Abstract
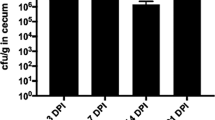
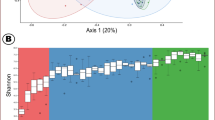
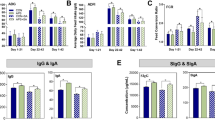
The gut microbiota makes important contributions to host immune system development and resistance to pathogen infections, especially during early life. However, studies addressing the immunomodulatory functions of gut microbial individuals or populations are limited. In this study, we explore the systemic impact of the ileal microbiota on immune cell development and function of chickens and identify the members of the microbiota involved in immune system modulation. We initially used a time-series design with six time points to prove that ileal microbiota at different succession stages is intimately connected to immune cell maturation. Antibiotics perturbed the microbiota succession and negatively affected immune development, whereas early exposure to the ileal commensal microbiota from more mature birds promoted immune cell development and facilitated pathogen elimination after Salmonella Typhimurium infection, illustrating that early colonization of gut microbiota is an important driver of immune development. Five bacterial strains, Blautia coccoides, Bacteroides xylanisolvens, Fournierella sp002159185, Romboutsia lituseburensis, and Megamonas funiformis, which are closely related to the immune system development of broiler chickens, were then screened out and validated for their immunomodulatory properties. Our results provide insight into poultry immune system–microbiota interactions and also establish a foundation for targeted immunological interventions aiming to combat infectious diseases and promote poultry health and production.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The raw 16S rRNA gene sequencing data of luminal microbiota from the antibiotic-untreated broiler chickens were obtained from NCBI BioProject PRJNA817429 (unpublished data from our own laboratory), and additional raw 16S rRNA gene sequencing data have been deposited in NCBI BioProject PRJNA904673. The genome data have been deposited in NCBI BioProject PRJNA903494 and PRJNA902159. The transcriptome data have been deposited in NCBI BioProject PRJNA904665. Raw datasets used for multicolor flow cytometry and qRT-PCR are available on FigShare (https://doi.org/10.6084/m9.figshare.21825042).
References
Skarp CPA, Hänninen ML, Rautelin HIK. Campylobacteriosis: the role of poultry meat. Clin Microbiol Infect. 2016;22:103–9.
Qi J, Li X, Zhang W, Wang H, Zhou G, Xu X. Influence of stewing time on the texture, ultrastructure and in vitro digestibility of meat from the yellow-feathered chicken breed. Anim Sci J. 2018;89:474–82.
Sedeik ME, El-Shall NA, Awad AM, Abd El-Hack ME, Alowaimer AN, Swelum AA. Comparative evaluation of HVT-IBD vector, immune complex, and live IBD vaccines against vvIBDV in commercial broiler chickens with high maternally derived antibodies. Animals. 2019;9:72.
El-Shall NA, Shewita RS, Abd El-Hack ME, AlKahtane A, Alarifi S, Alkahtani S, et al. Effect of essential oils on the immune response to some viral vaccines in broiler chickens, with special reference to Newcastle disease virus. Poult Sci. 2020;99:2944–54.
Maron DF, Smith TJS, Nachman KE. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Glob Health. 2013;9:48.
Favier CF, de Vos WM, Akkermans AD. Development of bacterial and bifidobacterial communities in feces of newborn babies. Anaerobe. 2003;9:219–29.
Wang S, Ryan CA, Boyaval P, Dempsey EM, Ross RP, Stanton C. Maternal vertical transmission affecting early-life microbiota development. Trends Microbiol. 2020;28:28–45.
Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–18.
Baumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535:85–93.
Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84.
Kim YG, Sakamoto K, Seo SU, Pickard JM, Gillilland MG 3rd, Pudlo NA, et al. Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science. 2017;356:315–9.
Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–93.
Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36:2336–46.
Tastan C, Karhan E, Zhou W, Fleming E, Voigt AY, Yao X, et al. Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T-cell presentation. Mucosal Immunol. 2018;11:1591–605.
