Abstract
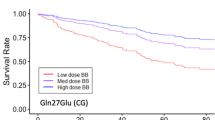
Digoxin is characterized by a small therapeutic window and a QT-interval shortening effect. Moreover, it has been shown that the genetic variants of the nitric oxide synthase-1 adaptor protein (NOS1AP) gene are associated with QT-interval prolongation. We investigated whether the rs10494366 variant of the NOS1AP gene decreases the QT-interval shortening effect of digoxin in patients using this drug. We included 10,057 individuals from the prospective population-based cohort of the Rotterdam Study during a median of 12.2 (interquartile range (IQR) 6.7–18.1) years of follow-up. At study entry, the mean age was 64 years and almost 59% of participants were women. A total of 23,179 ECGs were longitudinally recorded, of which 334 ECGs were from 249 individuals on digoxin therapy. The linear mixed model analysis was used to estimate the effect of the rs10494366 variant on the association between digoxin use and QT-interval duration, adjusted for age, sex, RR interval, diabetes, heart failure, and history of myocardial infarction. In non-users of digoxin, the GG genotype was associated with a significant 6.5 ms [95% confidence interval (CI) 5.5; 7.5] longer QT-interval duration than the TT variant. In current digoxin users, however, the GG variant was associated with a significantly −23.9 [95%CI −29.5; −18.5] ms shorter mean QT-interval duration than in those with the TT variant with −15.9 [95%CI −18.7; −13.1]. This reduction was strongest in the high digoxin dose category [≥0.250 mg/day] with the GG genotype group, with −40.8 [95%CI −52.5; −29.2] ms changes compared to non-users. Our study suggests that the minor homozygous GG genotype group of the NOS1AP gene rs10494366 variant is associated with a paradoxical increase of the QT-interval shortening effect of digoxin in a population of European ancestry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ehle M, Patel C, Giugliano RP. Digoxin: clinical highlights: a review of digoxin and its use in contemporary medicine. Crit Pathw Cardiol. 2011;10:93–8.
Lopes RD, Rordorf R, De Ferrari GM, Leonardi S, Thomas L, Wojdyla DM, et al. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018;71:1063–74.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498.
Digitalis Investigation G. The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl J Med. 1997;336:525–33.
Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403.
Aguirre Davila L, Weber K, Bavendiek U, Bauersachs J, Wittes J, Yusuf S, et al. Digoxin-mortality: randomized vs. observational comparison in the DIG trial. Eur Heart J. 2019;40:3336–41.
Gheorghiade M, Van Veldhuisen DJ, Colucci WS. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation. 2006;113:2556–64.
Newton-Cheh C, Larson MG, Corey DC, Benjamin EJ, Herbert AG, Levy D, et al. QT interval is a heritable quantitative trait with evidence of linkage to chromosome 3 in a genome-wide linkage analysis: the Framingham Heart Study. Heart Rhythm. 2005;2:277–84.
Straus SMJM, Sturkenboom MCJM, Bleumink GLS, Dieleman JP, Lei JVD, Graeff PAD, et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;26:2007–12.
Straus SMJM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–7.
Newton-Cheh C, Eijgelsheim M, Rice KM, de Bakker PI, Yin X, Estrada K, et al. Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat Genet. 2009;41:399–406.
Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M, et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–51.
Kao WH, Arking DE, Post W, Rea TD, Sotoodehnia N, Prineas RJ, et al. Genetic variations in nitric oxide synthase 1 adaptor protein are associated with sudden cardiac death in US white community-based populations. Circulation. 2009;119:940–51.
Eijgelsheim M, Newton-Cheh C, Aarnoudse AL, van Noord C, Witteman JC, Hofman A, et al. Genetic variation in NOS1AP is associated with sudden cardiac death: evidence from the Rotterdam Study. Hum Mol Genet. 2009;18:4213–8.
van Noord C, Aarnoudse A-JLHJ, Eijgelsheim M, Sturkenboom MCJM, Straus SMJM, Hofman A, et al. Calcium channel blockers, NOS1AP, and heart-rate-corrected QT prolongation. Pharmacogenet Genomics. 2009;19:260–6.
Eijgelsheim M, Aarnoudse AL, Rivadeneira F, Kors JA, Witteman JC, Hofman A, et al. Identification of a common variant at the NOS1AP locus strongly associated to QT-interval duration. Hum Mol Genet. 2009;18:347–57.
Aarnoudse AJ, Newton-Cheh C, de Bakker PI, Straus SM, Kors JA, Hofman A, et al. Common NOS1AP variants are associated with a prolonged QTc interval in the Rotterdam Study. Circulation. 2007;116:10–6.
