharmacogenomics for personalized precision medicine: barriers and solutions. J Pharm Sci. 2017;106:2368–79. https://doi.org/10.1016/j.xphs.2017.04.051.
Lauschke VM, Milani L, Ingelman-Sundberg M. Pharmacogenomic biomarkers for improved drug therapy—recent progress and future developments. AAPS J. 2017;20:4. https://doi.org/10.1208/s12248-017-0161-x.
Russell LE, Zhou Y, Almousa AA, Sodhi JK, Nwabufo CK, Lauschke VM. Pharmacogenomics in the era of next generation sequencing - from byte to bedside. Drug Metab Rev. 2021;53:253–78. https://doi.org/10.1080/03602532.2021.1909613.
Kroese M, Zimmern RL, Farndon P, Stewart F, Whittaker J. How can genetic tests be evaluated for clinical use? Experience of the UK Genetic Testing Network. Eur J Hum Genet. 2007;15:917–21. https://doi.org/10.1038/sj.ejhg.5201867.
Keeling NJ, Rosenthal MM, West-Strum D, Patel AS, Haidar CE, Hoffman JM. Preemptive pharmacogenetic testing: exploring the knowledge and perspectives of US payers. Genet Med. 2019;21:1224–32. https://doi.org/10.1038/gim.2017.181.
Marrero RJ, Cicali EJ, Arwood MJ, Eddy E, DeRemer D, Ramnaraign BH, et al. How to transition from single-gene pharmacogenetic testing to preemptive panel-based testing: A tutorial. Clin Pharm Ther. 2020;108:557–65. https://doi.org/10.1002/cpt.1912.
Hicks JK, Stowe D, Willner MA, Wai M, Daly T, Gordon SM, et al. Implementation of clinical pharmacogenomics within a large health system: from electronic health record decision support to consultation services. Pharmacotherapy. 2016;36:940–8. https://doi.org/10.1002/phar.1786.
van der Wouden CH, Cambon-Thomsen A, Cecchin E, Cheung KC, Dávila-Fajardo CL, Deneer VH, et al. Implementing pharmacogenomics in Europe: design and implementation strategy of the ubiquitous pharmacogenomics consortium. Clin Pharm Ther. 2017;101:341–58. https://doi.org/10.1002/cpt.602.
Reisberg S, Krebs K, Lepamets M, Kals M, Mägi R, Metsalu K, et al. Translating genotype data of 44,000 biobank participants into clinical pharmacogenetic recommendations: challenges and solutions. Genet Med. 2019;21:1345–54. https://doi.org/10.1038/s41436-018-0337-5.
Backman JD, Li AH, Marcketta A, Sun D, Mbatchou J, Kessler MD, et al. Exome sequencing and analysis of 454,787 UK Biobank participants. Nature. 2021;599:628–34. https://doi.org/10.1038/s41586-021-04103-z.
Greden JF, Parikh SV, Rothschild AJ, Thase ME, Dunlop BW, DeBattista C, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111:59–67. https://doi.org/10.1016/j.jpsychires.2019.01.003.
Bradley P, Shiekh M, Mehra V, Vrbicky K, Layle S, Olson MC, et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psychiatr Res. 2018;96:100–7. https://doi.org/10.1016/j.jpsychires.2017.09.024.
Hall-Flavin DK, Winner JG, Allen JD, Carhart JM, Proctor B, Snyder KA, et al. Utility of integrated pharmacogenomic testing to support the treatment of major depressive disorder in a psychiatric outpatient setting. Pharmacogenet Genomics. 2013;23:535–48. https://doi.org/10.1097/FPC.0b013e3283649b9a.
Pérez V, Salavert A, Espadaler J, Tuson M, Saiz-Ruiz J, Sáez-Navarro C, et al. Efficacy of prospective pharmacogenetic testing in the treatment of major depressive disorder: results of a randomized, double-blind clinical trial. BMC Psychiatry. 2017;17:250. https://doi.org/10.1186/s12888-017-1412-1.
Singh AB. Improved antidepressant remission in major depression via a pharmacokinetic pathway polygene pharmacogenetic report. Clin Psychopharmacol Neurosci. 2015;13:150–6. https://doi.org/10.9758/cpn.2015.13.2.150.
Bousman CA, Arandjelovic K, Mancuso SG, Eyre HA, Dunlop BW. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20:37–47. https://doi.org/10.2217/pgs-2018-0142.
Yoshida K, Müller DJ. Pharmacogenetics of antipsychotic drug treatment: Update and clinical implications. Mol Neuropsychiatry. 2020;5:1–26. https://doi.org/10.1159/000492332.
Author information
Authors and Affiliations
Contributions
VS: Conceptualization, Investigation, Data Curation, Writing—Original Draft, Writing-Review & Editing. IR: Conceptualization, Methodology, Validation, Investigation, Data Curation, Writing—Original Draft. MZ: Conceptualization, Formal Analysis, Writing—Original Draft, Visualization. VL: Conceptualization, Validation, Writing—Original Draft, Writing—Review & Editing. JF: Methodology, Writing—Original Draft, Supervision. EB: Writing—Review & Editing, Supervision. DS: Writing—Review & Editing, Supervision.
Corresponding author
Ethics declarations
Competing interests
VML is co is CEO and shareholder of HepaPredict AB, co-founder and shareholder of PersoMedix AB and discloses consultancy work for Enginzyme AB.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Skryabin, V., Rozochkin, I., Zastrozhin, M. et al. Meta-analysis of pharmacogenetic clinical decision support systems for the treatment of major depressive disorder. Pharmacogenomics J 23, 45–49 (2023). https://doi.org/10.1038/s41397-022-00295-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-022-00295-3