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;136:485–98.
Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6.
Verma R, Lee C, Jeun EJ, Yi J, Kim KS, Ghosh A, et al. Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of Foxp3+ regulatory T cells. Sci Immunol. 2018;3:eaat6975.
Dibner JJ, Knight CD, Kitchell ML, Atwell CA, Downs AC, Ivey FJ. Early feeding and development of the immune system in neonatal poultry. J Appl Poult Res. 1998;7:425–36.
Khadem A, Soler L, Everaert N, Niewold TA. Growth promotion in broilers by both oxytetracycline and Macleaya cordata extract is based on their anti-inflammatory properties. Br J Nutr. 2014;112:1110–8.
Pourabedin M, Guan L, Zhao X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome. 2015;3:15.
Varmuzova K, Kubasova T, Davidova-Gerzova L, Sisak F, Havlickova H, Sebkova A, et al. Composition of gut microbiota influences resistance of newly hatched chickens to Salmonella Enteritidis infection. Front Microbiol. 2016;7:957.
Zhang X, Akhtar M, Chen Y, Ma Z, Liang Y, Shi D, et al. Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome. 2022;10:107.
Freeman TC, Ivens A, Baillie JK, Beraldi D, Barnett MW, Dorward D, et al. A gene expression atlas of the domestic pig. BMC Biol. 2012;10:90.
Mach N, Berri M, Esquerré D, Chevaleyre C, Lemonnier G, Billon Y, et al. Extensive expression differences along porcine small intestine evidenced by transcriptome sequencing. PLoS ONE. 2014;9:e88515.
Lu J, Idris U, Harmon B, Hofacre C, Maurer JJ, Lee MD. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl Environ Microbiol. 2003;69:6816–24.
Oakley BB, Buhr RJ, Ritz CW, Kiepper BH, Berrang ME, Seal BS, et al. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet Res. 2014;10:282.
Oakley BB, Kogut MH. Spatial and temporal changes in the broiler chicken cecal and fecal microbiomes and correlations of bacterial taxa with cytokine gene expression. Front Vet Sci. 2016;3:11.
Hu J, Chen L, Tang Y, Xie C, Xu B, Shi M, et al. Standardized preparation for fecal microbiota transplantation in pigs. Front Microbiol. 2018;9:1328.
Withanage GS, Kaiser P, Wigley P, Powers C, Mastroeni P, Brooks H, et al. Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar typhimurium. Infect Immun. 2004;72:2152–9.
Beal RK, Wigley P, Powers C, Hulme SD, Barrow PA, Smith AL. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet Immunol Immunopathol. 2004;100:151–64.
Beal RK, Powers C, Wigley P, Barrow PA, Kaiser P, Smith AL. A strong antigen-specific T-cell response is associated with age and genetically dependent resistance to avian enteric Salmonellosis. Infect Immun. 2005;73:7509–16.
Kogut MH, Genovese KJ, He HQ, Swaggerty CL, Jiang YW. Modulation of chicken intestinal immune gene expression by small cationic peptides as feed additives during the first week posthatch. Clin Vaccin Immunol. 2013;20:1440–8.
Hoszowski A, Truszczynski M. Prevention of Salmonella typhimurium caecal colonisation by different preparations for competitive exclusion. Comp Immunol Microbiol Infect Dis. 1997;20:111–7.
Kramer J, Visscher AH, Wagenaar JA, Boonstra-Blom AG, Jeurissen SH. Characterization of the innate and adaptive immunity to Salmonella enteritidis PT1 infection in four broiler lines. Vet Immunol Immunopathol. 2001;79:219–33.
Kogut MH, Genovese KJ, He H, Li MA, Jiang YW. The effects of the BT/TAMUS 2032 cationic peptides on innate immunity and susceptibility of young chickens to extraintestinal Salmonella enterica serovar Enteritidis infection. Int Immunopharmacol. 2007;7:912–9.
