Abstract
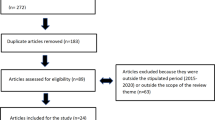
Nowadays, many relevant drug–gene associations have been discovered, but pharmacogenomics (PGx)-guided treatment needs to be cost-effective as well as clinically beneficial to be incorporated into standard health care. To address current challenges, this systematic review provides an update regarding previously published studies, which assessed the cost-effectiveness of PGx testing for the prescription of antidepressants and antipsychotics. From a total of 1159 studies initially identified by literature database querying, and after manual assessment and curation of all of them, a mere 18 studies met our inclusion criteria. Of the 18 studies evaluations, 16 studies (88.89%) drew conclusions in favor of PGx testing, of which 9 (50%) genome-guided interventions were cost-effective and 7 (38.9%) were less costly compared to standard treatment based on cost analysis. More precisely, supportive evidence exists for CYP2D6 and CYP2C19 drug–gene associations and for combinatorial PGx panels, but evidence is limited for many other drug–gene combinations. Amongst the limitations of the field are the unclear explanation of perspective and cost inputs, as well as the underreporting of study design elements, which can influence though the economic evaluation. Overall, the findings of this article demonstrate that although there is growing evidence on the cost-effectiveness of genome-guided interventions in psychiatric diseases, there is still a need for performing additional research on economic evaluations of PGx implementation with an emphasis on psychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hannah R, Max R. “Mental Health”. 2018. https://ourworldindata.org/mental-health. Accessed 10 Oct 2020.
WHO. Mental disorders. 2019. https://www.who.int/news-room/fact-sheets/detail/mental-disorders Accessed 10 Oct 2020.
National Institute of Mental Health. 2019. https://www.nimh.nih.gov/health/statistics/mental-illness.shtml. Accessed 10 Oct 2020.
John A, McGregor J, Jones I, Lee SC, Walters JTR, Owen MJ, et al. Premature mortality among people with severe mental illness—new evidence from linked primary care data. Schizophr Res. 2018;199:154–62.
Liu NH, Daumit GL, Dua T, Aquila R, Charlson F, Cuijpers P, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16:30–40.
Berto P, D’Ilario D, Ruffo P, Virgilio RDI, Rizzo F. Depression: cost-of-illness studies in the international literature, a review. J Ment Health Policy Econ. 2000;3:3–10.
Wang PS, Simon G, Kessler RC. The economic burden of depression and the cost-effectiveness of treatment. Int J Methods Psychiatr Res. 2003;12:22–33.
Trautmann S, Rehm J, Wittchen HU. The economic costs of mental disorders: Do our societies react appropriately to the burden of mental disorders? EMBO Rep. 2016;17:1245–9.
OECD. OECD Health at a Glance 2019. In OECD iLibrary. 2019. https://www.oecd-ilibrary.org/sites/health_glance_eur-2018-4-en/index.html?itemId=/content/component/health_glance_eur-2018-4-en.
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858.
Fabbri C, Corponi F, Souery D, Kasper S, Montgomery S, Zohar J, et al. The genetics of treatment-resistant depression: a critical review and future perspectives. Int J Neuropsychopharmacol. 2019;22:93–104.
Anderson IM, Haddad PM, Scott J. Bipolar disorder. BMJ. 2012;345:e8508.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17.
Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: Thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37:851–64.
Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449–59.
Mrazek MD, Mooneyham BW, Schooler JW. Insights from quiet minds: the converging fields of mindfulness and mind-wandering. 2014. https://doi.org/10.1007/978-3-319-01634-4_13.
Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15:473–500.
Laje G, Allen AS, Akula N, Manji H, John Rush A, McMahon FJ. Genome-wide association study of suicidal ideation emerging during citalopram treatment of depressed outpatients. Pharmacogenet Genomics. 2009;19:666–74.
Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–57.
Villafuerte SM, Vallabhaneni K, Śliwerska E, McMahon FJ, Young EA, Burmeister M. SSRI response in depression may be influenced by SNPs in HTR1B and HTR1A. Psychiatr Genet. 2009;19:281–91.
Alagoz O, Durham D, Kasirajan K. Cost-effectiveness of one-time genetic testing to minimize lifetime adverse drug reactions. Pharmacogenomics J. 2016;16:129–36. 2016
Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014;32:335–46.
