Abstract
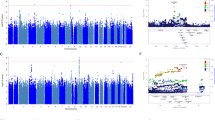
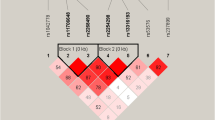
Stimulation of the serotonin (5-HT)1A receptor (HTR1A) has been shown to contribute to the mechanism of action of some atypical antipsychotic drugs (APDs), including clozapine and lurasidone. A meta-analysis of rs6295, a functional polymorphism located at the promoter region of HTR1A, showed association with clinical response in schizophrenic patients treated with atypical APD. We have now tested whether other SNPs related to rs6295 predict response to lurasidone. We first evaluated whether rs358532 and rs6449693, tag SNPs for rs6295, predicted response to lurasidone, using data from two clinical trials of acutely psychotic schizophrenia patients with European (EUR, n = 171) or African (AFR, n = 131) ancestry; we then determined if those findings could be replicated in a third trial of lurasidone of similar design. Weekly changes (up to 6 weeks) in the Positive and Negative Syndrome Scale (PANSS) Total score and its five subscales were used to assess response. In EUR, a significant association, or trends for association, were observed for PANSS Total (p = 0.035), positive (p = 0.039), negative (p = 0.004), and disorganization (p = 0.0087) subscales, at week 1–6. There was a trend for replication with PANNS Total (p = 0.036) in the third trial. No significant association was observed in AFR or the placebo group. Meta-analysis of five studies, including the three with lurasidone, showed that rs6295 was associated with improvement in positive (p = 0.023) and negative (p ≤ 0.0001) symptoms in EUR patients with schizophrenia. This is the first study to show a significant association between functional HTR1A polymorphisms and treatment response to lurasidone. The meta-analysis provides additional evidence that rs6295 could be a race-dependent biomarker for predicting treatment response to APDs in schizophrenic patients with European Ancestry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
McClay JL, Adkins DE, Aberg K, Stroup S, Perkins DO, Vladimirov VI, et al. Genome-wide pharmacogenomic analysis of response to treatment with antipsychotics. Mol Psychiatry. 2011;16:76–85.
McClay JL, Adkins DE, Aberg K, Bukszar J, Khachane AN, Keefe RS, et al. Genome-wide pharmacogenomic study of neurocognition as an indicator of antipsychotic treatment response in schizophrenia. Neuropsychopharmacology. 2011;36:616–26.
Arranz MJ, de Leon J. Pharmacogenetics and pharmacogenomics of schizophrenia: a review of last decade of research. Mol Psychiatry. 2007;12:707–47.
Charlab R, Zhang L. Pharmacogenomics: historical perspective and current status. In: Pharmacogenomics. Springer; 2013. pp 3–22.
Taylor DL, Tiwari AK, Lieberman JA, Potkin SG, Meltzer HY, Knight J, et al. Genetic association analysis of N-methyl-d-aspartate receptor subunit gene GRIN2B and clinical response to clozapine. Hum Psychopharmacol: Clin Exp. 2016;31:121–34.
Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393–406.
Meltzer HY. Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology. 1989;99:S18–S27.
Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:1159–72.
Kapur S, Zipursky R, Jones C, Shammi C, Remington G, Seeman P. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry. 2000;57:553–9.
Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17:263.
Huang M, Li Z, Ichikawa J, Dai J, Meltzer HY. Effects of divalproex and atypical antipsychotic drugs on dopamine and acetylcholine efflux in rat hippocampus and prefrontal cortex. Brain Res. 2006;1099:44–55.
Need AC, Keefe RS, Ge D, Grossman I, Dickson S, McEvoy JP, et al. Pharmacogenetics of antipsychotic response in the CATIE trial: a candidate gene analysis. Eur J Hum Genet. 2009;17:946–57.
Ikeda M, Yamanouchi Y, Kinoshita Y, Kitajima T, Yoshimura R, Hashimoto S, et al. Variants of dopamine and serotonin candidate genes as predictors of response to risperidone treatment in first-episode schizophrenia. 2008.
Hwang R, Shinkai T, De Luca V, Müller DJ, Ni X, Macciardi F, et al. Association study of 12 polymorphisms spanning the dopamine D2 receptor gene and clozapine treatment response in two treatment refractory/intolerant populations. Psychopharmacology. 2005;181:179–87.
Zhang J-P, Lencz T, Malhotra AK D2 receptor genetic variation and clinical response to antipsychotic drug treatment: a meta-analysis. Am J Psychiatry. 2010;167:763–72.
Lencz T, Robinson DG, Xu K, Ekholm J, Sevy S, Gunduz-Bruce H, et al. DRD2 promoter region variation as a predictor of sustained response to antipsychotic medication in first-episode schizophrenia patients. Am J Psychiatry. 2006;163:529–31.
Kim Y-K, Yoon H-K. Effect of serotonin-related gene polymorphisms on pathogenesis and treatment response in Korean schizophrenic patients. Behav Genet. 2011;41:709–15.
