Abstract
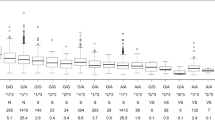
We assessed the predictive accuracy of the Warfarin Pharmacogenetics Consortium (IWPC) algorithm in a prospective cohort of 376 high-risk elderly patients (≥65 years) who required new treatment with warfarin for either medical (non valvular atrial fibrillation) or surgical conditions (heart valve replacement), had ≥1 comorbid conditions, and regularly used ≥2 other drugs. Follow-up visits were performed according to clinical practice and lasted for a maximum of 1 year. Two hundred and eighty-three (75%) patients achieved a stable maintenance dose. Warfarin maintenance doses were low on average (median 20.3 mg/week, interquartile range, 14.1–27.7 mg/week) and were substantially overestimated by the IWPC algorithm. Overall the percentage of patients whose predicted dose of warfarin was within 20% of the actual stable dose was equal to 37.5%, (95% CI 32.0–43.3%). IWPC algorithm explained only 31% of the actual warfarin dose variability. Modifications of the IWPC algorithm are needed in high-risk elderly people.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Friberg L, Rosenqvist M, Lip GYH. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298–307.
Franchini M, Mengoli C, Cruciani M, Bonfanti C, Mannucci PM. Effects on bleeding complications of pharmacogenetic testing for initial dosing of vitamin K antagonists: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:1480–7.
Mannucci PM, Franchini M. Old and new anticoagulant drugs: a minireview. Ann Med. 2011;43:116–23.
Franchini M, Mannucci PM. New anticoagulants for treatment of venous thromboembolism. Eur J Intern Med. 2012;23:692–5.
Johnson JJA, Caudle KE, Gong L, Whirl-Carrillo M, Stein CM, Scott SSA, et al. Clinical Pharmacogenetics Implementation Consortium (Cpic) Guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharm Ther. 2017;102:1–30.
International Warfarin Pharmacogenetics Consortium, Klein TE, Altman RB, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–64.
Roper N, Storer B, Bona R, Fang M. Validation and comparison of pharmacogenetics-based warfarin dosing algorithms for application of pharmacogenetic testing. J Mol Diagn. 2010;12:283–91.
Sagreiya H, Sagrieya H, Berube C, Wen A, Ramakrishnan R, Mir A, et al. Extending and evaluating a warfarin dosing algorithm that includes CYP4F2 and pooled rare variants of CYP2C9. Pharmacogenet Genomics. 2010;20:407–13.
Shaw PB, Donovan JL, Tran MT, Lemon SC, Burgwinkle P, Gore J. Accuracy assessment of pharmacogenetically predictive warfarin dosing algorithms in patients of an academic medical center anticoagulation clinic. J Thromb Thrombolysis. 2010;30:220–5.
Takeuchi F, Kashida M, Okazaki O, Tanaka Y, Fukuda S, Kashima T, et al. Evaluation of pharmacogenetic algorithm for warfarin dose requirements in Japanese patients. Circ J. 2010;74:977–82.
Cho H-J, On Y-K, Bang OY, Kim J-W, Huh W, Ko J-W, et al. Development and comparison of a warfarin-dosing algorithm for Korean patients with atrial fibrillation. Clin Ther. 2011;33:1371–80.
Schwartz JB, Kane L, Moore K, Wu AHB. Failure of pharmacogenetic-based dosing algorithms to identify older patients requiring low daily doses of warfarin. J Am Med Dir Assoc. 2011;12:633–8.
Zambon C-F, Pengo V, Padrini R, Basso D, Schiavon S, Fogar P, et al. VKORC1, CYP2C9 and CYP4F2 genetic-based algorithm for warfarin dosing: an Italian retrospective study. Pharmacogenomics. 2011;12:15–25.
Bazan NS, Sabry NA, Rizk A, Mokhtar S, Badary O. Validation of pharmacogenetic algorithms and warfarin dosing table in Egyptian patients. Int J Clin Pharm. 2012;34:837–44.
