Abstract
Ocean warming (OW) and acidification (OA) are recognized as two major climatic conditions influencing phytoplankton growth and nutritional or toxin content. However, there is limited knowledge on the responses of harmful algal bloom species that produce toxins. Here, the study provides quantitative and mechanistic understanding of the acclimation and adaptation responses of the domoic acid (DA) producing diatom Pseudo-nitzschia multiseries to rising temperature and pCO2 using both a one-year in situ bulk culture experiment, and an 800-day laboratory acclimation experiment. Ocean warming showed larger selective effects on growth and DA metabolism than ocean acidification. In a bulk culture experiment, increasing temperature +4 °C above ambient seawater temperature significantly increased DA concentration by up to 11-fold. In laboratory when the long-term warming acclimated samples were assayed under low temperatures, changes in growth rates and DA concentrations indicated that P. multiseries did not adapt to elevated temperature, but could instead rapidly and reversibly acclimate to temperature shifts. However, the warming-acclimated lines showed evidence of adaptation to elevated temperatures in the transcriptome data. Here the core gene expression was not reversed when warming-acclimated lines were moved back to the low temperature environment, which suggested that P. multiseries cells might adapt to rising temperature over longer timescales. The distinct strategies of phenotypic plasticity to rising temperature and pCO2 demonstrate a strong acclimation capacity for this bloom-forming toxic diatom in the future ocean.
Similar content being viewed by others
Introduction
One of the great public health challenges for marine ecosystems is determining how oceanic primary producers and their nutritional or toxin content will change in response to global change [1, 2]. Some species of the diatom genus Pseudo-nitzschia are well known to produce the neurotoxin domoic acid (DA). This toxin can harm wild and cultured marine animals, and human consumers through the food chain [3]. To date, 58 Pseudo-nitzschia species have been described, 26 of which are known to be toxigenic and global distribution patterns have been recorded, covering both coastal and open ocean regions [4,5,6]. However, no general uniform response of harmful algal bloom (HAB) events to environmental changes has yet been found at a global scale [7]. Evidence suggests that bloom dynamics and toxin concentration of Pseudo-nitzschia will be altered under future climate change conditions at regional and local scales [8,9,10].
While many investigations aim to study the effect of environmental factors on the eco-physiology of Pseudo-nitzschia, DA regulation has primarily been investigated using field observations of discrete bloom events and in laboratory-based short-term culture experiments [5, 11,12,13,14,15,16,17,18]. For example, the well documented toxic Pseudo-nitzschia bloom which affected nearly the entire West Coast of North America during 2015–2016, was reported to be driven by the “warm blob” of ocean water and anomalous nutrient concentrations present throughout this period [18, 19]. Culture experiments further found that warming increases toxicity and abundance of the harmful bloom diatoms P. australis and P. delicatissima in California coastal waters [13]. In addition to ocean warming, Wingert and Cochlan reported that the increasingly acidic seawater caused by elevated pCO2 influences cellular growth, photosynthetic performance, and domoic acid production of a strain of P. australis isolated from the California Current System [15]. Sun et al. found that the DA content of a toxic P. multiseries isolate from eastern Canada was regulated by both pCO2 and phosphate availability. P-limited cultures had 30–50 times higher DA concentrations compared to P-replete cultures at ambient pCO2, and increasing pCO2 further stimulated DA concentration, by up to 4-fold, under both nutrient conditions [12]. Tatters et al. confirmed that high pCO2 and silicate limitation synergistically increased the toxicity of P. fraudulenta isolated from southern California [20]. However, others have also reported that elevated pH resulted in a 70-fold increase in cellular DA concentrations in Japanese and eastern Canadian strains of P. multiseries [21, 22]. While cultures typically acclimate to experimental conditions after 3–5 consecutive transfers or 4–6 weeks of semicontinuous incubation, little is known about the fate of Pseudo-nitzschia diatoms under complex climate change scenarios, and especially about their potential for evolutionary adaptation to global change conditions.
The molecular basis of DA biosynthesis in P. multiseries has been delineated by Brunson et al. [23]. Four candidate genes have been implicated in the DA biosynthetic pathway, annotated as DabA (terpene cyclase), DabB (hypothetical protein), DabC (ɑ-ketoglutarate (ɑKG)–dependent dioxygenase), and DabD (CYP450) involved in terpenoid and redox biochemistry [23]. Additionally, the SLC6 amino acid transporter was previously shown to be involved in the depuration of DA [24]. This genetic knowledge offers the opportunity for a deeper understanding of the basis and diversity of Pseudo-nitzschia toxicity, the physiological functions of DA, and the environmental conditions that promote harmful algal blooms (HABs). However, the mechanisms that underpin acclimation or evolution of DA metabolism and genetic responses involved in the DA biosynthetic pathway under multiple climate-relevant conditions remain unclear. To address this issue, both short-term (more than 20 generations) and long-term (approximately 800 generations) responses of P. multiseries to projected ocean warming were studied in a factorial design with ocean acidification, using both semi-natural bulk culture experiments and laboratory acclimation experiments. Changes in their reproductive fitness were evaluated using growth rate measurements and quantification of changes in DA concentrations, as well as other physiological traits including cell size, cellular quotas and ratios of particulate organic carbon (POC) and nitrogen (PON), cellular biogenic silica (BSi), and Si/C. Since the magnitude of growth and DA metabolic responses under ocean warming was much larger than the responses under ocean acidification, a reciprocal transfer or shift experiment was conducted to test for evolutionary adaptation in warming-selected cell lines by transferring them to the higher or lower temperature ranges, and comparing them with ambient temperature selected ones. To understand the molecular evolutionary mechanisms involved, transcriptome and western blot analyses were applied to identify genetic divergence and expression variations between the ancestral lineage and selected populations under different warming and acidification regimes. This study aimed to address the following questions: 1) What is the strategy that P. multiseries employs to respond to rising temperature and increasing CO2 emissions, individually and in combination? and 2) What are the molecular mechanisms that underpin these phenotypic changes?
