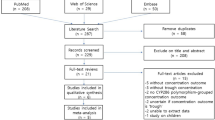
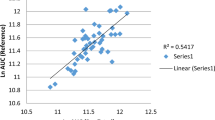
Abstract
Clinical data on the relationships of cytochrome P450 (CYP2) B6 516G>T polymorphisms with efavirenz-induced central nervous system (CNS) side effects and virological response in HIV-infected adults are controversial. We sought to analyze the associations by meta-analysis. To identify eligible studies, we systematically searched PubMed, Embase, ScienceDirect, and Web of Science. The strength of the associations was measured by odds ratio (OR) and effect size (ES) with 95% confidence interval (CI). Seventeen studies comprising a total of 3598 HIV-infected adults were included. The results showed that the CYP2B6-516 GG genotype was significantly associated with a decreased risk of efavirenz-induced CNS side effects compared with the GT and TT genotypes (GG + GT vs. TT: OR = 0.60, 95% CI = 0.41–0.87, P = 0.006; GG vs. GT + TT: OR = 0.68, 95% CI = 0.51–0.91, P = 0.008; GG vs. GT: OR = 0.70, 95% CI = 0.51–0.94, P = 0.018), and there was no significant association between the genetic variants GT and TT (GT vs. TT: OR = 0.82, 95% CI = 0.54–1.26, P = 0.372). However, there was no significant association between CYP2B6-516 GG and GT + TT genotypes in virological response (GT + TT vs. GG: ES = 1.06, 95% CI = 0.95–1.18, P = 0.321; OR = 1.01, 95% CI = 0.65–1.58, P = 0.963). Taken together, our results demonstrated that compared with the normal efavirenz clearance genotype CYP2B6-516 GG, the slow and very slow efavirenz clearance genotypes GT and TT were significantly associated with an increased risk of efavirenz-induced CNS side effects but not an increased virological response. To promote the tolerance of efavirenz, it is better to adjust the dosage of efavirenz according to the polymorphisms of CYP2B6-516 in HIV-infected adults.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Seckinelgin H. The politics of global AIDS. Switzerland: Springer, Cham; 2017.
Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, Esplugues JV. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother. 2015;70:2693–708.
Leutscher PDC, Stecher C, Storgaard M, Larsen CS. Discontinuation of efavirenz therapy in HIV patients due to neuropsychiatric adverse effects. Scand J Infect Dis. 2013;45:645–51.
Puls RL, Amin J, Losso M, Phanuphak P, Nwizu C, Orrell C, et al. Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet. 2014;383:1474–82.
Wyen C, Hendra H, Siccardi M, Platten M, Jaeger H, Harrer T, et al. Cytochrome P450 2B6 (CYP2B6) and constitutive androstane receptor (CAR) polymorphisms are associated with early discontinuation of efavirenz-containing regimens. J Antimicrob Chemother. 2011;66:2092–8.
Staszewski S, Morales-Ramirez J, Tashima KT, Rachlis A, Skiest D, Stanford J, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999;341:1865–73.
Mollan KR, Smurzynski M, Eron JJ, Daar ES, Campbell TB, Sax PE. et al. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide an analysis of trial data. Ann Intern Med. 2014;161:1–10.
Napoli AA, Wood JJ, Coumbis JJ, Soitkar AM, Seekins DW, Tilson HH. No evident association between efavirenz use and suicidality was identified from a disproportionality analysis using the FAERS database. J Int Aids Soc. 2014;17:19214.
Smith C, Ryom L, Monforte AD, Reiss P, Mocroft A, El-Sadr W, et al. Lack of association between use of efavirenz and death from suicide: evidence from the D:A:D study. J Int Aids Soc. 2014;17:17–8.
World Health Organization Interim Guidelines. Update recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. 2018. https://apps.who.int/iris/bitstream/handle/10665/277395/WHO-CDS-HIV-18.51-eng.pdf?ua=1.
European AIDS Clinical Society Guidelines. 2016. http://www.eacsociety.org/files/2015_eacsguidelines_8.0-english_rev-20151221.pdf.
Geneva: World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd edition. Geneva: WHO Press; 2016.
Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharm Exp Ther. 2003;306:287–300.
Gounden V, van Niekerk C, Snyman T, George JA. Presence of the CYP2B6 516G> T polymorphism, increased plasma Efavirenz concentrations and early neuropsychiatric side effects in South African HIV-infected patients. AIDS Res Ther. 2010;7:32.
Swart M, Skelton M, Ren Y, Smith P, Takuva S, Dandara C. High predictive value of CYP2B6 SNPs for steady-state plasma efavirenz levels in South African HIV/AIDS patients. Pharmacogenet Genom. 2013;23:415–27.
Hui KH, Lee SS, Lam TN. Dose optimization of efavirenz based on individual CYP2B6 polymorphisms in Chinese patients positive for HIV. CPT Pharmacomet Syst Pharm. 2016;5:182–91.
Neary M, Lamorde M, Olagunju A, Darin KM, Merry C, Byakika-Kibwika P, et al. The effect of gene variants on levonorgestrel pharmacokinetics when combined with antiretroviral therapy containing efavirenz or nevirapine. Clin Pharm Ther. 2017;102:529–36.
Reay R, Dandara C, Viljoen M, Rheeders M. CYP2B6 haplotype predicts efavirenz plasma concentration in black South African HIV-1-infected children: a longitudinal pediatric pharmacogenomic study. OMICS. 2017;21:465–73.
To KW, Liu ST, Cheung SW, Chan DP, Chan RC, Lee SS. Pharmacokinetics of plasma efavirenz and CYP2B6 polymorphism in southern Chinese. Ther Drug Monit. 2009;31:527–30.
Chang JL, Lee SA, Tsai AC, Musinguzi N, Muzoora C, Bwana B, et al. CYP2B6 genetic polymorphisms, depression, and viral suppression in adults living with hiv initiating efavirenz-containing antiretroviral therapy regimens in Uganda: pooled analysis of two prospective studies. AIDS Res Hum Retroviruses. 2018;34:982–92.
Mukonzo JK, Owen JS, Ogwal-Okeng J, Kuteesa RB, Nanzigu S, Sewankambo N, et al. Pharmacogenetic-based efavirenz dose modification: suggestions for an African population and the different CYP2B6 genotypes. PLoS ONE. 2014;9:e86919.
de Almeida TB, de Azevedo M, Pinto J, Ferry FRA, da Silva GAR, de Castro IJ, et al. Drug metabolism and transport gene polymorphisms and efavirenz adverse effects in Brazilian HIV-positive individuals. J Antimicrob Chemother. 2018;73:2460–7.
Johnson DH, Gebretsadik T, Shintani A, Mayo G, Acosta EP, Stein CM, et al. Neuropsychometric correlates of efavirenz pharmacokinetics and pharmacogenetics following a single oral dose. Br J Clin Pharm. 2013;75:997–1006.
Aurpibul L, Chotirosniramit N, Sugandhavesa P, Kosashunhanan N, Thetket S, Supindham T, et al. Correlation of CYP2B6-516G > T polymorphism with plasma efavirenz concentration and depression in HIV-infected adults in Northern Thailand. Curr HIV Res. 2012;10:653–60.
Muller TE, Ellwanger JH, Michita RT, Matte MCC, Renner JDP. CYP2B6 516 G>T polymorphism and side effects of the central nervous system in HIV-positive individuals under Efavirenz treatment: Study of a sample from southern Brazil. Acad Bras Cienc. 2017;89:497–504.
Vujkovic M, Bellamy SL, Zuppa AF, Gastonguay MR, Moorthy GS, Ratshaa B, et al. Polymorphisms in cytochrome P450 are associated with extensive efavirenz pharmacokinetics and CNS toxicities in an HIV cohort in Botswana. Pharmacogenomics J. 2018;18:678–88.
Lee SS, To KW, Lee MP, Wong NS, Chan DP, Li PC, et al. Sleep quality in efavirenz-treated Chinese HIV patients - comparing between GT and GG genotype of CYP2B6-516 G/T polymorphisms. Int J STD AIDS. 2014;25:193–200.