Cini M, Legnani C, Cosmi B, Guazzaloca G, Valdrè L, Frascaro M, et al. A new warfarin dosing algorithm including VKORC1 3730 G>A polymorphism: comparison with results obtained by other published algorithms. Eur J Clin Pharm. 2012;68:1167–74.
Marin-Leblanc M, Perreault S, Bahroun I, Lapointe M, Mongrain I, Provost S, et al. Validation of warfarin pharmacogenetic algorithms in clinical practice. Pharmacogenomics. 2012;13:21–9.
Pathare A, Al Khabori M, Alkindi S, Al Zadjali S, Misquith R, Khan H, et al. Warfarin pharmacogenetics: development of a dosing algorithm for Omani patients. J Hum Genet. 2012;57:665–9.
Ramirez AH, Shi Y, Schildcrout JS, Delaney JT, Xu H, Oetjens MT, et al. Predicting warfarin dosage in European-Americans and African-Americans using DNA samples linked to an electronic health record. Pharmacogenomics. 2012;13:407–18.
Ramos AS, Seip RL, Rivera-Miranda G, Felici-Giovanini ME, Garcia-Berdecia R, Alejandro-Cowan Y, et al. Development of a pharmacogenetic-guided warfarin dosing algorithm for Puerto Rican patients. Pharmacogenomics. 2012;13:1937–50.
Tan SL, Li Z, Song GB, Liu LM, Zhang W, Peng J, et al. Development and comparison of a new personalized warfarin stable dose prediction algorithm in Chinese patients undergoing heart valve replacement. Pharmazie. 2012;67:930–7.
Ekladious SMM, Issac MSM, El-Atty Sharaf SA, Abou-Youssef HS. Validation of a proposed warfarin dosing algorithm based on the genetic make-up of Egyptian patients. Mol Diagn Ther. 2013;17:381–90.
Bosch LÁB. A proposal for an individualized pharmacogenetic-guided warfarin dosage regimen for Puerto Rican patients commencing anticoagulation therapy. J Pharmacogen Pharmacoproteom 2014; 5. https://doi.org/10.4172/2153-0645.T-001.
Hernandez W, Gamazon ER, Aquino-Michaels K, Patel S, O’Brien TJ, Harralson AF, et al. Ethnicity-specific pharmacogenetics: the case of warfarin in African Americans. Pharmacogenomics J. 2014;14:223–8.
Pavani A, Naushad SM, Uma A, Kutala VK. Methodological issues in the development of a pharmacogenomic algorithm for warfarin dosing: comparison of two regression approaches. Pharmacogenomics. 2014;15:1125–32.
Zhao L, Chen C, Li B, Dong L, Guo Y, Xiao X, et al. Verification of pharmacogenetics-based warfarin dosing algorithms in Han-Chinese patients undertaking mechanic heart valve replacement. PLoS ONE. 2014;9:1–8.
Karaca S, Bozkurt NC, Cesuroglu T, Karaca M, Bozkurt M, Eskioglu E, et al. International warfarin genotype-guided dosing algorithms in the Turkish population and their preventive effects on major and life-threatening hemorrhagic events. Pharmacogenomics. 2015;16:1109–18.
Peng Q, Huang S, Chen X, Yuan Y, Yu Y, Tao L, et al. Validation of warfarin pharmacogenetic algorithms in 586 Han Chinese patients. Pharmacogenomics. 2015;16:1465–74.
Saffian SM, Wright DFB, Roberts RL, Duffull SB. Methods for predicting warfarin dose requirements. Ther Drug Monit. 2015;37:531–8.
Santos PCJL, Marcatto LR, Duarte NE, Gadi Soares RA, et al. Development of a pharmacogenetic-based warfarin dosing algorithm and its performance in Brazilian patients: highlighting the importance of population-specific calibration. Pharmacogenomics. 2015;16:865–76.
Xu H, Su S, Tang W, Wei M, Wang T, Wang D, et al. Comparison of the performance of the warfarin pharmacogenetics algorithms in patients with surgery of heart valve replacement and heart valvuloplasty. Thromb Res. 2015;136:552–9.