Materials and methods
Algal isolation and culture
An experimental culture of Pseudo-nitzschia multiseries was isolated from the coastal waters of Sungo Bay in the Yellow Sea, China (37°02′N, 122°33′E) (Fig. S1), at an ambient surface seawater temperature of 19.8 °C and ambient seawater pH of 8.23. Cells were isolated using a glass micro-pipette under an inverted Eclipse Ti-U microscope (Nikon, Tokyo, Japan). In the laboratory, monoclonal cultures were established by serial dilution and streaking the isolate onto agar plates infused with f/2 medium. Colonies were then picked under a microscope and transferred back into liquid axenic culture with modified f/2 medium containing 100 μmol L−1N, 6 μmol L−1P, and 100 μmol L−1Si, made with 0.2 μM-filtered seawater collected from the same location that the cultures were sampled from. Cultures were kept at 20 ± 1 °C and 120 μmol photons m−2 s−1 with a 12 h/12 h light–dark cycle (light on at 08:00 and off at 20:00) using cool white fluorescent bulbs. To study the effects of ocean warming and acidification on physiological performance and DA concentration of P. multiseries, three sets of experiments were conducted: 1) bulk culture experiments in the field, 2) long-term acclimation experiments and 3) shift experiments in the laboratory (see the details of laboratory experiments in Fig. S2).
Bulk culture experiments to study acclimation responses to ocean warming and acidification under semi-natural conditions
The mesocosm facility for Climate Change Impacts Study of Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences consists of 20 independently operated culture units made out of acrylic, each holding 100 L (Fig. S3). The site is located in the southeastern Sungo Bay in the Yellow Sea, China (37°02′N, 122°33′E), where P. multiseries was isolated. Six treatments were replicated in triplicate to simulate ocean warming and acidification in accordance with the Intergovernmental Panel on Climate Change (IPCC) representative concentration pathway (RCP) 8.5 “business-as-usual” emissions scenario: 1) +4 °C at 400 μatm pCO2, 2) +4 °C at 1000 μatm pCO2, 3) +4 °C at 2000 μatm pCO2, 4) ambient temperature at 400 μatm pCO2, 5) ambient temperature at 1000 μatm pCO2 and 6) ambient temperature at 2000 μatm pCO2 (Eighteen culture units in total; Fig. S3B). Warming was achieved by an electronic heating element connected to a thermocouple (MC-6; Pengyu, Qingdao, China), which monitored and recorded the temperature variations (a constant 4 degrees above ambient temperature was maintained). The pCO2 levels of 400 μatm, 1000 μatm, and 2000 μatm were established by bubbling the medium with air or air/CO2 premixed gas using a CO2 Enrichlor (CE-100B; Wuhan Ruihua Instrument & 25 Equipment Ltd).
To compare the responses between different seasons, the effects of ocean warming and acidification on growth rates and domoic acid (DA) concentrations of P. multiseries was investigated at four time ponts from 2018 to 2019. Experiment dates include, March 15 to April 15 (spring, ambient seawater surface temperature (ASST) 6.93 ± 1.13 °C and warming temperature (WT) 10.86 ± 1.28 °C); June 16 to July 16 (summer, ASST 19.22 ± 0.60 °C and WT 23.64 ± 0.78 °C); August 25 to September 25 (autumn, ASST 23.33 ± 0.40 °C and WT 27.79 ± 0.71 °C); January 29 to February 29 (winter, ASST 2.99 ± 0.72 °C and WT 7.21 ± 0.32 °C). The ancestral P. multiseries lineage was inoculated into 18 independent 100 L culture units with CO2-modified natural seawater at a starting density of 2 × 104 cells mL−1. The seawater was vacuum-filtered through GF/F filters (47 mm, Whatman), but without sterilization. After acclimation under ambient and elevated pCO2 for 10 days (two batch cycles), three replicates per pCO2 level were warmed gradually by 4 °C (+1 °C day−1), while the other three were kept at the ambient natural temperature. The experiments lasted for 30 days, and algal cells were diluted into fresh medium every five days (six batch cycles) at 2 × 104 cells mL−1 to maintain stable carbonate chemistry. The seawater was supplemented with modified f/2 medium (100 μmol L−1 N, 6 μmol L−1P, and 100 μmol L−1 Si) to prevent nutrient limitation during each transfer. The pH in each culture medium treatment was measured every day using a pH meter (Orion ROSS, Fisher Scientific Instruments) calibrated with National Bureau of Standards (NBS) buffers (pH variations <± 0.05). Temperature, salinity, and total alkalinity (TA) were also measured simultaneously throughout the experiment. Other carbonate system parameters were calculated with CO2SYS using pH and TA (Fig. S4) [25]. In the bulk culture experiments, temperature and pCO2 were the two principal abiotic factors; other variables such as nutrient availability, light, and day length were consistent between different temperature and pCO2 combinations.
Cell density, particulate organic carbon (POC), particulate DA (pDA) and dissolved DA (dDA) were measured on the 4th day of each batch cycle during exponential growth phase. One 2 mL sample was collected at the same time (11:00 AM to12:00 PM) across different seasons. Samples were preserved in Lugol’s solution to estimate growth using a 1 mL Sedgewick-Rafter counting chamber using an optical microscope (Nikon, Tokyo, Japan). At the beginning of each batch cycle, the initial concentration was measured using a particle counter (Z2, Beckman Instruments). Exponential growth rate (day−1) was calculated as follows:
where N1 and N0 represent cell density at times t1 and t0, respectively. At the same sampling time, 100 mL cultures filtered onto pre-combusted (500 °C for 6 h) Whatman GF/F filters and used for particulate carbon content (POC) determination. A further 100 mL was filterd onto un-combusted Whatman GF/F filters for pDA analysis, and 50 mL filtrate samples were collected for dDA analysis. The DA concentrations presented in the results are the sum of the pDA and dDA, which represents approximately 90% and 10% of total DA, respectively. The averages for growth rate, particulate organic carbon (POC), and total DA concentration, over six batch cycles, were calculated and presented as acclimation responses to ocean warming and acidification.