Crippen TL, Bischoff KM, Lowry VK, Kogut MH. rP33 activates bacterial killing by chicken peripheral blood heterophils. J Food Prot. 2003;66:787–92.
Rychlik I, Elsheimer-Matulova M, Kyrova K. Gene expression in the chicken caecum in response to infections with non-typhoid Salmonella. Vet Res. 2014;45:119.
Kashiwagi M, Hosoi J, Lai J-F, Brissette J, Ziegler SF, Morgan BA, et al. Direct control of regulatory T cells by keratinocytes. Nat Immunol. 2017;18:334–43.
Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell. 2015;163:367–80.
Lécuyer E, Rakotobe S, Lengliné-Garnier H, Lebreton C, Picard M, Juste C, et al. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity. 2014;40:608–20.
Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218–28.
Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41.
Quandt D, Rothe K, Baerwald C, Rossol M. GPRC6A mediates Alum-induced Nlrp3 inflammasome activation but limits Th2 type antibody responses. Sci Rep. 2015;5:16719.
Blander JM. The comings and goings of MHC class I molecules herald a new dawn in cross-presentation. Immunol Rev. 2016;272:65–79.
Shirakawa K, Endo J, Kataoka M, Katsumata Y, Yoshida N, Yamamoto T, et al. IL (Interleukin)-10-STAT3-Galectin-3 axis is essential for osteopontin-producing reparative macrophage polarization after myocardial infarction. Circulation. 2018;138:2021–35.
Hörhold F, Eisel D, Oswald M, Kolte A, Röll D, Osen W, et al. Reprogramming of macrophages employing gene regulatory and metabolic network models. PLoS Comput Biol. 2020;16:e1007657.
Dillmann C, Mora J, Olesch C, Brüne B, Weigert A. S1PR4 is required for plasmacytoid dendritic cell differentiation. Biol Chem. 2015;396:775–82.
Liu J, Zhang X, Chen K, Cheng Y, Liu S, Xia M, et al. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1α-mediated glycolysis. Immunity. 2019;50:600–15.
Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–13.
Zhao D-M, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, et al. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184:1191–9.
Xu W-D, Wang J, Yuan T-L, Li Y-H, Yang H, Liu Y, et al. Interactions between canonical Wnt signaling pathway and MAPK pathway regulate differentiation, maturation and function of dendritic cells. Cell Immunol. 2016;310:170–7.
Chen D, Jin D, Huang S, Wu J, Xu M, Liu T, et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020;469:456–67.
Richards-Rios P, Fothergill J, Bernardeau M, Wigley P. Development of the ileal microbiota in three broiler breeds. Front Vet Sci. 2020;7:17.
Borda-Molina D, Vital M, Sommerfeld V, Rodehutscord M, Camarinha-Silva A. Insights into broilers’ gut microbiota fed with phosphorus, calcium, and phytase supplemented diets. Front Microbiol. 2016;7:2033.
Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 2015;6:8292.
Hansson GC. Mucins and the microbiome. Annu Rev Biochem. 2020;89:769–93.
Chen CH, Gobel TW, Kubota T, Cooper MD. T cell development in the chicken. Poult Sci. 1994;73:1012–8.
Lowenthal JW, Connick TE, McWaters PG, York JJ. Development of T cell immune responsiveness in the chicken. Immunol Cell Biol. 1994;72:115–22.
Toivanen P, Toivanen A, Good RA. Ontogeny of bursal function in chicken. 3. Immunocompletent cell for humoral immunity. J Exp Med. 1972;136:816–31.
Chen CH, Six A, Kubota T, Tsuji S, Kong FK, Göbel TW, et al. T cell receptors and T cell development. Curr Top Microbiol Immunol. 1996;212:37–53.
Tang MS, Poles J, Leung JM, Wolff MJ, Davenport M, Lee SC, et al. Inferred metagenomic comparison of mucosal and fecal microbiota from individuals undergoing routine screening colonoscopy reveals similar differences observed during active inflammation. Gut Microbes. 2015;6:48–56.