Cho S-M, Lee K-Y, Choi JR, Lee K-A. Development and comparison of warfarin dosing algorithms in stroke patients. Yonsei Med J. 2016;57:635.
Duconge J, Ramos AS, Claudio-Campos K, Rivera-Miranda G, Bermúdez-Bosch L, Renta JY, et al. A novel admixture-based pharmacogenetic approach to refine warfarin dosing in Caribbean Hispanics. PLoS ONE. 2016;11:e0145480.
Lin M, Yu L, Qiu H, Wang Q, Zhang J, Song H. Verification of five pharmacogenomics-based warfarin administration models. Indian J Pharm. 2016;48:258–63.
Stack G, Maurice CB. Warfarin pharmacogenetics reevaluated: subgroup analysis reveals a likely underestimation of the maximum pharmacogenetic benefit by clinical trials. Am J Clin Pathol. 2016;145:671–86.
Saffian S, Duffull S, Wright D. Warfarin dosing algorithms underpredict dose requirements in patients requiring ≥7 mg daily: a systematic review and meta-analysis. Clin Pharm Ther. 2017;102:297–304.
Anderson JL, Horne BD, Stevens SM, Woller SC, Samuelson KM, Mansfield JW, et al. A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II). Circulation. 2012;125:1997–2005.
Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–6.
Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4.
1000 Genomes Project Consortium RA, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74.
Mazzaccara C, Conti V, Liguori R, Simeon V, Toriello M, Severini A, et al. Warfarin anticoagulant therapy: a Southern Italy pharmacogenetics-based dosing model. PLoS ONE. 2013;8:e71505.
Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37:1075–80.
Chappell J, He J, Knadler MP, Mitchell M, Lee D, Lobo E. Effects of duloxetine on the pharmacodynamics and pharmacokinetics of warfarin at steady state in healthy subjects. J Clin Pharm. 2009;49:1456–66.
Chappell JC, Dickinson G, Mitchell MI, Haber H, Jin Y, Lobo ED. Evaluation of methods for achieving stable INR in healthy subjects during a multiple-dose warfarin study. Eur J Clin Pharm. 2012;68:239–47.
Roberts GW, Quinn S, Druskeit T, Helboe T, Jørgensen LE, Johansen C. Post-operative warfarin re-initiation—modelling loading dose strategy. J Pharm Pr Res. 2013;43:276–8.
Yan H, Yin JY, Zhang W, Li X. Possible strategies to make warfarin dosing algorithm prediction more accurately in patients with extreme dose. Clin. Pharmacol. Ther. 2018. https://doi.org/10.1002/cpt.800.
Burmester JK, Berg RL, Yale SH, Rottscheit CM, Glurich IE, Schmelzer JR, et al. A randomized controlled trial of genotype-based Coumadin initiation. Genet Med. 2011;13:509–18.
Verhoef TI, Ragia G, de Boer A, Barallon R, Kolovou G, Kolovou V, et al. A randomized trial of genotype-guided dosing of acenocoumarol and phenprocoumon. N. Engl J Med. 2013;369:2304–12.
Iung B, Rodés-cabau J. The optimal management of anti-thrombotic therapy after valve replacement: certainties and uncertainties. Eur Heart J. 2014;35:2942–9.
Acknowledgements
The study was fully supported by the Italian Drug Agency (Agenzia Italiana per il Farmaco) study code FARM9JNT9Y. The sponsor had no role in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. VS has been supported by Programma VALERE, University of Campania “Luigi Vanvitelli”. The authors thank Flavia Lo Passo for assistance with data management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the ethics committees of all participating centers. All participants gave written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Filippelli, A., Signoriello, S., Bancone, C. et al. Prospective validation of the International Warfarin Pharmacogenetics Consortium algorithm in high-risk elderly people (VIALE study). Pharmacogenomics J 20, 451–461 (2020). https://doi.org/10.1038/s41397-019-0129-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-019-0129-6