Laboratory acclimation experiment to study long-term responses to ocean warming and acidification
Long-term acclimation experiments
To study long-term responses of P. multiseries to ocean warming and acidification, algal cultures were maintained under semi-continuous culture conditions, under three temperatures of 20 °C, 25 °C, and 30 °C, combined with three pCO2 levels (400, 1,000, and 2,000 μatm) at each temperature (Fig. S2). Triplicate populations for each regime (9 × 3 = 27 cultures) were maintained in 1000 mL Schott Duran flasks containing 800 mL sterile seawater enriched with modified f/2 medium (100 μmol L−1 N, 6 μmol L−1 P, and 100 μmol L−1 Si). The control culture conditions were maintained at 20 °C at 400 μatm pCO2, consistent with the in situ conditions at the time of sampling. Clonal ancestral lineages in exponential growth phase were split into three independent cultures and semi-continuously cultured under different CO2 conditions (400, 1000, and 2000 μatm) at 20 °C, for 30 days (more than 20 generations). For each pCO2 condition, cultures were then split into three independent replicates under one of three different temperature conditions (20 °C, 25 °C, and 30 °C), resulting in nine experimental units in total. Cultures maintained at 25 °C and 30 °C were gradually elevated by 1 °C per day. The acclimation experiment lasted for 800 days (160 batch cycles) corresponding to 251 ± 12.1 to 750 ± 3.5 generations, depending on different seasonal growth rates under different experimental conditions (Table S1).
During the acclimation phase, 2 × 104 cells were transferred into fresh medium every five days to initiate the next batch cycle. Culture medium was bubbled either with air (~400 μatm) or air/CO2 mixed gas in a programmed CO2 chamber (HP1000G-D, China) to achieve the desired pCO2 levels and temperature treatments. Samples were shaken gently three times daily to avoid settling and biofilm formation. The seawater carbonate system parameters were maintained at stable levels by adjusting the aeration rate, and by maintaining cell concentrations within the range of 2.0–30 × 104cells mL−1 (pH variations <± 0.05). The pH in each treatment was measured before and after each transfer using a pH meter (Orion ROSS, Fisher Scientific Instruments) that was calibrated with National Bureau of Standards (NBS) buffers. Temperature, salinity, and total alkalinity (TA) were also measured periodically (but not at every transfer due to logistical limitations) throughout the experiment. Other carbonate system parameters were calculated with CO2SYS using the average pH and TA (Table S2).
On the 4th day of each batch cycle, 2 mL samples were collected to monitor cell concentrations using a 1 mL Sedgewick-Rafter counting chamber under an optical microscope (Nikon, Tokyo, Japan). At the same sampling time, 50 mL samples were filtered onto Whatman GF/F filters to collect algal cells. The filters and filtrates were stored at −20 °C and used for particulate DA (pDA) and dissolved DA (dDA) analysis. 20 mL cultures were filtered onto pre-combusted (450 °C, 5 h) GF/F glass filters for POC and PON determination in the same time interval. The average values of cell concentrations and total DA concentrations across 20 days (4 batch cycles) are presented as the physiological responses to different acclimation treatments. To examine the potential bacterial effects on DA content, bacterial abundance was monitored regularly via staining with SYBR gold and enumerating with flow cytometry (FACS Calibur, BD, USA) throughout the acclimation period. The proportion of bacteria remained stable at low quantities and contributed <1% of total biomass throughout.
To study and compare other phenotypic changes between the short-term acclimation (30 days) and long-term acclimation (800 days) experiments, particulate organic carbon (POC) and nitrogen (PON) content, cellular biogenic silica (BSi), and cell size were measured. For POC and PON, 20 mL samples were filtered onto pre-combusted (500 °C, 6 h) GF/F glass filters and placed onto pre-combusted aluminum foil and analysed using a a Vario EL III automatic elemental analyzer (Elementar Analysensysteme). For BSi, samples of 20 mL were collected onto 0.6 μM polycarbonate filters and analyzed according to the spectrophotometric method of Brzezinski and Nelson [26]. Algal cell length was monitored using a Eclipse 80i microscope (Nikon, Tokyo, Japan) with NISelements BR software (Nikon Co., Japan).
Shift experiments after long-term acclimation
Changes in physiological responses following long-term acclimation to temperature were measured across a gradient of seven temperatures (5–35 °C), including growth rates and DA concentrations. The low and high temperatures of 5 °C and 35 °C were chosen to coincide with the lower and upper thermal tolerance limits for P. multiseries. Pilot experiments revealed that the culture almost stopped growing above 30 °C (Table S3), while the chosen experimental temperature was well within the environmental range for this widely distributed species. As in Schlüter et al. [27], due to logistical restrictions and a prohibitively large number of replicates temperature switch experiments were only performed for samples selected under low (20 °C), and high (30 °C) temperature at respective pCO2 values of ambient (400 μatm), moderate (1000 μatm), and high (2000 μatm) levels (Fig. S2). After having been grown under ambient and elevated temperature and pCO2 regimes for 800 days, these lineages were transferred into 800 mL fresh media across a temperature gradient of 5 °C, 10 °C, 15 °C, 20 °C, 25 °C, 30 °C, and 35 °C at their respective selection pCO2. Ancestral populations were also challenged under the same conditions to distinguish plastic and evolved responses. After acclimation for 35 days (seven batch cycles), growth rates and DA concentrations were measured in the last (7th) batch cycle when no significant changes were found with longer culture periods. The pH, temperature, salinity, and total alkalinity (TA) were also measured periodically (but not at every transfer due to logistical restrictions) throughout the experiment, and carbonate system parameters were calculated with CO2SYS (Table S4).