Takekita Y, Fabbri C, Kato M, Nonen S, Sakai S, Sunada N, et al. Serotonin 7 receptor variants are not associated with response to second-generation antipsychotics in Japanese schizophrenia patients. Neuropsychobiology. 2015;72:118–25.
Kishi T, Okochi T, Tsunoka T, Okumura T, Kitajima T, Kawashima K, et al. Serotonin 1A receptor gene, schizophrenia and bipolar disorder: an association study and meta-analysis. Psychiatry Res. 2011;185:20–26.
Pazos A, Palacios J. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–30.
Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O'laughlin IA, Meltzer HY. 5‐HT2A and D2 receptor blockade increases cortical DA release via 5‐HT1A receptor activation: a possible mechanism of atypical antipsychotic‐induced cortical dopamine release. J Neurochem. 2001;76:1521–31.
Díaz-Mataix L, Scorza MC, Bortolozzi A, Toth M, Celada P, Artigas F. Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. J Neurosci. 2005;25:10831–43.
Sumiyoshi T, Stockmeier CA, Overholser JC, Dilley GE, Meltzer HY. Serotonin 1A receptors are increased in postmortem prefrontal cortex in schizophrenia. Brain Res. 1996;708:209–14.
Yasuno F, Suhara T, Ichimiya T, Takano A, Ando T, Okubo Y. Decreased 5-HT 1A receptor binding in amygdala of schizophrenia. Biol Psychiatry. 2004;55:439–44.
Sumiyoshi T, Matsui M, Nohara S, Yamashita I, Kurachi M, Sumiyoshi C, et al. Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment. Am J Psychiatry. 2001;158:1722–5.
Sumiyoshi T, Matsui M, Yamashita I, Nohara S, Kurachi M, Uehara T, et al. The effect of tandospirone, a serotonin 1A agonist, on memory function in schizophrenia. Biol Psychiatry. 2001;49:861–8.
Sumiyoshi T, Matsui M, Yamashita I, Nohara S, Uehara T, Kurachi M, et al. Effect of adjunctive treatment with serotonin-1A agonist tandospirone on memory functions in schizophrenia. J Clin Psychopharmacol. 2000;20:386–8.
Meltzer HY, Sumiyoshi T. Does stimulation of 5-HT 1A receptors improve cognition in schizophrenia? Behav Brain Res. 2008;195:98–102.
Sumiyoshi T, Meltzer HY. Serotonin 1A receptors in memory function. Am J Psychiatry. 2004;161:1505–1505.
Serretti A, Artioli P, Lorenzi C, Pirovano A, Tubazio V, Zanardi R. The C (−1019) G polymorphism of the 5-HT1A gene promoter and antidepressant response in mood disorders: preliminary findings. Int J Neuropsychopharmacol. 2004;7:453–60.
Kato M, Fukuda T, Wakeno M, Okugawa G, Takekita Y, Watanabe S, et al. Effect of 5‐HT1A gene polymorphisms on antidepressant response in major depressive disorder. Am J Med Genet Part B: Neuropsychiatr Genet. 2009;150:115–23.
Wasserman D, Geijer T, Sokolowski M, Rozanov V, Wasserman J. The serotonin 1A receptor C (-1019) G polymorphism in relation to suicide attempt. Behav Brain Funct. 2006;2:14.
Wang L, Chen L, Ma J, Qiao Z, Qiu X, Yang X, et al. Polymorphisms in 5-HTR1A and coupled G-proteins in association with negative life events increase susceptibility to suicide attempt. Int J Clin Exp Pathol. 2017;10:6189–97.
Tang H, Dalton CF, Srisawat U, Zhang ZJ, Reynolds GP. Methylation at a transcription factor-binding site on the 5-HT1A receptor gene correlates with negative symptom treatment response in first episode schizophrenia. Int J Neuropsychopharmacol. 2014;17:645–9.
Le François B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55:977–85.
Wu X, Yao J, Ding M, Shi Z-s, Xu F-l, Zhang J-j, et al. 5-HT1A receptor (HTR1A) 5′ region haplotypes significantly affect protein expression in vitro. Neurosci Lett. 2017;638:51–54.
Drago A, De Ronchi D, Serretti A. 5-HT1A gene variants and psychiatric disorders: a review of current literature and selection of SNPs for future studies. Int J Neuropsychopharmacol. 2008;11:701–21.
Takekita Y, Fabbri C, Kato M, Nonen S, Sakai S, Sunada N, et al. HTR1A gene polymorphisms and 5-HT1A receptor partial agonist antipsychotics efficacy in schizophrenia. J Clin Psychopharmacol. 2015;35:220–7.
Wesnes KA, Hopkins SC, Brooker HJ, Koblan KS. Differences in memory function between 5-HT1A receptor genotypes in patients with major depressive disorder. CNS Spectr. 2016;21:379–84.
Takekita Y, Fabbri C, Kato M, Koshikawa Y, Tajika A, Kinoshita T, et al. HTR1A polymorphisms and clinical efficacy of antipsychotic drug treatment in schizophrenia: a meta-analysis. Int J Neuropsychopharmacol. 2016;19:1–10.