Vegter S, Jansen E, Postma MJ, Boersma C. Economic evaluations of pharmacogenetic and genomic screening programs: update of the literature. Drug Dev Res. 2010;71:492–501.
Vegter S, Boersma C, Rozenbaum M, Wilfert B, Navis G, Postma J. Pharmacoeconomic evaluations of pharmacogenetic and genomic screening programmes: a systematic review on content and adherence to guidelines. Pharmacoeconomics. 2008;26:569–87.
Goetz MP, Sangkuhl K, Guchelaar HJ, Schwab M, Province M, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and tamoxifen therapy. Clin Pharm Ther. 2018;103:770–7.
Johnson JA, Cavallari LH. Pharmacogenetics and cardiovascular disease-implications for personalized medicine’. Pharm Rev. 2013;65:987–1009.
Mahungu T, Owen A. Current progress in the pharmacogenetics of infectious disease therapy. In: Tibayrenc M., editor. Genetics and Evolution of Infectious Disease. 2nd ed. Elsevier: Amsterdam; 2011.
Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N. Engl J Med. 2008;358:568–79.
Hicks JK, Sangkuhl K, Swen JJ, Ellingrod VL, Müller DJ, Shimoda K, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharm Ther. 2017;102:37–44.
Westergaard N, Søgaard Nielsen R, Jørgensen S, Vermehren C. Drug use in Denmark for drugs having pharmacogenomics (PGx) based dosing guidelines from CPIC or DPWG for CYP2D6 and CYP2C19 drug–gene pairs: Perspectives for introducing PGx test to polypharmacy patients. J Pers Med. 2020;10:3.
Kordou Z, Skokou M, Tsermpini EE, Chantratita W, Fukunaga K, Mushiroda T, et al. Discrepancies and similarities in the genome-informed guidance for psychiatric disorders amongst different regulatory bodies and research consortia using next generation sequencing-based clinical pharmacogenomics data [published online ahead of print, 2021 Mar 9]. Pharm Res. 2021;167:105538.
Hiemke C, Bergemann N, Clement HW, Conca A, Deckert J, Domschke K, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51:9–62.
Bousman CA, Bengesser SA, Aitchison KJ, Amare AT, Aschauer H, Baune BT, et al. Review and consensus on pharmacogenomic testing in psychiatry. Pharmacopsychiatry. 2021;54:5–17.
Berm EJJ, De Looff M, Wilffert B, Boersma C, Annemans L, Vegter S, et al. Economic evaluations of pharmacogenetic and pharmacogenomic screening tests: a systematic review. Second update of the literature. PLoS ONE 2016;11:e0146262.
Peterson K, Dieperink E, Anderson J, Boundy E, Ferguson L, Helfand M. Rapid evidence review of the comparative effectiveness, harms, and cost-effectiveness of pharmacogenomics-guided antidepressant treatment versus usual care for major depressive disorder. Psychopharmacology. 2017;234:1649–61.
Chiou CF, Hay JW, Wallace JF, Bloom BS, Neumann PJ, Sullivan SD, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Med Care. 2003;41:32–44.
Djalalov S, Musa Z, Mendelson M, Siminovitch K, Hoch J. A review of economic evaluations of genetic testing services and interventions (2004–2009). Genet Med. 2011;13:89–94.
Wong WB, Carlson JJ, Thariani R, Veenstra DL. Cost effectiveness of pharmacogenomics: a critical and systematic review. Pharmacoeconomics. 2010;28:1001–13.
Zhu Y, Swanson KM, Rojas RL, Wang Z, St. Sauver JL, Visscher SL, et al. Systematic review of the evidence on the cost-effectiveness of pharmacogenomics-guided treatment for cardiovascular diseases. Genet Med. 2020;22:475–86.
King KR, Grazette LP, Paltoo DN, McDevitt JT, Sia SK, Barrett PM, et al. Point-of-care technologies for precision cardiovascular care and clinical research: National Heart, Lung, and Blood Institute Working Group. JACC Basic Transl Sci. 2016;1:73–86.
Benitez J, Cool CL, Scotti DJ. Use of combinatorial pharmacogenomic guidance in treating psychiatric disorders. Per Med. 2018;15:481–94.
Berm EJJ, Gout-Zwart JJ, Luttjeboer J, Wilffert B, Postma MJ. A model based cost-effectiveness analysis of routine genotyping for CYP2D6 among older, depressed inpatients starting nortriptyline pharmacotherapy. PLoS ONE. 2016;11:e0169065.