Thermal tolerance curves for growth rate were fitted using the four-parameter Sharpe–Schoolfield model [28], which assumes that there is a single rate-limiting enzymatic reaction that is reversibly inhibited by temperature:
Where b(T) represents the per capita metabolic rate, Ea represents an activation energy (in eV) during the metabolic process, k is Boltzmann’s constant (8.62 × 10−5 ev K−1), T represents Kelvin temperature (K), Th is the temperature when half of the enzyme is inactivated, and Eh indicates the temperature above Th-induced inactivation of enzyme kinetics. b(Tc) represents the rate of metabolism normalized to an arbitrary reference temperature, and Topt is the maximum growth rate at an optimum temperature, which was calculated from the following equation.
The parameters b(Tc), Ea, Eh, Th, and Topt represent traits that together characterize the metabolic thermal response. Parameters of this model were considered to be traits that were expected to vary between different acclimation regimes [28].
Molecular responses involved in DA metabolic pathways of P. multiseries
To explore the potential for genetic adaptation using transcriptomes from the different warming and acidification regimes and between the acclimated and ancestral lineages, transcriptome resequencing was performed at the beginning (t0) and after 800 days (t800) of acclimation in the following five groups in triplicate: 1) ancestor (algal isolate without any acclimation at t0), 2) low temperature 20 °C and low pCO2 400 μatm at t800 (20/400), 3) low temperature 20 °C and high pCO2 1000 μatm at t800 (20/1000), 4)bhigh temperature 30 °C and low pCO2 400 μatm at t800 (30/400), and 5) high temperature 30 °C and high pCO2 1000 μatm at t800 (30/1000). During exponential growth phase, 250 mL of algal culture was sampled from the ancestral and long-term acclimation lineages, 30 h after inoculation during a batch cycle (at 14:00 pm on the second day). A pilot study showed that P. multiseries produced a peak in gene expression at this time point. Algal cells were centrifuged at 10,000 g, 4 °C for 5 min and immediately frozen in liquid nitrogen and stored at −80 °C for RNA extraction, transcriptional sequencing, and gene RT-qPCR. The transcriptome data have been deposited in the Sequence Read Archive (SRA) at the National Center for Biotechnology Information (NCBI) and are accessible through accession number PRJNA686847.
Gene sequences involved in the DA metabolic pathways including DabA (terpene cyclase), DabB (hypothetical protein), DabC (ɑ-ketoglutarate (ɑKG)–dependent dioxygenase), DabD (CYP450), and SLC6 amino acid transporter were identified in the transcriptome data. The housekeeping gene Histone H3 was selected as a reference gene and used for RT-qPCR of P. multiseries [24]. The primers designed for reference and target genes are listed in Table S5. To determine protein expression involved in DA metabolic pathway, total protein from ancestral P. multiseries cells and the long-term acclimation lineages (same samples as used in transcriptome analysis) was extracted using a Protein Extraction Kit (KeyGEN, Nanjing, China). Proteins were separated by SDS-PAGE on a 10% gel and electrotransferred to a polyvinylidene fluoride membrane for western blot analysis. Actin served as an internal control. To further test for any adaptation due to long-term warming acclimation, the relative expression of the core genes DabA, DabB, DabC, DabD, and SLC6 was measured in the shift experiment at their respective pCO2, and differences in expression were compared between treatments: of 20 °C/400 pCO2, 20 °C /1000 pCO2, 30 °C /400 pCO2, 30 °C /1000 pCO2, 20 °C /400 pCO2 to 30 °C /400 pCO2, 20 °C /1000 pCO2 to 30 °C /1000 pCO2, 30 °C /400 pCO2to 20 °C /400 pCO2, and 30 °C /1000 pCO2 to 20 °C /1000 pCO2.
DA determination
Whatman GF/F glass fiber filters (0.45 μM, 25 mm diameter, Whatman, Germany) were used to collect cells and filtrate from the cultures to determine particulate DA (pDA) and dissolved DA (dDA). Particulate DA (pDA) was extracted using 10% methanol/water (8 mL methanol:water, 1:9, v/v) [29]. Each filter was placed in a 10 mL plastic conical centrifuge tubes and stored at −20 °C until analysis. 8 mL of 10% methanol/water was added to the 10 mL plastic conical centrifuge tubes. The filtrates were homogenized by vortexing at room temperature for 10 min. Cells were disrupted to release intracellular DA into solution by sonication using a 650 W sonic disrupter at 30% amplitude, for 2 min on an ice slurry. The extraction was centrifuged at 4500 r min−1 for 5 min and then filtered with a 0.22 μM MCM syringe filter into an LC vial for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. Dissolved DA (dDA) was measured in the filtrate without cells. 50 mL filtered cultures were placed into 50 mL plastic conical centrifuge tubes and acidified with 20% aqueous formic acid to yield 0.2% formic acid in the filtered cultures. After vortex mixing, the filtered cultures were transferred into the pre-activated solid-phase extraction (Bond Elut C18 LRC10 mL column, 200 mg, 40 μM; Agilent) for desalination and extraction. Nest, the 50 mL plastic conical centrifuge tubes were washed with 10 mL 0.2% aqueous formic acid, and this was added to the solid-phase extraction and eluted with 1.5 mL 50% methanol/water into a 2 mL microcentrifuge tube. The eluate was filtered through a 0.22 MCM syringe filter into an LC vial for LC-MS/MS analysis.
LC-MS/MS analysis was performed using an U3000 HPLC system (Thermo Scientific, USA) linked to a Thermo TSQ Endura mass spectrometer equipped with an ESI source operated in positive ionization mode. The chromatographic system was equipped with a binary pump and an autosampler with a 100 µL sample loop. To obtain maximum abundance of molecular ions and generate higher sensitivity for analyte, optimization of MS/MS parameters was necessary. Using flow injection analysis at 10 μL min−1, the mass spectrometer was tuned using the CRM-DA-f DA standard solution (1 μg mL−1) and the final ion source conditions were as follows: spray voltage (kV) of +3.50, sheath gas flow 25 (arb), auxilliary gas flow 15 (arb), sweep gas flow (arb), ion transfer tube temperature of 300 °C, and vaporizer temperature of 250 °C.
