Abstract
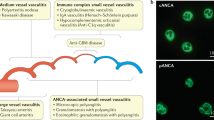
The introduction of immunosuppressive therapy for ANCA-associated vasculitis (AAV) has greatly improved outcomes, though patients now accumulate damage from vasculitis activity and adverse effects of treatment. Prediction of treatment outcomes using gene variants might help reduce this damage by allowing for personalized treatment. Several studies have studied genetic polymorphisms in relation to treatment outcomes of AAV. This review gives an overview of these studies, discussing both gene polymorphisms associated with inflammatory pathways (potentially influencing disease outcomes such as activity, severity, and relapse risk) and pharmacogenetics (potentially influencing drug metabolism and/or drug response). Subsequently, potential benefits of testing genetic variants for AAV and the steps needed for its implementation in clinical practice are discussed. The conclusion of this review is that measurement of most polymorphisms is currently not indicated in clinical practice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603–6.
Costenbader KH, Gay S, Alarcon-Riquelme ME, Iaccarino L, Doria A. Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev. 2012;11:604–9.
Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum. 2009;60:661–8.
Kuo C-F, Grainge MJ, Valdes AM, See L-C, Luo S-F, Yu K-H, et al. Familial aggregation of systemic lupus erythematosus and coaggregation of autoimmune diseases in affected families. JAMA Intern Med. 2015;175:1518–26.
Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–23.
Fujimoto S, Watts RA, Kobayashi S, Suzuki K, Jayne DR, Scott DG, et al. Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the U.K. Rheumatology. 2011;50:1916–20.
Liu L-J, Chen M, Yu F, Zhao M-H, Wang H-Y. Evaluation of a new algorithm in classification of systemic vasculitis. Rheumatology. 2008;47:708–12.
Popa ER, Stegeman CA, Kallenberg CG, Tervaert JW. Staphylococcus aureus and Wegener’s granulomatosis. Arthritis Res. 2002;4:77–9.
Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med. 1996;335:16–20.
Gatenby PA, Lucas RM, Engelsen O, Ponsonby AL, Clements M. Antineutrophil cytoplasmic antibody-associated vasculitides: could geographic patterns be explained by ambient ultraviolet radiation? Arthritis Rheum. 2009;61:1417–24.
Tervaert JW, Stegeman CA, Kallenberg CG. Silicon exposure and vasculitis. Curr Opin Rheumatol. 1998;10:12–7.
Aldo MA, Benson MD, Comerford FR, Cohen AS. Treatment of Wegener’s granulomatosis with immunosuppressive agents. Description of renal ultrastructure. Arch Intern Med. 1970;126:298–305.
Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98:76–85.
Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniene J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44.
Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32.
Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–80.
Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75:1583–94.
Seo P, Min YI, Holbrook JT, Hoffman GS, Merkel PA, Spiera R, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET). Arthritis Rheum. 2005;52:2168–78.
Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Hoglund P, et al. Damage in the anca-associated vasculitides: long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis. 2015;74:177–84.
Tavakolpour S, Darvishi M, Ghasemiadl M. Pharmacogenetics: a strategy for personalized medicine for autoimmune diseases. Clin Genet. 2018;93:481–97.
Franceschini N, Frick A, Kopp JB. Genetic testing in clinical settings. Am J Kidney Dis. 2018;72:569–81.
van Schaik RH. CYP450 pharmacogenetics for personalizing cancer therapy. Drug Resist Updat. 2008;11:77–98.
Takada K, Arefayene M, Desta Z, Yarboro CH, Boumpas DT, Balow JE, et al. Cytochrome P450 pharmacogenetics as a predictor of toxicity and clinical response to pulse cyclophosphamide in lupus nephritis. Arthritis Rheum. 2004;50:2202–10.
Singh G, Saxena N, Aggarwal A, Misra R. Cytochrome P450 polymorphism as a predictor of ovarian toxicity to pulse cyclophosphamide in systemic lupus erythematosus. J Rheumatol. 2007;34:731–3.
Ngamjanyaporn P, Thakkinstian A, Verasertniyom O, Chatchaipun P, Vanichapuntu M, Nantiruj K, et al. Pharmacogenetics of cyclophosphamide and CYP2C19 polymorphism in Thai systemic lupus erythematosus. Rheumatol Int. 2011;31:1215–8.