Brown LC, Lorenz RA, Li J, Dechairo BM. Economic utility: combinatorial pharmacogenomics and medication cost savings for mental health care in a primary care setting. Clin Ther. 2017;39:592–602.e1.
Fagerness J, Fonseca E, Hess GP, Scott R, Gardner KR, Koffler M, et al. Pharmacogenetic-guided psychiatric intervention associated with increased adherence and cost savings. Am J Manag Care. 2014;20:e146–e156.
Girardin FR, Poncet A, Perrier A, Vernaz N, Pletscher M, F. Samer C, et al. Cost-effectiveness of HLA-DQB1/HLA-B pharmacogenetic-guided treatment and blood monitoring in US patients taking clozapine. Pharmacogenomics J. 2019;19:211–8.
Groessl EJ, Tally SR, Hillery N, Maciel A, Garces JA. Cost-effectiveness of a pharmacogenetic test to guide treatment for major depressive disorder. J Manag Care Spec Pharm. 2018;24:726–34.
Herbild L, Andersen SE, Werge T, Rasmussen HB, Jürgens G. Does pharmacogenetic testing for CYP450 2D6 and 2C19 among patients with diagnoses within the schizophrenic spectrum reduce treatment costs? Basic Clin Pharm Toxicol. 2013;113:266–72.
Hornberger J, Li Q, Quinn B. Cost-effectiveness of combinatorial pharmacogenomic testing for treatment-resistant major depressive disorder patients. Am J Manag Care. 2015;21:e357–e365.
Maciel A, Cullors A, Alukowiak A, Garces J. Estimating cost savings of pharmacogenetic testing for depression in real-world clinical settings. Neuropsychiatr Dis Treat. 2018;14:225–30.
Najafzadeh M, Garces JA, Maciel A. Economic evaluation of implementing a novel pharmacogenomic test (IDgenetix®) to guide treatment of patients with depression and/or anxiety. Pharmacoeconomics. 2017;35:1297–310.
Olgiati P, Bajo E, Bigelli M, De Ronchi D, Serretti A. Should pharmacogenetics be incorporated in major depression treatment? Economic evaluation in high- and middle-income European countries. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:147–54.
Perlis RH, Ganz DA, Avorn J, Schneeweiss S, Glynn RJ, Smoller JW, et al. Pharmacogenetic testing in the clinical management of schizophrenia: A decision-analytic model. J Clin Psychopharmacol. 2005;25:427–34.
Perlis RH, Patrick A, Smoller JW, Wang PS. When is pharmacogenetic testing for antidepressant response ready for the clinic? A cost-effectiveness analysis based on data from the STAR*D study. Neuropsychopharmacology. 2009;34:2227–36.
Rejon-Parrilla JC, Nuijten M, Redekop WK, Gaultney JG (2014). Economic evaluation of the use of a pharmacogenetic diagnostic test in schizophrenia. Health Policy Technol. 2014. https://doi.org/10.1016/j.hlpt.2014.08.004.
Serretti A, Olgiati P, Bajo E, Bigelli M, De Ronchi D. A model to incorporate genetic testing (5-HTTLPR) in pharmacological treatment of major depressive disorders. World J Biol Psychiatry. 2011;12:501–15.
Sluiter RL, Janzing JGE, van der Wilt GJ, Kievit W, Teichert M. An economic model of the cost-utility of pre-emptive genetic testing to support pharmacotherapy in patients with major depression in primary care. Pharmacogenomics J. 2019;19:480–9.
Winner JG, Carhart JM, Altar CA, Goldfarb S, Allen JD, Lavezzari G, et al. Combinatorial pharmacogenomic guidance for psychiatric medications reduces overall pharmacy costs in a 1 year prospective evaluation. Curr Med Res Opin. 2015;31:1633–43.
Winner JG, Carhart JM, Altar CA, Allen JD, Dechairo BM. A prospective, randomized, double-blind study assessing the clinical impact of integrated pharmacogenomic testing for major depressive disorder. Disco Med. 2013;16:219–27.
Mitropoulou C, Fragoulakis V, Rakicevic LB, Novkovic MM, Vozikis A, Matic DM, et al. Economic analysis of pharmacogenomic-guided clopidogrel treatment in Serbian patients with myocardial infarction undergoing primary percutaneous coronary intervention. Pharmacogenomics. 2016;17:1775–84.