Tomás M, Napolitano C, De Giuli L, Bloise R, Subirana I, Malovini A, et al. Polymorphisms in the NOS1AP gene modulate QT interval duration and risk of arrhythmias in the long QT syndrome. J Am Coll Cardiol. 2010;55:2745–52.
Earle N, Yeo Han D, Pilbrow A, Crawford J, Smith W, Shelling AN, et al. Single nucleotide polymorphisms in arrhythmia genes modify the risk of cardiac events and sudden death in long QT syndrome. Heart Rhythm. 2014;11:76–82.
Zang X, Li S, Zhao Y, Chen K, Wang X, Song W, et al. Systematic meta-analysis of the association between a common NOS1AP genetic polymorphism, the QTc interval, and sudden death. Int Heart J. 2019;60:1083–90.
Hofman A, Grobbee DE, De Jong P, Van, den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7:403–22.
Ikram MA, Brusselle G, Ghanbari M, Goedegebure A, Ikram MK, Kavousi M, et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol. 2020;35:483–517.
World Health Organization. ATC/DDD Index. 2020. http://wwwwhoccno/atc_ddd_index.
Van Bemmel JH, Kors JA, Van Herpen G. Methodology of the modular ECG analysis system MEANS. Methods Inf Med. 1990;29:346–53.
Willems JL, Abreu-Lima C, Arnaud P, van Bemmel JH, Brohet C, Degani R, et al. The diagnostic performance of computer programs for the interpretation of electrocardiograms. N Engl J Med. 1991;325:1767–73.
Woosley RL, Black K, Heise CW, Romero K. CredibleMeds. org: what does it offer? Trends Cardiovasc Med. 2018;28:94–9.
Leening MJG, Kavousi M, Heeringa J, van Rooij FJA, Verkroost-van Heemst J, Deckers JW, et al. Methods of data collection and definitions of cardiac outcomes in the Rotterdam Study. Eur J Epidemiol. 2012;27:173–85.
Smith TW, Antman EM, Friedman PL, Blatt CM, Marsh JD. Part II Digitalis glycosides: mechanisms and manifestations of toxicity. Prog Cardiovasc Dis. 1984;26:495–540.
Bers DM, Berlin JR. Kinetics of [Ca] i decline in cardiac myocytes depend on peak [Ca] i. Am J Physiol-Cell Physiol. 1995;268:C271–C7.
Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, et al. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res. 2003;92:e52–e9.
Chang KC, Barth AS, Sasano T, Kizana E, Kashiwakura Y, Zhang Y, et al. CAPON modulates cardiac repolarization via neuronal nitric oxide synthase signaling in the heart. Proc Natl Acad Sci USA. 2008;105:4477–82.
Ashley EA, Sears CE, Bryant SM, Watkins HC, Casadei B. Cardiac nitric oxide synthase 1 regulates basal and β-adrenergic contractility in murine ventricular myocytes. Circulation. 2002;105:3011–6.
Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci. 1999;96:657–62.
Ronchi C, Bernardi J, Mura M, Stefanello M, Badone B, Rocchetti M, et al. NOS1AP polymorphisms reduce NOS1 activity and interact with prolonged repolarization in arrhythmogenesis. Cardiovasc Res. 2021;117:472–83.
Earle NJ, Poppe KK, Pilbrow AP, Cameron VA, Troughton RW, Skinner JR, et al. Genetic markers of repolarization and arrhythmic events after acute coronary syndromes. Am Heart J. 2015;169:579–86 e3.
Souza DS, Menezes-Filho JER, Santos-Miranda A, Jesus ICG, Silva Neto JA, Guatimosim S, et al. Calcium overload-induced arrhythmia is suppressed by farnesol in rat heart. Eur J Pharm. 2019;859:172488.
Hauptman PJ, Kelly RA. Digitalis. Circulation. 1999;99:1265–70.
Auton A, Abecasis GR, Altshuler DM, Durbin RM, Abecasis GR, Bentley DR, et al. A global reference for human genetic variation. Nature. 2015;526:68–74.
Funding
“This Project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 116030. This Joint Understanding receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.”
Author information
Authors and Affiliations
Contributions
BHS, FA, and AJA are responsible for the study concept and design; NS performed the statistical analyses, data interpretation and prepared the manuscript/revision; AJA, MK, JAK, MAI, CN, FA, and BHS critically edited the manuscript for intellectual content. BHS and FA are responsible for the final content, interpretation of the results and contributed to the revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soroush, N., Aarnoudse, AJ., Kavousi, M. et al. A NOS1AP gene variant is associated with a paradoxical increase of the QT-interval shortening effect of digoxin. Pharmacogenomics J 22, 55–61 (2022). https://doi.org/10.1038/s41397-021-00256-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-021-00256-2