Chang TK, Yu L, Goldstein JA, Waxman DJ. Identification of the polymorphically expressed CYP2C19 and the wild-type CYP2C9-ILE359 allele as low-Km catalysts of cyclophosphamide and ifosfamide activation. Pharmacogenetics. 1997;7:211–21.
Roy P, Yu LJ, Crespi CL, Waxman DJ. Development of a substrate-activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 profiles. Drug Metab Dispos. 1999;27:655–66.
Schirmer JH, Bremer JP, Moosig F, Holle JU, Lamprecht P, Wieczorek S, et al. Cyclophosphamide treatment-induced leukopenia rates in ANCA-associated vasculitis are influenced by variant CYP450 2C9 genotypes. Pharmacogenomics. 2016;17:367–74.
Cartin-Ceba R, Indrakanti D, Specks U, Stone JH, Hoffman GS, Kallenberg CG, et al. The pharmacogenomic association of Fcgamma receptors and cytochrome P450 enzymes with response to rituximab or cyclophosphamide treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2017;69:169–75.
Asadov C, Aliyeva G, Mustafayeva K. Thiopurine S-methyltransferase as a pharmacogenetic biomarker: significance of testing and review of major methods. Cardiovasc Hematol Agents Med Chem. 2017;15:23–30.
Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharm Ther. 2011;89:387–91.
Coenen MJ, de Jong DJ, van Marrewijk CJ, Derijks LJ, Vermeulen SH, Wong DR, et al. Identification of patients with variants in TPMT and dose reduction reduces hematologic events during thiopurine treatment of inflammatory bowel disease. Gastroenterology. 2015;149:907–17.e7.
Stassen PM, Derks RP, Kallenberg CG, Stegeman CA. Thiopurinemethyltransferase (TPMT) genotype and TPMT activity in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis: relation to azathioprine maintenance treatment and adverse effects. Ann Rheum Dis. 2009;68:758–9.
Hessels AC, Rutgers A, Sanders JSF, Stegeman CA. Thiopurine methyltransferase genotype and activity cannot predict outcomes of azathioprine maintenance therapy for antineutrophil cytoplasmic antibody associated vasculitis: a retrospective cohort study. PLoS One 2018;13:e0195524.
Wong L, Harper L, Little MA. Getting the balance right: adverse events of therapy in anti-neutrophil cytoplasm antibody vasculitis. Nephrol Dial Transplant. 2015;30 Suppl 1:i164–70.
Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Hoglund P, et al. Glucocorticoid treatment and damage in the anti-neutrophil cytoplasm antibody-associated vasculitides: long-term data from the European Vasculitis Study Group trials. Rheumatology. 2015;54:471–81.
Little MA, Nightingale P, Verburgh CA, Hauser T, De Groot K, Savage C, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis. 2010;69:1036–43.
Manenschijn L, van den Akker EL, Lamberts SW, van Rossum EF. Clinical features associated with glucocorticoid receptor polymorphisms. Overv Ann N Y Acad Sci. 2009;1179:179–98.
Koper JW, van Rossum EF, van den Akker EL. Glucocorticoid receptor polymorphisms and haplotypes and their expression in health and disease. Steroids 2014;92:62–73.
Hessels AC, Tuin J, Sanders JSF, Huitema MG, van Rossum EFC, Koper JW, et al. Clinical outcome in anti-neutrophil cytoplasmic antibody-associated vasculitis and gene variants of 11beta-hydroxysteroid dehydrogenase type 1 and the glucocorticoid receptor. Rheumatology. 2018;58:447–54.
Quax RA, Manenschijn L, Koper JW, Hazes JM, Lamberts SW, van Rossum EF, et al. Glucocorticoid sensitivity in health and disease. Nat Rev. 2013;9:670–86.
van Rossum EF, Roks PH, de Jong FH, Brinkmann AO, Pols HA, Koper JW, et al. Characterization of a promoter polymorphism in the glucocorticoid receptor gene and its relationship to three other polymorphisms. Clin Endocrinol. 2004;61:573–81.
