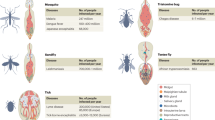
Abstract
Mosquito-borne diseases like dengue and malaria cause a significant global health burden. Unfortunately, current insecticides and environmental control strategies aimed at the vectors of these diseases are only moderately effective in decreasing disease burden. Understanding and manipulating the interaction between the mosquito holobiont (i.e., mosquitoes and their resident microbiota) and the pathogens transmitted by these mosquitoes to humans and animals could help in developing new disease control strategies. Different microorganisms found in the mosquito’s microbiota affect traits related to mosquito survival, development, and reproduction. Here, we review the physiological effects of essential microbes on their mosquito hosts; the interactions between the mosquito holobiont and mosquito-borne pathogen (MBP) infections, including microbiota-induced host immune activation and Wolbachia-mediated pathogen blocking (PB); and the effects of environmental factors and host regulation on the composition of the microbiota. Finally, we briefly overview future directions in holobiont studies, and how these may lead to new effective control strategies against mosquitoes and their transmitted diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, Guan-Hong Wang, upon reasonable request.
References
Manzoor KN, Javed F. The global emergence of Chikungunya infection: an integrated view. Rev Med Virol. 2021;32:e2287.
Kolimenakis A, Heinz S, Wilson ML, Winkler V. The role of urbanisation in the spread of Aedes mosquitoes and the diseases they transmit-A systematic review. PLoS Negl Trop Dis. 2021;15:e0009631.
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–07.
Organization WH. World Malaria Day 2022: Harness innovation to reduce the malaria disease burden and save lives 2022.
Demok S, Endersby-Harshman N, Vinit R, Timinao L, Robinson LJ, Susapu M, et al. Insecticide resistance status of Aedes aegypti and Aedes albopictus mosquitoes in papua new guinea. Parasit Vectors. 2019;12:333.
Altinli M, Schnettler E, Sicard M. Symbiotic interactions between mosquitoes and mosquito viruses. Front Cell Infect Microbiol. 2021;11:694020.
Reshef L, Koren O, Loya Y, Zilber-Rosenberg I, Rosenberg E. The coral probiotic hypothesis. Environ Microbiol. 2006;8:2068–73.
Guégan M, Zouache K, Démichel C, Minard G, Tran Van V, Potier P, et al. The mosquito holobiont: fresh insight into mosquito-microbiota interactions. Microbiome. 2018;6:49.
Gao H, Cui C, Wang L, Jacobs-Lorena M, Wang S. Mosquito microbiota and implications for disease control. Trends Parasitol. 2020;36:98–111.
Caragata EP, Short SM. Vector microbiota and immunity: modulating arthropod susceptibility to vertebrate pathogens. Curr Opin Insect Sci. 2022;50:100875.
Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098.
Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci USA. 2009;106:17841–46.
Bahia AC, Dong Y, Blumberg BJ, Mlambo G, Tripathi A, BenMarzouk-Hidalgo OJ, et al. Exploring Anopheles gut bacteria for Plasmodium blocking activity. Environ Microbiol. 2014;16:2980–94.
Bai L, Wang L, Vega-Rodríguez J, Wang G, Wang S. A gut symbiotic bacterium Serratia marcescens renders mosquito resistance to Plasmodium infection through activation of mosquito immune responses. Front Microbiol. 2019;10:1580.
Gao H, Bai L, Jiang Y, Huang W, Wang L, Li S, et al. A natural symbiotic bacterium drives mosquito refractoriness to Plasmodium infection via secretion of an antimalarial lipase. Nat Microbiol. 2021;6:806–17.
Cappelli A, Damiani C, Mancini MV, Valzano M, Rossi P, Serrao A, et al. Asaia activates immune genes in mosquito eliciting an anti-Plasmodium response: Implications in malaria control. Front Genet. 2019;10:836.
Yu S, Wang J, Luo X, Zheng H, Wang L, Yang X, et al. Transmission-blocking strategies against malaria parasites during their mosquito stages. Front Cell Infect Microbiol. 2022;12:820650.
Gabrieli P, Caccia S, Varotto-Boccazzi I, Arnoldi I, Barbieri G, Comandatore F, et al. Mosquito trilogy: microbiota, immunity and pathogens, and their implications for the control of disease transmission. Front Microbiol. 2021;12:630438.
Djihinto OY, Medjigbodo AA, Gangbadja ARA, Saizonou HM, Lagnika HO, Nanmede D, et al. Malaria-transmitting vectors microbiota: Overview and interactions with anopheles mosquito biology. Front Microbiol. 2022;13:891573.