Dhoro M, Ngara B, Kadzirange G, Nhachi C, Masimirembwa C. Genetic variants of drug metabolizing enzymes and drug transporter (ABCB1) as possible biomarkers for adverse drug reactions in an HIV/AIDS cohort in Zimbabwe. Curr HIV Res. 2013;11:481–90.
Leger P, Chirwa S, Turner M, Richardson DM, Baker P, Leonard M, et al. Pharmacogenetics of efavirenz discontinuation for reported central nervous system symptoms appears to differ by race. Pharmacogenet Genomics. 2016;26:473–80.
Ribaudo HJ, Liu H, Schwab M, Schaeffeler E, Eichelbaum M, Motsinger-Reif AA, et al. Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS Clinical Trials Group study. J Infect Dis. 2010;202:717–22.
Gallien S, Journot V, Loriot MA, Sauvageon H, Morlat P, Reynes J, et al. Cytochrome 2B6 polymorphism and efavirenz-induced central nervous system symptoms: a substudy of the ANRS ALIZE trial. HIV Med. 2017;18:537–45.
Dickinson L, Amin J, Else L, Boffito M, Egan D, Owen A, et al. Comprehensive pharmacokinetic, pharmacodynamic and pharmacogenetic evaluation of once-daily efavirenz 400 and 600 mg in treatment-naive HIV-infected patients at 96 weeks: results of the ENCORE1 study. Clin Pharmacokinet. 2016;55:861–73.
Sanchez Martin A, Cabrera Figueroa S, Cruz Guerrero R, Hurtado LP, Hurle AD, Carracedo Alvarez A. Impact of pharmacogenetics on CNS side effects related to efavirenz. Pharmacogenomics. 2013;14:1167–78.
Cabrera Figueroa S, Fernandez de Gatta M, Hernandez Garcia L, Dominguez-Gil Hurle A, Bustos Bernal C, Sepulveda Correa R, et al. The convergence of therapeutic drug monitoring and pharmacogenetic testing to optimize efavirenz therapy. Ther Drug Monit. 2010;32:579–85.
Frasco MA, Mack WJ, Van Den Berg D, Aouizerat BE, Anastos K, Cohen M, et al. Underlying genetic structure impacts the association between CYP2B6 polymorphisms and response to efavirenz and nevirapine. AIDS. 2012;26:2097–106.
Vujkovic M, Bellamy SL, Zuppa AF, Gastonguay M, Moorthy GS, Ratshaa BR, et al. Brief report: CYP2B6 516G>T minor allele protective of late virologic failure in efavirenz-treated HIV-infected patients in botswana. J Acquir Immune Defic Syndr. 2017;75:488–91.
Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an adult AIDS clinical trials group study. AIDS. 2004;18:2391–400.
Haas DW, Severe P, Jean Juste MA, Pape JW, Fitzgerald DW. Functional CYP2B6 variants and virologic response to an efavirenz-containing regimen in Port-au-Prince, Haiti. J Antimicrob Chemother. 2014;69:2187–90.
Glass TR, Rotger M, Telenti A, Decosterd L, Csajka C, Bucher HC, et al. Determinants of sustained viral suppression in HIV-infected patients with self-reported poor adherence to antiretroviral therapy. PLoS ONE. 2012;7:e29186.
Gross R, Bellamy SL, Ratshaa B, Han X, Vujkovic M, Aplenc R, et al. CYP2B6 genotypes and early efavirenz-based HIV treatment outcomes in Botswana. AIDS. 2017;31:2107–13.
Orrell C, Bienczak A, Cohen K, Bangsberg D, Wood R, Maartens G, et al. Effect of mid-dose efavirenz concentrations and CYP2B6 genotype on viral suppression in patients on first-line antiretroviral therapy. Int J Antimicrob Agents. 2016;47:466–72.
Masebe TM, Bessong PO, Nwobegahay J, Ndip RN, Meyer D. Prevalence of MDR1 C3435T and CYP2B6 G516T polymorphisms among HIV-1 infected South African patients. Dis Markers. 2012;32:43–50.