Full scan MS in the positive ion mode gave the predominant peak at [M + H] + m/z 312.0, which was selected as precursor ion. The SRM transitions from the protonated DA ion were m/z 312.0 > 266.0 ([M − HCOOH + H]+) at collision energy (CE) = 15 V used for quantitative analysis, and two confirmatory transitions of m/z 312.0 > 248.0 ([M − HCOOH–H2O + H]+) at CE = 16 V and m/z 312.0 > 161.0 ([M-HCOOH-C2H3O2N-H2O-CH2 + H]+) at CE = 22 V, all with a RF lens of 142 V, a dwell time of 250 msec and a collision induced dissociation gas setting of 1.5 mTorr. Resolution of both quadrupoles Q1 and Q3 were set at 0.7 FWHM. Chromeleon and Xcalibur software were used for the entire MS tune, instrument control, data acquisition and data analysis. The sample of 10 µL was injected into a LC Kinetex C18 column (2.1 × 100 mm, 2.6 µm, Phenomenex, USA). The system was operated under the following gradient elution program: solution A (2 mmol L−1 ammonium formate in H2O) and solution B (100% MeOH) at a flow rate of 0.3 mL min-1 as follows: 0–1 min, 20% B, 1–3 min, 20–90% B, 3.0–3.1 min, 90–20% B, 3.1–8.0 min, 20% B. The column and the autosampler were maintained at 35 °C and 15 °C, respectively. The retention time of DA was around 2.86 min. The flow was diverted to waste by a six-way valve for the 0–2.2 min and 3.5–8.0 min of each run to prevent early eluting matrix material from blocking the heated capillary region of the MS system and contaminating the ion source. DA quantitation was obtained using a calibration curve of DA standard reference material. Because of the variation of cell size during the culture period, the total DA concentration was normalized to algal particulate organic carbon (POC) and expressed as mg DA gC−1.
Statistical analysis
All statistics were conducted in R (version 3.5.1; The R Foundation for Statistical Computing, Vienna, Austria) and model selection was based on Akaike Information Criterion (AIC) using the “MuMIn” package. The effects of temperature, pCO2, and the combined effects of increasing temperature and pCO2 on growth rate and DA concentration for the bulk culture experiment were analyzed with a mixed effects model using the lmer function within the package lme4 and lmerTest. When modelling the individual effect of temperature or pCO2 alone, temperature or pCO2 was used as a fixed factor, and when modelling the combined effects of temperature and pCO2, both temperature and pCO2 were used as fixed factors. Using the lm function within R, the interactions between temperature, pCO2, and season were included in a linear model (the additive effects between temperature, pCO2, and season were dropped from the model based on AIC). Growth rate or DA concentration were used as random factors in both models.
In the laboratory acclimation experiment, the lm function was used to test the differences in growth rates or DA contents over acclimation times of 160 batch cycles for P. multiseries acclimated under different temperatures and pCO2. The effect of acclimation time, temperature, pCO2, and the interaction between time, temperature, pCO2 were included in the model and used as fixed factors. The effect of measurement type, either growth rate or DA content or other phenotypic parameters, were used as random factors. Additionally, the individual effects of acclimation time, temperature, or pCO2, or the combined effects between two of them were analyzed using a mixed effects model with the lmer function.
Results
Acclimation responses of toxic P. multiseries to ocean warming and acidification under semi-natural conditions
Controlled laboratory studies have investigated the effects of warming, elevated CO2, or nutrient limitation on growth and toxin production of Pseudo-nitzschia species [12, 20, 30]. However, there is no consensus regarding whether any one factor or combination of factors leads to predictable DA concentration in a realistic ecological context [4, 31]. These results reveal that changes in specific growth rate (SGR) and DA concentration are driven by temperature and pCO2 across all four seasons (Fig. 1, Table S6). P. multiseries showed maximal SGR in the summer when ambient seawater temperature was 19.2 ± 0.1 °C. Warming treatments (OW) decreased SGR by 61% and 54% when ambient seawater temperatures were higher than 19.2 °C in summer and autumn, respectively (Fig. 1A; F1, 104 = 1341.9, p < 0.001 for summer, and F1, 104 = 271.9, p < 0.001 for autumn). However, warming increased SGR in spring and winter when ambient seawater temperature was less than 6.9 °C (Fig. S5; F1, 106 = 75.6, p < 0.001 for spring and F1, 106 = 409.1, p < 0.001 for winter). Although elevated pCO2 showed no significant effects on SGR in spring, autumn and winter, it increased SGR by 17% in summer when ambient seawater was around 19 °C. The DA concentration increased more than four orders of magnitude in the high temperature seasons of summer and autumn (Fig. 1B) compared to the low temperature seasons of spring and winter (Fig. S5B). OW consistently increased DA concentrations by 30.5%, 1045.9%, 2339.2%, and 12.5% in spring, summer, autumn, and winter, respectively. While OA showed no significant effect on DA concentration in spring and winter, DA levels increased by 52.6% and 46.6% respectively in summer and autumn. Moreover, OW and OA synergistically increased DA concentration in the high temperature seasons of summer and autumn as the maximal level occurred in autumn at 26.8 °C and 2000 μatm pCO2 (Fig. 1B; F15, 416 = 1140.1, p < 0.001).