Sanford M. Lurasidone. CNS Drugs. 2013;27:67–80.
Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Therapeutics. 2010;334:171–81.
Huang M, Horiguchi M, Felix AR, Meltzer HY. 5-HT1A and 5-HT7 receptors contribute to lurasidone-induced dopamine efflux. Neuroreport. 2012;23:436–40.
Rajagopal L, Massey BW, Michael E, Meltzer HY. Serotonin (5-HT) 1A receptor agonism and 5-HT7 receptor antagonism ameliorate the subchronic phencyclidine-induced deficit in executive functioning in mice. Psychopharmacology. 2016;233:649–60.
Nasrallah HA, Silva R, Phillips D, Cucchiaro J, Hsu J, Xu J, et al. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res. 2013;47:670–7.
Loebel A, Cucchiaro J, Xu J, Sarma K, Pikalov A, Kane JM. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res. 2013;147:95–102.
Meltzer HY, Cucchiaro J, Silva R, Ogasa M, Phillips D, Xu J, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo-and olanzapine-controlled study. Am J Psychiatry. 2011;168:957–67.
Li J, Yoshikawa A, Brennan MD, Ramsey TL, Meltzer HY Genetic predictors of antipsychotic response to lurasidone identified in a genome wide association study and by schizophrenia risk genes. Schizophr. Res. 2017.
Li J, Loebel A, Meltzer HY Identifying the genetic risk factors for treatment response to lurasidone by genome-wide association study: a meta-analysis of samples from three independent clinical trials. Schizophr Res. 2018;199:203–13.
Levine SZ, Rabinowitz J. Revisiting the 5 dimensions of the Positive and Negative Syndrome Scale. J Clin Psychopharmacol. 2007;27:431–6.
Loebel A, Cucchiaro J, Sarma K, Xu L, Hsu C, Kalali AH, et al. Efficacy and safety of lurasidone 80mg/day and 160mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo-and active-controlled trial. Schizophr Res. 2013;145:101–9.
Purcell S, Chang C. PLINK 1.9. 2015. www.cog-genomicsorg/plink2
Groothuis-Oudshoorn K, Van Buuren S. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67.
Reynolds GP, Arranz B, Templeman LA, Fertuzinhos S, San L. Effect of 5-HT 1A receptor gene polymorphism on negative and depressive symptom response to antipsychotic treatment of drug-naive psychotic patients. Am J Psychiatry. 2006;163:1826–9.
Mössner R, Schuhmacher A, Kühn K-U, Cvetanovska G, Rujescu D, Zill P, et al. Functional serotonin 1A receptor variant influences treatment response to atypical antipsychotics in schizophrenia. Pharmacogenet Genom. 2009;19:91–94.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–14.
Higgins J. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0. 2011. The Cochrane Collaboration. www.cochrane-handbook.org
Huang M, Kwon S, Rajagopal L, He W, Meltzer HY. 5-HT 1A parital agonism and 5-HT 7 antagonism restore episodic memory in subchronic phencyclidine-treated mice: role of brain glutamate, dopamine, acetylcholine and GABA. Psychopharmacology. 2018;235:2795–808.
Drago A, Giegling I, Schäfer M, Hartmann AM, Friedl M, Konte B, et al. AKAP13, CACNA1, GRIK4 and GRIA1 genetic variations may be associated with haloperidol efficacy during acute treatment. Eur Neuropsychopharmacol. 2013;23:887–94.
Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–86.
Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res. 2008;172:177–97.
Zheng H, Onoda K, Wada Y, Mitaki S, Nabika T, Yamaguchi S. Serotonin-1A receptor C-1019G polymorphism affects brain functional networks. Sci Rep. 2017;7:12536.
Schreiber R, Newman-Tancredi A. Improving cognition in schizophrenia with antipsychotics that elicit neurogenesis through 5-HT 1A receptor activation. Neurobiol Learn Mem. 2014;110:72–80.
Albert PR. Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philos Trans R Soc B. 2012;367:2402–15.
Sakaue M, Somboonthum P, Nishihara B, Koyama Y, Hashimoto H, Baba A, et al. Postsynaptic 5-hydroxytryptamine1A receptor activation increases in vivo dopamine release in rat prefrontal cortex. Br J Pharmacol. 2000;129:1028–34.
Acknowledgements
The authors would like to thank all the patients, healthy volunteers, and psychiatrists who intensively cooperated with us to recruit patients in this study. This work was supported by a grant awarded to HYM by Sunovion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HYM is a stockholder in ACADIA. HYM receives grant support from ACADIA, Allergan, Aptinyx, Eli Lilly, Janssen, Lundbeck, Neurocrine, Sumitomo Dainippon, and Sunovion. Other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoshikawa, A., Li, J. & Meltzer, H.Y. A functional HTR1A polymorphism, rs6295, predicts short-term response to lurasidone: confirmation with meta-analysis of other antipsychotic drugs. Pharmacogenomics J 20, 260–270 (2020). https://doi.org/10.1038/s41397-019-0101-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-019-0101-5