Bolling BG, Weaver SC, Tesh RB, Vasilakis N. Insect-specific virus discovery: significance for the arbovirus community. Viruses-Basel. 2015;7:4911–28.
Halbach R, Junglen S, van Rij RP. Mosquito-specific and mosquito-borne viruses: evolution, infection, and host defense. Curr Opin Insect Sci. 2017;22:16–27.
Stollar V, Thomas VL. An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology. 1975;64:367–77.
White AV, Fan M, Mazzara JM, Roper RL, Richards SL. Mosquito-infecting virus Espirito Santo virus inhibits replication and spread of dengue virus. J Med Virol. 2021;93:3362–73.
Feng Y, Gou Q-Y, Yang W-H, Wu W-C, Wang J, Holmes EC, et al. A time-series meta-transcriptomic analysis reveals the seasonal, host, and gender structure of mosquito viromes. Virus Evolut. 2022;8:veac006.
Du J, Li F, Han Y, Fu S, Liu B, Shao N, et al. Characterization of viromes within mosquito species in China. Sci China-Life Sci. 2020;63:1089–92.
Coatsworth H, Bozic J, Carrillo J, Buckner EA, Rivers AR, Dinglasan RR, et al. Intrinsic variation in the vertically transmitted core virome of the mosquito Aedes aegypti. Mol Ecol. 2022;31:2545–61.
Wang L, Rosas ALR, De Coninck L, Shi C, Bouckaert J, Matthijnssens J, et al. Establishment of Culex modestus in Belgium and a Glance into the Virome of Belgian Mosquito Species. Msphere. 2021;6:e01229–20.
Saraiva RG, Fang J, Kang S, Angleró-Rodríguez YI, Dong Y, Dimopoulos G. Aminopeptidase secreted by Chromobacterium sp. Panama inhibits dengue virus infection by degrading the E protein. PLoS Negl Trop Dis. 2018;12:e0006443.
Wu P, Sun P, Nie K, Zhu Y, Shi M, Xiao C, et al. A gut commensal bacterium promotes mosquito permissiveness to arboviruses. Cell Host Microbe. 2019;25:101–12.e5.
Apte-Deshpande AD, Paingankar MS, Gokhale MD, Deobagkar DN. Serratia odorifera mediated enhancement in susceptibility of Aedes aegypti for chikungunya virus. Indian J Med Res. 2014;139:762–68.
Shaw WR, Marcenac P, Childs LM, Buckee CO, Baldini F, Sawadogo SP, et al. Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nat Commun. 2016;7:11772.
Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–51.
O’Neill EBSL, Hoffmann AA, Werren JH Influential Passengers: Microorganisms and Invertebrate Reproduction. Oxford University Press: Oxford 1997.
Lau MJ, Ross PA, Hoffmann AA. Infertility and fecundity loss of Wolbachia-infected Aedes aegypti hatched from quiescent eggs is expected to alter invasion dynamics. PLoS Negl Trop Dis. 2021;15:e0009179.
Ant TH, Herd C, Louis F, Failloux AB, Sinkins SP. Wolbachia transinfections in Culex quinquefasciatus generate cytoplasmic incompatibility. Insect Mol Biol. 2020;29:1–8.
Walker T, Quek S, Jeffries CL, Bandibabone J, Dhokiya V, Bamou R, et al. Stable high-density and maternally inherited Wolbachia infections in Anopheles moucheti and Anopheles demeilloni mosquitoes. Curr Biol. 2021;31:2310–20.e5.
Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–57.
Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, et al. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2012;109:E23–31.
Martins M, Ramos LFC, Murillo JR, Torres A, de Carvalho SS, Domont GB, et al. Comprehensive quantitative proteome analysis of Aedes aegypti identifies proteins and pathways involved in Wolbachia pipientis and Zika virus interference phenomenon. Front Physiol. 2021;12:642237.
Mao W, Zeng Q, She L, Yuan H, Luo Y, Wang R, et al. Wolbachia utilizes lncRNAs to activate the anti-dengue Toll pathway and balance Reactive Oxygen Species stress in Aedes aegypti through a competitive endogenous RNA network. Front Cell Infect Microbiol. 2021;11:823403.
Wong ZS, Brownlie JC, Johnson KN. Oxidative stress correlates with Wolbachia-mediated antiviral protection in Wolbachia-Drosophila associations. Appl Environ Microbiol. 2015;81:3001–05.