Ambient seawater temperatures were 19.2 °C and 23.3 °C, respectively. In each season, treatments were a matrix of three CO2 levels (400, 1000 and 2000 uatm) and two temperatures, ambient and warming (ambient +4 °C). Boxplot symbols indicate ambient (open symbols) and warmer (filled symbols). A Specific growth rates (SGR, day-1). B DA concentrations expressed as mg DA gC−1. Boxplots show the bold line indicating the median, and the tops and bottoms of the boxes corresponding to the 25th and 75th percentiles, and whiskers extending to highest and lowest values that are within 1 interquartile range (IQR) of lower/upper quartile. n = 3 for each combination of pCO2 and temperature. Boxplots marked with different letters are significantly different (p < 0.05).
Long term responses of toxic P. multiseries to ocean warming and acidification in laboratory acclimation experiments
Consistent with the bulk culture experiment, warming treatments (MT and HT) significantly reduced SGR at all three pCO2 levels across the acclimation period of 800 days (Fig. 2A; Table S7, F1, 356 = 1204.3, p < 0.001). Acidification treatments (HC and MC) generally increased SGR at low and medium temperature conditions (LT and MT, Fig. 2A; Table S7, F1, 356 = 21.1, p < 0.001), but showed no significant effect on SGR at high temperature (HT) at the end of acclimation. In contrast to SGR, warming treatments resulted in increased DA concentration in all three CO2 levels (Fig. 2B; Table S7, F1, 356 = 1171.8, p < 0.001); elevated pCO2 interacted with warming synergistically and up-regulated DA concentration significantly, so the maximal value of DA occurred in the HTHC combination condition (Table S7, F15, 416 = 325.2, p < 0.001).
Treatments were a matrix of three three CO2 levels (400, 1000 and 2000 uatm) and three temperatures (20 °C, 25 °C, 30 °C) A Specific growth rates (SGR, day-1). B DA concentrations expressed as mg DA gC−1. Fitted lines are based on an autoregressive moving average model that incorporates significant autocorrelation terms, and shaded areas depict 95% prediction intervals.
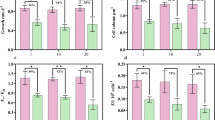
Next, changes in cell size and cell quotas under warming selection in combination with ocean acidification were anlaysed and compared response differences between short-term (30 days) and long-term (800 days) acclimation treatments (Fig. 3). Warming selection resulted in significant reduction of cell length after short-term acclimation (30 days) for all cells selected under ambient or moderate or high pCO2 (Fig. 3A; F1, 23 = 17.0, p < 0.001). Compared with the short-term acclimation, P. multiseries underwent progressive cell size reduction by 11.4% in length after long-term acclimation (Fig. 3A; F1, 48 = 103.5, p < 0.001). Although temperature and pCO2 showed significant effects on cell length after short-term acclimation (F3, 23 = 103.5, p = 0.003), there were no significant differences among treatments after long-term acclimation (F3, 23 = 0.6, p = 0.66).
Results are from the same experiment shown in Fig. 2, sampled at acclimation periods of 30 days and 800 days. A Cell lengths (μM). B Particulate organic carbon per cell (POC, ng cell−1). C Particulate organic nitrogen per cell (PON, pg cell−1). D Ratio of POC/PON. E Cellular biogenic silica (BSi, pg cell−1). F Ratio of Si/C. Values are displayed as means and ±1 SEM of triplicate cultures and symbols marked with different letters are significantly different. (p < 0.05).
As an immediate physiological response to ocean acidification, POC increased linearly with pCO2 under all temperatures (Fig. 3B; F1, 23 = 61.6, p < 0.001). However, short-term warming acclimation at all pCO2 levels produced a pronounced decline in POC content (Fig. 3B; F1, 23 = 77.9, p < 0.001). This response was smaller after long-term acclimation. Similar to POC, PON content increased by 7.91% and 11.96% under MC and HC at low temperature (p < 0.05), but elevated pCO2 did not change PON significantly at MT and LT (p > 0.05). After long-term acclimation, there were no significant differences in PON among treatments (Fig. 3C; F3, 23 = 2.7, p = 0.07). As a result of the simultaneous variation of POC and PON, the ratio of POC/PON did not change significantly with elevated pCO2 at low temperature (p > 0.05), but increased linearly with pCO2 under MT and LT (p < 0.05) (Fig. 3D). Under long-term acclimation, the ratio showed no significant variation among treatments, except for that between MC and HC at moderate temperature (25 °C).
Like other diatoms, P. multiseries requires silicic acid to produce its silica cell wall. In contrast to POC, cellular biogenic silica (BSi) decreased linearly with elevated pCO2 under both short-term (Fig. 3E; F1, 25 = 26.7, p < 0.001) and long-term acclimation (Fig. 3E; F1, 23 = 33.2, p < 0.001). Warming treatments did not influence BSi contents regardless of pCO2 (p > 0.05). Due to increasing POC quotas and decreasing BSi quotas, the ratio of Si/C decreased linearly with the increasing pCO2 under both short-term (Fig. 3F; F1, 23 = 63.6, p < 0.001) and long-term acclimation (Fig. 3F; F1, 25 = 58.0, p < 0.001).
Rapid reversible changes in neurotoxin concentrations of long-term acclimated P. multiseries in shift experiments
To test whether P. multiseries had the capacity to adapt to elevated temperatures after 800 generations, growth rates and DA concentrations were measured across a gradient of seven temperatures (5–35 °C) and responses were compared between different acclimation regimes. Due to a prohibitively large number of replicates, temperature transition experiments were performed for samples selected under low (20 °C), and high (30 °C) temperature at respective pCO2 values of ambient (400 μatm), moderate (1000 μatm), and high (2000 μatm) levels (Fig. 4). A typical “increase-peak-decline” pattern of specific growth rate (SGR) was found in thermal reaction norms of all selected cell lines (Fig. 4A). Under ambient pCO2 (400 μatm) conditions and high temperature ranges (25 °C, 30 °C, and 35 °C), growth rates were higher for the high temperature selected lines (30 °C) than the ancestral and low temperature selected lines (20 °C). However, high temperature selected lines had similar growth rates to ancestral or low temperature selected lines when assayed at low temperature ranges (5 °C, 10 °C, 15 °C, and 20 °C). While warming acclimation (30 °C) resulted in significantly higher values of Th (the upper temperature at which growth rates are 50% inactivated) and Topt (the thermal optimum) under ambient pCO2 (400 μatm), Th of the long-term warming acclimation lines varied under high pCO2, and the ancestral lines showed the highest Th level (Table S8). This indicates that although ocean warming had a larger effect on algal fitness than ocean acidification, the selective effect of warming was changed by elevated pCO2 [32]. This is supported by the significant interaction between selective ocean warming and acidification (mixed-effects model, interaction pCO2 × Temperature, p < 0.001).