Mitropoulou C, Fragoulakis V, Bozina N, Vozikis A, Supe S, Bozina T, et al. Economic evaluation of pharmacogenomic-guided warfarin treatment for elderly Croatian atrial fibrillation patients with ischemic stroke. Pharmacogenomics. 2015;16:137–48.
Cooper C, Dewe P. Well-being—Absenteeism, presenteeism, costs and challenges. Occup Med. 2008;58:522–4.
Kigozi J, Jowett S, Lewis M, Barton P, Coast J. The estimation and inclusion of presenteeism costs in applied economic evaluation: a systematic review. Value Health. 2017;20:496–506.
Bousman CA, Zierhut H, Müller DJ. Navigating the labyrinth of pharmacogenetic testing: a guide to test selection. Clin Pharm Ther. 2019;106:309–12.
Payne K, Gavan SP, Wright SJ, Thompson AJ. Cost-effectiveness analyses of genetic and genomic diagnostic tests. Nat Rev Genet. 2018;19:235–46.
Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: How are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7:518–28.
Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and care excellence cost-effectiveness threshold. Health Technol Assess. 2015;19:1–vi.
Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19:929–35.
Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004;13:437–52.
Simoens S. ‘Pricing and reimbursement of orphan drugs: The need for more transparency’. Orphanet J Rare Dis. 2011;6:42.
Birch S, Gafni A. The biggest bang for the buck or bigger bucks for the bang: the fallacy of the cost-effectiveness threshold. J Health Serv Res Policy. 2006;11:46–51.
Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharm Ther. 2013;138:103–41.
Petrović J, Pešić V, Lauschke VM. Frequencies of clinically important CYP2C19 and CYP2D6 alleles are graded across Europe. Eur J Hum Genet. 2020;28:88–94.
Zastrozhin MS, Grishina EA, Ryzhikova KA, Smirnov VV, Savchenko LM, Bryun EA, et al. The influence of CYP3A5 polymorphisms on haloperidol treatment in patients with alcohol addiction. Pharmgenomics Pers Med. 2017;11:1–5.
Ragia G, Dahl ML, Manolopoulos VG. Influence of CYP3A5 polymorphism on the pharmacokinetics of psychiatric drugs. Curr Drug Metab. 2016;17:227–36.
Patrinos GP, Mitropoulou C. Measuring the value of pharmacogenomics evidence. Clin Pharm Ther. 2017;102:739–41.
Acknowledgements
This study has been partly funded by the European Union’s Horizon 2020 research and innovation grant (H2020’ 668353; Ubiquitous Pharmacogenomics) to GPP and CM and, partly funded by the European Union’s Horizon 2020 research and innovation under the Marie Skłodowska - Curie grant agreement (H2020’ 860895; TranSYS) to GPP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. GPP is Full Member and national Representative of the European Medicines Agency, Committee for Human medicinal Product (CHMP) – Pharmacogenomics Working Party (Amsterdam, the Netherlands) and member of the Clinical Pharmacogenetics Implementation Consortium (CPIC). DJM is a member of CPIC and co-investigator in two pharmacogenetic studies where genetic test kits were provided as in-kind contribution by Myriad Neuroscience. No payment or any equity, stocks, or options from any pharmacogenetic testing company was obtained.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Karamperis, K., Koromina, M., Papantoniou, P. et al. Economic evaluation in psychiatric pharmacogenomics: a systematic review. Pharmacogenomics J 21, 533–541 (2021). https://doi.org/10.1038/s41397-021-00249-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-021-00249-1
This article is cited by
-
Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction of CYP2C9, HLA-A and HLA-B with anti-epileptic drugs
European Journal of Human Genetics (2024)
-
Meta-analysis of pharmacogenetic clinical decision support systems for the treatment of major depressive disorder
The Pharmacogenomics Journal (2023)
-
Comparative neurogenetics of dog behavior complements efforts towards human neuropsychiatric genetics
Human Genetics (2023)
-
A systematic review on the cost effectiveness of pharmacogenomics in developing countries: implementation challenges
The Pharmacogenomics Journal (2022)
-
Cost–Utility Analysis of Pharmacogenetic Testing Based on CYP2C19 or CYP2D6 in Major Depressive Disorder: Assessing the Drivers of Different Cost-Effectiveness Levels from an Italian Societal Perspective
Clinical Drug Investigation (2022)