Dekker MJ, van den Akker EL, Koper JW, Manenschijn L, Geleijns K, Ruts L, et al. Effect of glucocorticoid receptor gene polymorphisms in Guillain-Barre syndrome. J Peripher Nerv Syst. 2009;14:75–83.
van Oosten MJ, Dolhain RJ, Koper JW, van Rossum EF, Emonts M, Han KH, et al. Polymorphisms in the glucocorticoid receptor gene that modulate glucocorticoid sensitivity are associated with rheumatoid arthritis. Arthritis Res Ther. 2010;12:R159.
van Winsen LM, Manenschijn L, van Rossum EF, Crusius JB, Koper JW, Polman CH, et al. A glucocorticoid receptor gene haplotype (TthIII1/ER22/23EK/9beta) is associated with a more aggressive disease course in multiple sclerosis. J Clin Endocrinol Metab. 2009;94:2110–4.
Zou YF, Xu JH, Wang F, Liu S, Tao JH, Cai J, et al. Association study of glucocorticoid receptor genetic polymorphisms with efficacy of glucocorticoids in systemic lupus erythematosus: a prospective cohort study. Autoimmunity. 2013;46:531–6.
Quax RA, Koper JW, Huisman AM, Weel A, Hazes JM, Lamberts SW, et al. Polymorphisms in the glucocorticoid receptor gene and in the glucocorticoid-induced transcript 1 gene are associated with disease activity and response to glucocorticoid bridging therapy in rheumatoid arthritis. Rheumatol Int. 2015;35:1325–33.
Gabryel M, Skrzypczak-Zielinska M, Kucharski MA, Slomski R, Dobrowolska A. The impact of genetic factors on response to glucocorticoids therapy in IBD. Scand J Gastroenterol. 2016;18:1–12.
Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, et al. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–66.
Dekker MJ, Tiemeier H, Luijendijk HJ, Kuningas M, Hofman A, de Jong FH, et al. The effect of common genetic variation in 11beta-hydroxysteroid dehydrogenase type 1 on hypothalamic-pituitary-adrenal axis activity and incident depression. J Clin Endocrinol Metab. 2012;97:E233–7.
Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369:417–27.
Terrier B, Pagnoux C, Perrodeau E, Karras A, Khouatra C, Aumaitre O, et al. Long-term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann Rheum Dis. 2018;77:1150–6.
Alberici F, Smith RM, Fonseca M, Willcocks LC, Jones RB, Holle JU, et al. Association of a TNFSF13B (BAFF) regulatory region single nucleotide polymorphism with response to rituximab in antineutrophil cytoplasmic antibody-associated vasculitis. J Allergy Clin Immunol. 2017;139:1684–87.e10.
Robledo G, Davila-Fajardo CL, Marquez A, Ortego-Centeno N, Callejas Rubio JL, de Ramon Garrido E, et al. Association between −174 interleukin-6 gene polymorphism and biological response to rituximab in several systemic autoimmune diseases. DNA Cell Biol. 2012;31:1486–91.
Ruyssen-Witrand A, Rouanet S, Combe B, Dougados M, Le Loet X, Sibilia J, et al. Fcgamma receptor type IIIA polymorphism influences treatment outcomes in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis. 2012;71:875–7.
Lee YH, Bae SC, Song GG. Functional FCGR3A 158 V/F and IL-6 -174 C/G polymorphisms predict response to biologic therapy in patients with rheumatoid arthritis: a meta-analysis. Rheumatol Int. 2014;34:1409–15.
Stork AC, Notermans NC, van den Berg LH, Schellevis RD, Niermeijer JM, Nederend M, et al. Fcgamma receptor IIIA genotype is associated with rituximab response in antimyelin-associated glycoprotein neuropathy. J Neurol Neurosurg Psychiatry. 2014;85:918–20.
Ravani P, Ponticelli A, Siciliano C, Fornoni A, Magnasco A, Sica F, et al. Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int. 2013;84:1025–33.
Dijstelbloem HM, Scheepers RH, Oost WW, Stegeman CA, van der Pol WL, Sluiter WJ, et al. Fcgamma receptor polymorphisms in Wegener’s granulomatosis: risk factors for disease relapse. Arthritis Rheum. 1999;42:1823–7.
Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120:12–7.
Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64.