Results are from short-term transfers to assay conditions (5 °C–30 °C, 400, 1000 or 2000 uatm) for cell lines acclimated long term (800 d) to a matrix of temperatures (20 °C, 30 °C) and pCO2 levels. (400, 1000 and 2000 uatm). Comparisons to the ancestral cell line are also included. A Fitted lines of specific growth rates (SGR) at each acclimation condition across replicates (n = 3) derived from non-linear least squares regression using the modified Sharpe-Schoolfield model. B Carbon-normalized log DA concentrations (Log(DA, μg DA gC−1)) of P. multiseries when assayed under different temperature and pCO2 regimes. Values are displayed as means and ±1 SEM of triplicate cultures, and symbols marked with different letters among different acclimation regimes at each assay temperature are significantly different. (p < 0.05).
After thermal acclimation for 800 days, long-term 30 °C-selected P. multiseries cells showed higher concentrations of DA than 20 °C-selected cells (Fig. 4B). During the shift experiments, the long-term warming acclimated P. multiseries cells demonstrated rapid acclimation rather than adaptation to temperature from 5 °C to 30 °C (Fig. 4B). When the warming selected lines (30 °C) were transferred to the low temperature range from 5 to 25 °C, their DA concentration decreased linearly with significantly lower levels than at a selection temperature of 30 °C. Although the long-term 30 °C-selected cultures showed rapid acclimation, they still showed higher concentrations of DA than low temperature selected (20 °C) and ancestral lines when assayed under the temperature range from 10 to 25 °C. Therefore, it is unknown whether adaptation might have occurred later with longer acclimation time. In contrast, long-term moderate and high pCO2 acclimation did not change the DA trends across the assay temperature ranges, although they significantly increased DA levels compared with ambient pCO2 selected cultures (F1, 153 = 47.4, p < 0.001). Note that the DA concentration was log-normalized DA concentration (LogDA) because it showed relatively low values and was close to the detection limit in the low temperature range from 5 to 15 °C.
Molecular mechanisms underpinning increasing DA concentration of P. multiseries under climate change
Significant differences were found in the transcriptomes under different acclimation regimes, yielding a total of 8,102 differentially expressed genes (DEGs) (Fig. S6A) including eight main clusters (Fig. S6B). By comparing ancestral or 20 °C/400 pCO2 with 30 °C/400 pCO2 or 30 °C /1000 pCO2, it was found that most genes involved in carbon metabolism such as the CO2 concentration mechanism (CCM), calvin cycle, glycolysis and tricarboxylic acid (TCA) cycle were significantly downregulated (Fig. S7). Genes involved in nutrient metabolism such as NiT, NR, NiR, GS, PHT, and SiT were also significantly downregulated. In contrast, isocitrate dehydrogenase (ICDHm) and glutamine amidotransferase (GOGAT) were up-regulated under 30/400, likely to produce Glu as one of the substrates for DA biosynthesis. Based on the gene expression profile (Fig. 5A) and western blot analysis (Fig. 5B), all of the five core genes DabA, DabB, DabC, DabD, and SLC6 in the DA metabolic pathway were significantly up-regulated under high temperature and elevated pCO2 (Fig. 5C).
A Variation of core gene expression involved in the DA biosynthetic pathway under different warming and pCO2 combinations. The color of heatmap represents the z-score of the FPKM value. B Western blot analysis of core protein expression involved in the DA metabolic pathway using Actin as an internal control. C Diagram representing altered DA biosynthetic potential of P. multiseries by comparison between the ambient condition (low temperature and low pCO2) and HTHC (high temperature and high pCO2). The red and blue symbols represent up and downregulated genes or processes, respectively. CA carbonic anhydrase involved in CCM, ICDHm isocitrate dehydrogenase, NT nitrate transporter, NiT nitrite transporter; NR nitrate reductase, NiR nitrite reductase, AMT ammonia transporter; GS glutamine synthetase, GOGAT glutamine amidotransferase, PHT phosphate transporter, SIT silicate transporter, DabA terpene cyclase, DabB hypothetical protein, DabC ɑ-ketoglutarate (ɑKG)–dependent dioxygenase, DabD CYP450, SLC6 sodium and chloride-dependent amino acid transporter.
To test for any adaptive changes arising at the transcriptome level due to long-term warming acclimation, the long-term selected cultures were transferred from high to low or low to high temperature at their respective pCO2, and the relative expression levels of core genes involved in the DA metabolic pathway were determined. As expected, all core genes were significantly up-regulated when cultures acclimated in low temperature treatments were transferred to high temperature at respective pCO2 (20 °C/400 pCO2 to 30 °C/400 pCO2 and 20 °C/1000 pCO2 to 30 °C/1000 pCO2). Transfers between low temperature lines showed higher levels than warming-selected and high temperature grown lines (30 °C/400 pCO2and 30 °C/1000 pCO2) and warming-selected transferred to low temperature switch ones (30 °C/400 pCO2 to 20 °C/400 pCO2 and 30 °C/1000 pCO2 to 20 °C/1000 pCO2) (p < 0.05) (Fig. 6). However, when the warming-selected lines were transferred to low temperature conditions at respective pCO2 (30 °C/400 pCO2 to 20 °C/400 pCO2 and 30 °C/1000 pCO2 to 20 °C/1000 pCO2), the relative expression of DabA, DabB, DabC, DabD, and SLC6 were 34.8, 12.3, 75.2, 10.9, and 8.2 times higher, respectively, than those of low temperature-selected and low temperature grown ones (20 °C/400 pCO2 and 20 °C/1000 pCO2), but showed similar expression levels to the warming-selected and high temperature grown ones (30 °C/400 pCO2 and 30 °C/1000 pCO2). Thus, the warming-selected lines were unable to down-regulate the core gene expression when moved back to the low temperature level, which was suggestive of adaptation at the transcriptome level.