Lehmann DS, Ribaudo HJ, Daar ES, Gulick RM, Haubrich RH, Robbins GK, et al. Genome-wide association study of virologic response with efavirenz-containing or abacavir-containing regimens in AIDS clinical trials group protocols. Pharmacogenet Genomics. 2015;25:51–9.
Rotger M, Colombo S, Furrer H, Bleiber G, Buclin T, Lee BL, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5.
Queiroz MAF, Laurentino RV, da Silva Graca Amoras E, Araujo MSM, Gomes STM, Lima SS, et al. The CYP2B6 G516T polymorphism influences CD4(+) T-cell counts in HIV-positive patients receiving antiretroviral therapy in an ethnically diverse region of the Amazon. Int J Infect Dis. 2017;55:4–10.
Klein K, Lang T, Saussele T, Barbosa-Sicard E, Schunck WH, Eichelbaum M, et al. Genetic variability of CYP2B6 in populations of African and Asian origin: allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet Genomics. 2005;15:861–73.
Sandkovsky U, Podany AT, Fletcher CV, Owen A, Felton-Coleman A, Winchester LC, et al. Impact of efavirenz pharmacokinetics and pharmacogenomics on neuropsychological performance in older HIV-infected patients. J Antimicrob Chemother. 2017;72:200–4.
Dhoro M, Zvada S, Ngara B, Nhachi C, Kadzirange G, Chonzi P, et al. CYP2B6*6, CYP2B6*18, Body weight and sex are predictors of efavirenz pharmacokinetics and treatment response: population pharmacokinetic modeling in an HIV/AIDS and TB cohort in Zimbabwe. BMC Pharm Toxicol. 2015;16:4.
Ramachandran G, Kumar AK, Ponnuraja C, Ramesh K, Rajesh L, Chandrasekharan C, et al. Lack of association between plasma levels of non-nucleoside reverse transcriptase inhibitors & virological outcomes during rifampicin co-administration in HIV-infected TB patients. Indian J Med Res. 2013;138:955–61.
Habtewold A, Aklillu E, Makonnen E, Yimer G, Bertilsson L, Burhenne J, et al. Population pharmacokinetic model linking plasma and peripheral blood mononuclear cell concentrations of efavirenz and its metabolite, 8-hydroxy-efavirenz, in HIV patients. Antimicrob Agents Chemother. 2017;61:e00207–17.
Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K, et al. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics. 2007;8:547–58.
Vo TT, Varghese Gupta S. Role of cytochrome P450 2B6 pharmacogenomics in determining efavirenz-mediated central nervous system toxicity, treatment outcomes, and dosage adjustments in patients with human immunodeficiency virus infection. Pharmacotherapy. 2016;36:1245–54.
ENCORE1 Study Group, Carey D, Puls R, Amin J, Losso M, Phanupak P, et al. Efficacy and safety of efavirenz 400 mg daily versus 600 mg daily: 96-week data from the randomised, double-blind, placebo-controlled, non-inferiority ENCORE1 study. Lancet Infect Dis. 2015;15:793–802.
Gatanaga H, Hayashida T, Tsuchiya K, Yoshino M, Kuwahara T, Tsukada H, et al. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin Infect Dis. 2007;45:1230–7.
Acknowledgements
The research was supported by the Clinical Research Fund of the First Affiliated Hospital of Third Military Medical University (Grant No. SWH2016LCYB-16).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, L., Wang, Y., Li, X. et al. Meta-analysis of the associations of CYP2B6-516G>T polymorphisms with efavirenz-induced central nervous system side effects and virological outcome in HIV-infected adults. Pharmacogenomics J 20, 246–259 (2020). https://doi.org/10.1038/s41397-019-0112-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-019-0112-2
This article is cited by
-
Meta-analysis of the associations of IMPDH and UGT1A9 polymorphisms with rejection in kidney transplant recipients taking mycophenolic acid
European Journal of Clinical Pharmacology (2022)
-
Adverse events in Chinese human immunodeficiency virus (HIV) patients receiving first line antiretroviral therapy
BMC Infectious Diseases (2020)
-
Effects of CYP2B6 polymorphisms on plasma nevirapine concentrations: a systematic review and meta-analysis
Scientific Reports (2020)