Audsley MD, Seleznev A, Joubert DA, Woolfit M, O’Neill SL, McGraw EA. Wolbachia infection alters the relative abundance of resident bacteria in adult Aedes aegypti mosquitoes, but not larvae. Mol Ecol. 2018;27:297–309.
Zhang G, Hussain M, O’Neill SL, Asgari S. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc Natl Acad Sci USA. 2013;110:10276–81.
McFarlane M, Almire F, Kean J, Donald CL, McDonald A, Wee B, et al. The Aedes aegypti domino ortholog p400 regulates antiviral exogenous small interfering RNA pathway activity and ago-2 expression. mSphere. 2020;5:e00081–20.
Sucupira PHFFÁ, Leite THJF, de Mendonça SF, Ferreira FV, Rezende FO, Marques JT, et al. The RNAi pathway is important to control mayaro virus infection in Aedes aegypti but not for Wolbachia-mediated protection. Viruses. 2020;12:871.
Terradas G, McGraw EA. Wolbachia-mediated virus blocking in the mosquito vector Aedes aegypti. Curr Opin Insect Sci. 2017;22:37–44.
Lindsey ARI. BT, Newton ILG, Hardy RW. Conflict in the intracellular lives of endosymbionts and viruses: A mechanistic look at Wolbachia-mediated pathogen-blocking. Viruses. 2018;10:141.
Zheng X, Zhang D, Li Y, Yang C, Wu Y, Liang X, et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature. 2019;572:56–61.
Caputo B, Moretti R, Manica M, Serini P, Lampazzi E, Bonanni M, et al. A bacterium against the tiger: preliminary evidence of fertility reduction after release of Aedes albopictus males with manipulated Wolbachia infection in an Italian urban area. Pest Manag Sci. 2020;76:1324–32.
Indriani C, Tantowijoyo W, Rancès E, Andari B, Prabowo E, Yusdi D, et al. Reduced dengue incidence following deployments of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia: a quasi-experimental trial using controlled interrupted time series analysis. Gates Open Res. 2020;4:50.
Garcia GA, Sylvestre G, Aguiar R, da Costa GB, Martins AJ, Lima JBP, et al. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl Trop Dis. 2019;13:e0007023.
Pinto SB, Riback TIS, Sylvestre G, Costa G, Peixoto J, Dias FBS, et al. Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niterói, Brazil: A quasi-experimental study. PLoS Negl Trop Dis. 2021;15:e0009556.
Ryan PA, Turley AP, Wilson G, Hurst TP, Retzki K, Brown-Kenyon J, et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 2019;3:1547.
Nazni WA, Hoffmann AA, NoorAfizah A, Cheong YL, Mancini MV, Golding N, et al. Establishment of Wolbachia Strain wAlbB in Malaysian Populations of Aedes aegypti for Dengue Control. Curr Biol. 2019;29:4241–8.e5.
Gao L, Wang H, Liu Z, Liu S, Zhao G, Xu B, et al. The initial analysis of a serine proteinase gene (AccSp10) from Apis cerana cerana: Possible involvement in pupal development, innate immunity and abiotic stress responses. Cell Stress Chaperones. 2017;22:867–77.
Bongio NJ, Lampe DJ. Inhibition of Plasmodium berghei development in mosquitoes by effector proteins secreted from Asaia sp. Bacteria using a novel native secretion signal. PloS One. 2015;10:e0143541.
Fang W, Vega-Rodríguez J, Ghosh AK, Jacobs-Lorena M, Kang A, St Leger RJ. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science. 2011;331:1074–7.
Ren X, Hoiczyk E, Rasgon JL. Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog. 2008;4:e1000135.
Huang W, Cha SJ, Jacobs-Lorena M. New weapons to fight malaria transmission: A historical view. Entomol Res. 2022;52:235–40.
Coon KL, Valzania L, McKinney DA, Vogel KJ, Brown MR. Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc Natl Acad Sci USA. 2017;114:E5362–69.
Wang Y, Gilbreath TM 3rd, Kukutla P, Yan G, Xu J. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS One. 2011;6:e24767.
Buck M, Nilsson LK, Brunius C, Dabiré RK, Hopkins R, Terenius O. Bacterial associations reveal spatial population dynamics in Anopheles gambiae mosquitoes. Sci Rep. 2016;6:22806.
Saab SA, Dohna HZ, Nilsson LKJ, Onorati P, Nakhleh J, Terenius O. The environment and species affect gut bacteria composition in laboratory co-cultured Anopheles gambiae and Aedes albopictus mosquitoes. Sci Rep. 2020;10:3352.
Minard G, Mavingui P, Moro CV. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit Vectors. 2013;6:146.