Sanders JS, Huitma MG, Kallenberg CG, Stegeman CA. Plasma levels of soluble interleukin 2 receptor, soluble CD30, interleukin 10 and B cell activator of the tumour necrosis factor family during follow-up in vasculitis associated with proteinase 3-antineutrophil cytoplasmic antibodies: associations with di. Ann Rheum Dis. 2006;65:1484–9.
Bader L, Koldingsnes W, Nossent J. B-lymphocyte activating factor levels are increased in patients with Wegener’s granulomatosis and inversely correlated with ANCA titer. Clin Rheumatol. 2010;29:1031–5.
Krumbholz M, Specks U, Wick M, Kalled SL, Jenne D, Meinl E. BAFF is elevated in serum of patients with Wegener’s granulomatosis. J Autoimmun. 2005;25:298–302.
Ruyssen-Witrand A, Rouanet S, Combe B, Dougados M, Le Loet X, Sibilia J, et al. Association between -871C>T promoter polymorphism in the B-cell activating factor gene and the response to rituximab in rheumatoid arthritis patients. Rheumatology. 2013;52:636–41.
Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2017;69:1016–27.
Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Discov. 2018;17:395–412.
Fabris M, Quartuccio L, Lombardi S, Saracco M, Atzeni F, Carletto A, et al. The CC homozygosis of the -174G>C IL-6 polymorphism predicts a lower efficacy of rituximab therapy in rheumatoid arthritis. Autoimmun Rev. 2012;11:315–20.
Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–50.
Marquez A, Davila-Fajardo CL, Robledo G, Rubio JL, de Ramon Garrido E, Garcia-Hernandez FJ, et al. IL2/IL21 region polymorphism influences response to rituximab in systemic lupus erythematosus patients. Mol Biol Rep. 2013;40:4851–6.
Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604.
Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79.
Jonsson N, Erlandsson E, Gunnarsson L, Pettersson A, Ohlsson S. Monocyte chemoattractant protein-1 in antineutrophil cytoplasmic autoantibody-associated vasculitis: biomarker potential and association with polymorphisms in the MCP-1 and the CC chemokine receptor-2 gene. Mediators Inflamm. 2018;2018:6861257.
Hadzik-Blaszczyk M, Zdral A, Zielonka TM, Rozy A, Krupa R, Falkowski A, et al. SERPINA1 gene variants in granulomatosis with polyangiitis. Adv Exp Med Biol. 2018;1070:9–18.
Hilhorst M, Arndt F, Joseph Kemna M, Wieczorek S, Donner Y, Wilde B, et al. HLA-DPB1 as a risk factor for relapse in antineutrophil cytoplasmic antibody-associated vasculitis: a cohort study. Arthritis Rheumatol. 2016;68:1721–30.
Isenberg D, Appel GB, Contreras G, Dooley MA, Ginzler EM, Jayne D, et al. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology. 2010;49:128–40.
Liu C, Batliwalla F, Li W, Lee A, Roubenoff R, Beckman E, et al. Genome-wide association scan identifies candidate polymorphisms associated with differential response to anti-TNF treatment in rheumatoid arthritis. Mol Med. 2008;14:575–81.
Lee KS, Kronbichler A, Pereira Vasconcelos DF, Pereira da Silva FR, Ko Y, Oh YS, et al. Genetic variants in antineutrophil cytoplasmic antibody-associated vasculitis: a bayesian approach and systematic review. J Clin Med. 2019;8:266. https://www.ncbi.nlm.nih.gov/pubmed/30795559.
Walsh M, Merkel PA, Peh CA, Szpirt W, Guillevin L, Pusey CD, et al. Plasma exchange and glucocorticoid dosing in the treatment of anti-neutrophil cytoplasm antibody associated vasculitis (PEXIVAS): protocol for a randomized controlled trial. Trials. 2013;14:73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hessels, A.C., Sanders, J.S.F., Rutgers, A. et al. Gene variants and treatment outcomes in antineutrophil cytoplasmic antibody-associated vasculitis. Pharmacogenomics J 20, 749–759 (2020). https://doi.org/10.1038/s41397-020-0176-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-020-0176-z
This article is cited by
-
Update on Maintenance Therapies for ANCA-Associated Vasculitis
Current Treatment Options in Rheumatology (2021)