Sonnenburg JL, Angenent LT, Gordon JI. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nat Immunol. 2004;5:569–73.
Ennamorati M, Vasudevan C, Clerkin K, Halvorsen S, Verma S, Ibrahim S, et al. Intestinal microbes influence development of thymic lymphocytes in early life. Proc Natl Acad Sci USA. 2020;117:2570–8.
Legoux F, Bellet D, Daviaud C, El Morr Y, Darbois A, Niort K, et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science. 2019;366:494–9.
Zegarra-Ruiz DF, Kim DV, Norwood K, Kim M, Wu W-JH, Saldana-Morales FB, et al. Thymic development of gut-microbiota-specific T cells. Nature. 2021;594:413–7.
Kundu P, Blacher E, Elinav E, Pettersson S. Our gut microbiome: the evolving inner self. Cell. 2017;171:1481–93.
Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3:148–58.
Schwartz DJ, Langdon AE, Dantas G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020;12:82.
Gao P, Ma C, Sun Z, Wang L, Huang S, Su X, et al. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome. 2017;5:91.
Vicente-Dueñas C, Janssen S, Oldenburg M, Auer F, González-Herrero I, Casado-García A, et al. An intact gut microbiome protects genetically predisposed mice against leukemia. Blood. 2020;136:2003–17.
Kawaguchi-Miyashita M, Shimizu K, Nanno M, Shimada S, Watanabe T, Koga Y, et al. Development and cytolytic function of intestinal intraepithelial T lymphocytes in antigen-minimized mice. Immunology. 1996;89:268–73.
Imaoka A, Matsumoto S, Setoyama H, Okada Y, Umesaki Y. Proliferative recruitment of intestinal intraepithelial lymphocytes after microbial colonization of germ-free mice. Eur J Immunol. 1996;26:945–8.
Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2011;12:9–23.
Fulde M, Hornef MW. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol Rev. 2014;260:21–34.
Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, et al. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect Immun. 2011;79:2755–63.
Yang H, Cai R, Zhang YH, Chen YY, Gu B. Gold nanoclusters as an antibacterial alternative against Clostridium difficile. Int J Nanomed. 2020;15:6401–8.
Liu X, Mao B, Gu J, Wu J, Cui S, Wang G, et al. Blautia-a new functional genus with potential probiotic properties? Gut Microbes. 2021;13:1–21.
Vaiserman AM, Koliada AK, Marotta F. Gut microbiota: a player in aging and a target for anti-aging intervention. Ageing Res Rev. 2017;35:36–45.
Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, et al. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017;168:928–43.
Koyama A, Wallenstein MD, Simpson RT, Moore JC. Soil bacterial community composition altered by increased nutrient availability in Arctic tundra soils. Front Microbiol. 2014;5:516.
Hitch TCA, Riedel T, Oren A, Overmann J, Lawley TD, Clavel T. Automated analysis of genomic sequences facilitates high-throughput and comprehensive description of bacteria. ISME Commun. 2021;1:1–16.
Rohart F, Gautier B, Singh A, Lê Cao K-A. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol. 2017;13:e1005752.
Wise MG, Siragusa GR. Quantitative analysis of the intestinal bacterial community in one- to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J Appl Microbiol. 2007;102:1138–349.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32172685), the National Key Research and Development Program of China (2022YFD1300400) and the 2115 Talent Development Program of China Agricultural University. Figure 1 was produced with BioRender.
Author information
Authors and Affiliations
Contributions
DL designed the project. YL carried out the experimental work and drafted the manuscript. MZ carried out bioinformatic analyses. YF, XY, ZL, PL and FW collected samples. All the other authors revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All the experiments were reviewed and approved by China Agricultural University Animal Care and Use Committee (statement no. AW30112202-1-1).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Feng, Y., Yang, X. et al. Mining chicken ileal microbiota for immunomodulatory microorganisms. ISME J 17, 758–774 (2023). https://doi.org/10.1038/s41396-023-01387-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-023-01387-z