MacLeod HJ, Dimopoulos G, Short SM. Larval diet abundance influences size and composition of the midgut microbiota of Aedes aegypti mosquitoes. Front Microbiol. 2021;12:645362.
Lin D, Zheng X, Sanogo B, Ding T, Sun X, Wu Z. Bacterial composition of midgut and entire body of laboratory colonies of Aedes aegypti and Aedes albopictus from Southern China. Parasit Vectors. 2021;14:586.
Cirimotich CM, Dong Y, Clayton AM, Sandiford SL, Souza-Neto JA, Mulenga M, et al. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science. 2011;332:855–8.
Muturi EJ, Njoroge TM, Dunlap C, Cáceres CE. Blood meal source and mixed blood-feeding influence gut bacterial community composition in Aedes aegypti. Parasit Vectors. 2021;14:83.
Telang A, Skinner J. Effects of host blood meal source on reproductive output, nutrient reserves and gut microbiome of West Nile virus vector Culex quinquefasciatus. J Insect Physiol. 2019;114:15–22.
Alfano N, Tagliapietra V, Rosso F, Manica M, Arnoldi D, Pindo M, et al. Changes in microbiota across developmental stages of Aedes koreicus, an invasive mosquito vector in Europe: Indications for microbiota-based control strategies. Front Microbiol. 2019;10:2832.
Moll RM, Romoser WS, Modrzakowski MC, Moncayo AC, Lerdthusnee K. Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J Med Entomol. 2001;38:29–32.
Romoli O, Schönbeck JC, Hapfelmeier S, Gendrin M. Production of germ-free mosquitoes via transient colonisation allows stage-specific investigation of host-microbiota interactions. Nat Commun. 2021;12:942.
Bottino-Rojas V, Talyuli OA, Jupatanakul N, Sim S, Dimopoulos G, Venancio TM, et al. Heme signaling impacts global gene expression, immunity and dengue virus infectivity in Aedes aegypti. PLoS One. 2015;10:e0135985.
Kakani P, Gupta L, Kumar S. Heme-peroxidase 2, a peroxinectin-like gene, regulates bacterial homeostasis in Anopheles stephensi midgut. Front Physiol. 2020;11:572340.
Kajla M, Choudhury TP, Kakani P, Gupta K, Dhawan R, Gupta L, et al. Silencing of Anopheles stephensi heme peroxidase HPX15 activates diverse immune pathways to regulate the growth of midgut bacteria. Front Microbiol. 2016;7:1351.
Ross PA, Ritchie SA, Axford JK, Hoffmann AA. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl Trop Dis. 2019;13:e0007357.
Hixson B, Bing XL, Yang X, Bonfini A, Nagy P, Buchon N. A transcriptomic atlas of Aedes aegypti reveals detailed functional organization of major body parts and gut regional specializations in sugar-fed and blood-fed adult females. Elife. 2022;11:e76132.
Pan X, Pike A, Joshi D, Bian G, McFadden MJ, Lu P, et al. The bacterium Wolbachia exploits host innate immunity to establish a symbiotic relationship with the dengue vector mosquito Aedes aegypti. ISME J. 2018;12:277–88.
Stathopoulos S, Neafsey DE, Lawniczak MK, Muskavitch MA, Christophides GK. Genetic dissection of Anopheles gambiae gut epithelial responses to Serratia marcescens. PLoS Pathog. 2014;10:e1003897.
Xiao X, Yang L, Pang X, Zhang R, Zhu Y, Wang P, et al. A Mesh-Duox pathway regulates homeostasis in the insect gut. Nat Microbiol. 2017;2:17020.
Williams M, Contet A, Hou CD, Levashina EA, Baxter R. Anopheles gambiae TEP1 forms a complex with the coiled-coil domain of LRIM1/APL1C following a conformational change in the thioester domain. PloS One. 2019;14:e0218203.
Short SM, Mongodin EF, MacLeod HJ, Talyuli OAC, Dimopoulos G. Amino acid metabolic signaling influences Aedes aegypti midgut microbiome variability. PLoS Negl Trop Dis. 2017;11:e0005677.
Wang GH, Gamez S, Raban RR, Marshall JM, Alphey L, Li M, et al. Combating mosquito-borne diseases using genetic control technologies. Nat Commun. 2021;12:4388.
Murray JV, Jansen CC, De Barro P. Risk associated with the release of Wolbachia-infected Aedes aegypti mosquitoes into the environment in an effort to control dengue. Front Public Health. 2016;4:43.
Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell. 2009;139:1268–78.
Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326:134–6.
