Abstract
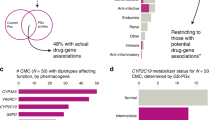
We aimed to determine the potential value of panel-based pharmacogenetic (PGx) testing in patients with chronic pain or gastroesophageal reflux disease (GERD) who underwent single-gene PGx testing to guide opioid or proton pump inhibitor (PPI) therapy, respectively. Of 448 patients included (chronic pain, n = 337; GERD, n = 111), mean age was 57 years, 68% were female, and 73% were white. Excluding opiates for the pain cohort and PPIs for the GERD cohort, 76.6% of patients with pain and 71.2% with GERD were prescribed at least one additional medication with a high level of PGx evidence, most commonly ondansetron or selective serotonin reuptake inhibitors. The most common genes that could inform PGx drug prescribing were CYP2C19, CYP2D6, CYP2C9, and SLCO1B1. Our findings suggest that patients with chronic pain or GERD are commonly prescribed drugs with a high level of evidence for a PGx-guided approach, supporting panel-based testing in these populations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
U.S. Food and Drug Administration. Table of Pharmacogenomic Biomarkers in Drug Labeling. 2020. https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling (accessed 21 Jan 2020).
U.S. Food & Drug Administration. Table of Pharmacogenetic Associations. 2020. https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations (accessed 23 Jun 2020).
Clinical Pharmacogenetics Implementation Consortium (CPIC). Guidelines. https://cpicpgx.org/guidelines/ (accessed 23 Jun 2020).
Clinical Pharmacogenetics Implementation Consortium (CPIC). Prioritization. https://cpicpgx.org/prioritization/#diagram (accessed 1 Jul 2020).
Schildcrout JS, Denny JC, Bowton E, Gregg W, Pulley JM, Basford MA, et al. Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther. 2012;92:235–42.
Haga SB, Kantor A. Horizon scan of clinical laboratories offering pharmacogenetic testing. Health Aff (Proj Hope). 2018;37:717–23.
El Rouby N, Alrwisan A, Langaee T, Lipori G, Angiolillo DJ, Franchi F, et al. Clinical utility of pharmacogene panel-based testing in patients undergoing percutaneous coronary intervention. Clin Transl Sci. 2020;13:473–81.
van der Wouden CH, Bank PCD, Özokcu K, Swen JJ, Guchelaar HJ. Pharmacist-initiated pre-emptive pharmacogenetic panel testing with clinical decision support in primary care: record of PGx results and real-world impact. Genes (Basel). 2019;10:416.
Smith DM, Peshkin BN, Springfield TB, Brown RP, Hwang E, Kmiecik S, et al. Pharmacogenetics in practice: estimating the clinical actionability of pharmacogenetic testing in perioperative and ambulatory settings. Clin Transl Sci. 2020;13:618–27.
Marrero RJ, Cicali EJ, Arwood MJ, Eddy E, DeRemer D, Ramnaraign BH, et al. How to transition from single gene pharmacogenetic testing to preemptive panel-based testing: a tutorial. Clin Pharmacol Ther. 2020;108:557–65.
Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morb Mortal Wkly Rep. 2018;67:1001–6.
Sonnenberg A. Esophageal diseases. In: Everhart JE, editor. Digestive diseases in the United States: epidemiology and impact. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, NIH Publication No. 94–1447. Washington, DC: US Government Printing Office; 1994. p. 299–355.
Institute of Medicine Committee on Advancing Pain Research, Care, Education. The National Academies Collection: Reports funded by National Institutes of Health. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press (US) Copyright © 2011, National Academy of Sciences; 2011.
Gawron AJ, French DD, Pandolfino JE, Howden CW. Economic evaluations of gastroesophageal reflux disease medical management. Pharmacoeconomics. 2014;32:745–58.
Bilgi MM, Vardar R, Yıldırım E, Veznedaroğlu B, Bor S. Prevalence of psychiatric comorbidity in symptomatic gastroesophageal reflux subgroups. Dig Dis Sci. 2017;62:984–93.
Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization study in primary care. JAMA. 1998;280:147–51.
Lu CY, Loomer S, Ceccarelli R, Mazor KM, Sabin J, Clayton EW, et al. Insurance coverage policies for pharmacogenomic and multi-gene testing for cancer. J Personalized Med. 2018;8:19.
Smith DM, Weitzel KW, Elsey AR, Langaee T, Gong Y, Wake DT, et al. CYP2D6-guided opioid therapy improves pain control in CYP2D6 intermediate and poor metabolizers: a pragmatic clinical trial. Genet Med. 2019;21:1842–50.
Cicali EJ, Weitzel KW, Elsey AR, Orlando FA, Vinson M, Mosley S, et al. Challenges and lessons learned from clinical pharmacogenetic implementation of multiple gene-drug pairs across ambulatory care settings. Genet Med. 2019;21:2264–74.
Clinical Pharmacogenetics Implementation Consortium (CPIC).Genes-Drugs. https://cpicpgx.org/genes-drugs/.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81.
Bain KT, Schwartz EJ, Knowlton OV, Knowlton CH, Turgeon J. Implementation of a pharmacist-led pharmacogenomics service for the Program of All-Inclusive Care for the Elderly (PHARM-GENOME-PACE). J Am Pharm Assoc. 2018;58:281–9.e1.
Grice GR, Seaton TL, Woodland AM, McLeod HL. Defining the opportunity for pharmacogenetic intervention in primary care. Pharmacogenomics. 2006;7:61–5.
Fuentes AV, Pineda MD, Venkata KCN. Comprehension of top 200 prescribed drugs in the US as a resource for pharmacy teaching, training and practice. Pharmacy (Basel) 2018;6:43.
Youssef E, Kirkdale CL, Wright DJ, Guchelaar HJ, Thornley T. Estimating the potential impact of implementing pre-emptive pharmacogenetic testing in primary care across the UK. Br J Clin Pharmacol. 2021; e-pub ahead of print 19 Jan 2021; https://doi.org/10.1111/bcp.14704.
Crews KR, Monte AA, Huddart R, Caudle KE, Kharasch ED, Gaedigk A, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2D6, OPRM1, and COMT genotypes and select opioid therapy. Clin Pharmacol Ther. 2021; e-pub ahead of print 2 Jan 2021; https://doi.org/10.1002/cpt.2149.
Theken KN, Lee CR, Gong L, Caudle KE, Formea CM, Gaedigk A, et al. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and nonsteroidal anti-inflammatory drugs. Clin Pharmacol Ther. 2020;108:191–200.
Lima JJ, Thomas CD, Barbarino J, Desta Z, Van Driest SL, El Rouby N, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2C19 and proton pump inhibitor dosing. Clin Pharmacol Ther. 2020;109:1417–23.
Ho RH, Choi L, Lee W, Mayo G, Schwarz UI, Tirona RG, et al. Effect of drug transporter genotypes on pravastatin disposition in European- and African-American participants. Pharmacogenet Genomics. 2007;17:647–56.
Hu M, Mak VW, Tomlinson B. Intronic variants in SLCO1B1 related to statin-induced myopathy are associated with the low-density lipoprotein cholesterol response to statins in Chinese patients with hyperlipidaemia. Pharmacogenet Genomics. 2012;22:803–6.
Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharm Ther. 2007;82:726–33.
Acknowledgements
NIH/NCATS UF CTSA UL1 TR000064 and UL1TR001427, IGNITE Network grant U01 HG007269, and substantial institutional support from University of Florida and University of Florida Health. We would also like to thank the following physicians for their efforts Elvira Mercado, Jeffrey Budd, Melanie Hagen, Ashleigh Wright, and Ying Nagoshi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Elchynski, A.L., Cicali, E.J., Ferrer del Busto, M.C. et al. Determining the potential clinical value of panel-based pharmacogenetic testing in patients with chronic pain or gastroesophageal reflux disease. Pharmacogenomics J 21, 657–663 (2021). https://doi.org/10.1038/s41397-021-00244-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-021-00244-6