Kozlova EV, Hegde S, Roundy CM, Golovko G. Microbial interactions in the mosquito gut determine Serratia colonization and blood-feeding propensity. ISME J. 2021;15:93–108.
Dong Y, Morton JC Jr., Ramirez JL, Souza-Neto JA, Dimopoulos G. The entomopathogenic fungus Beauveria bassiana activate toll and JAK-STAT pathway-controlled effector genes and anti-dengue activity in Aedes aegypti. Insect Biochem Mol Biol. 2012;42:126–32.
Angleró-Rodríguez YI, Talyuli OA, Blumberg BJ, Kang S, Demby C, Shields A, et al. An Aedes aegypti-associated fungus increases susceptibility to dengue virus by modulating gut trypsin activity. Elife. 2017;6:e28844.
Coon KL, Brown MR, Strand MR. Gut bacteria differentially affect egg production in the anautogenous mosquito Aedes aegypti and facultatively autogenous mosquito Aedes atropalpus (Diptera: Culicidae). Parasit Vectors. 2016;9:375.
Ant TH, Sinkins SPA. Wolbachia triple-strain infection generates self-incompatibility in Aedes albopictus and transmission instability in Aedes aegypti. Parasit Vectors. 2018;11:295.
Mancini MV, Damiani C, Short SM, Cappelli A, Ulissi U, Capone A, et al. Inhibition of Asaia in adult mosquitoes causes male-specific mortality and diverse transcriptome changes. Pathogens. 2020;9:380.
Pelloquin B, Kristan M, Edi C, Meiwald A, Clark E, Jeffries CL, et al. Overabundance of Asaia and Serratia bacteria is associated with deltamethrin insecticide susceptibility in Anopheles coluzzii from Agboville, Côte d’Ivoire. Microbiol Spectr. 2021;9:e0015721.
Wang S, Ghosh AK, Bongio N, Stebbings KA, Lampe DJ, Jacobs-Lorena M. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc Natl Acad Sci USA. 2012;109:12734–39.
Angleró-Rodríguez YI, Blumberg BJ, Dong Y, Sandiford SL, Pike A, Clayton AM, et al. A natural Anopheles-associated Penicillium chrysogenum enhances mosquito susceptibility to Plasmodium infection. Sci Rep. 2016;6:34084.
Bando H, Okado K, Guelbeogo WM, Badolo A, Aonuma H, Nelson B, et al. Intra-specific diversity of Serratia marcescens in Anopheles mosquito midgut defines Plasmodium transmission capacity. Sci Rep. 2013;3:1641.
Wei G, Lai Y, Wang G, Chen H, Li F, Wang S. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc Natl Acad Sci USA. 2017;114:5994–99.
Valzano M, Cecarini V, Cappelli A, Capone A, Bozic J, Cuccioloni M, et al. A yeast strain associated to Anopheles mosquitoes produces a toxin able to kill malaria parasites. Malar J. 2016;15:21.
Bian GW, Joshi D, Dong YM, Lu P, Zhou GL, Pan XL, et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013;340:748–51.
Gnambani EJBE, Sanou A, Dabiré RK, Diabaté A. Infection of highly insecticide-resistant malaria vector Anopheles coluzzii with entomopathogenic bacteria Chromobacterium violaceum reduces its survival, blood feeding propensity and fecundity. Malar J. 2020;19:352.
Díaz-Nieto LM, C DA, Perotti MA, Berón CM. Culex pipiens development is greatly influenced by native bacteria and exogenous yeast. PLoS One. 2016;11:e0153133.
Acknowledgements
This work was supported by the CAS strategic funding via CAS-CSIRO funding scheme (152111KYSB20210011), the National Key R&D Program of China (2022YFF0710603), the National Science Foundation of China (32270538), and the Natural Science Foundation of Beijing (6222046) awarded to G.H.W. The work was also supported by CSIRO strategic funding via CAS-CSIRO funding scheme to PNP. We thank Lei Jiang and Wenxin Ma for their helpful edits on earlier versions of Figs. 2 and 3.
Author information
Authors and Affiliations
Contributions
All authors critically reviewed the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, R., Wang, Q., Wu, R. et al. Holobiont perspectives on tripartite interactions among microbiota, mosquitoes, and pathogens. ISME J 17, 1143–1152 (2023). https://doi.org/10.1038/s41396-023-01436-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-023-01436-7
This article is cited by
-
Generating prophylactic immunity against arboviruses in vertebrates and invertebrates
Nature Reviews Immunology (2024)
-
Wolbachia dominance influences the Culex quinquefasciatus microbiota
Scientific Reports (2023)