Transcriptome results are from the same temperature and CO2 acclimation/shift experiments shown in Fig. 4. DabA: terpene cyclase; DabB: hypothetical protein; DabC: ɑ-ketoglutarate (ɑKG)–dependent dioxygenase; DabD: CYP450; SLC6: sodium and chloride-dependent amino acid transporter. Values are displayed as means and ±1 SEM of triplicate cultures and symbols marked with different letters among different acclimation regimes at each assay temperature are significantly different. (p < 0.05).
Discussion
Biomass and DA productivity are among the most important traits indicating the ecological roles of toxigenic microorganisms, yet it remains difficult to predict how these traits may change under future global climate change [5, 7, 23]. The current understanding is based on simplified, short-term laboratory experiments [12, 33, 34], that may underestimate or overestimate the evolutionary potential of marine phytoplankton to adapt to global change [28, 35,36,37]. This work builds on this knowledge based on one-year semi-natural field experiments and 800-day long-term acclimation laboratory experiments using the model toxin producing diatom P. multiseries. Long-term acclimation experiments showed that P. multiseries acclimate rapidly to elevated temperatures and pCO2 resulting in changes in algal growth and increased DA concentration. Warming by 4 °C or 5 °C had greater selective effects on physiological responses than acidification under 1000 μatm pCO2, regardless of field or laboratory experiments. While long-term warming improved algal growth rate and DA concentration under high temperature conditions, this trend was reversed and both growth rates and DA declined when cultures were switched back to low temperature regimes in a shift experiments. In contrast to physiological acclimation, the warming-selected lines showed adaptation at the transcriptome level, where the core gene expression was unable to decline when moved back to the low temperature level. Thus, results suggest that P. multiseries cells might adapt to rising temperature with longer acclimation.
The combined effects of ocean warming and acidification on P. multiseries
Bulk culture experiments highlight that ocean warming and acidification could stimulate P. multiseries growth or even promote blooms at a suitable temperature range, but inhibit growth when warming and acidification becomes too high (27.8 °C and 2000 μatm pCO2). Ocean warming and acidification are two major anthropogenic perturbations that influence phytoplankton physiology and productivity [32, 34, 38]. Complex interactions between CO2 concentration and temperature have been reported to cause antagonistic, synergistic, or additive effects on different phytoplankton species [27, 39]. Schlüter et al. reported that temperature adaptation of the coccolithophore Emiliania huxleyi occurred independently of ocean acidification levels [27]. In two other diatom species Phaeodactylum tricornutum and Thalassiosira weissflogii, Xu et al. found that the effect of ocean acidification on phytoplankton growth depends on growth temperature [39]. Here, our results using semi-natural bulk culture experiments found that ocean acidification had different effects on the growth rate of P. multiseries, depending on the ocean temperature and under different seasonal and warming scenarios. In the low temperature seasons of spring and winter when ambient seawater temperature was less than 6.9 °C, there were no significant effects of elevated pCO2 on growth rate under either ambient seawater temperature or warming (+4 °C) scenarios, whereas warming stimulated growth at all pCO2 levels. Similar to the findings of Xu et al., high pCO2 enhanced growth rates in summer when seawater temperatures ranged from 19.2 °C to 23.6 °C, suggesting ocean acidification and warming had antagonistic effects [39]. However, when temperatures reached 27.8 °C in autumn, high pCO2 interacted synergistically with warming to reduce growth rate by 22.0% and 42.0% at 1000 μatm and 2000 μatm, respectively.
Acclimation and adaptation potential of P. multiseries to ocean warming and acidification
Long-term acclimation experiments demonstrated that warming temperatures showed larger selective effects on growth and DA metabolism than elevated pCO2, and temperature and CO2 interact synergistically. It is important to study the acclimation and adaptation of HAB species to fully understand their responses to future climate change [40]. Acclimation usually involves phenotypic changes without genetic variation, while adapation is generally associated with irreversible evolution [41]. Many studies have found different responses between short-term acclimation and long-term adaptation of phytoplankton to CO2 and temperature perturbation [42,43,44]. This is the first study to test the adaptive response of Pseudo-nitzschia to ocean warming and acidification, individually and in combination, however, this finding is in agreement with other studies. Schlüter et al. found that the adaptation of E. huxleyi to a elevated temperatures at the upper thermal tolerance was up to six times faster than to elevated CO2 [27]. Increasing temeprature has been highlighted as a key factor in determining phytoplankton distribution, production and metabolic function [45,46,47]. Since most enzymes are temperature dependent, there is possibly a larger mutational target for thermal adaptation than CO2 adaptation, accelerating the speed of thermal adaptation [27, 47].
Reciprocal transplant experiments (also called shift experiments) can be used to compare trait optima between ancestral and evolved populations after long-term selection [48]. Our shift experiments further suggested that long-term warming acclimation resulted in the entire thermal reaction norm shifting to higher temperatures. However, the lower fitness and DA concentration of the high-temperature acclimation lines when moved back to low temperature regimes indicate that P. multiseries cells use rapid plastic acclimation to respond to temperature changes, even after an 800-day acclimation period. Similar expression levels of core genes involved in the DA metabolic pathway suggest adaptation at the transcriptome level. Plastic acclimation or evolutionary adapatation have been extensively debated in different phytoplankton taxa in response to environmental changes, and both are viable ways to maintain fitness under long-term climatic acclimation [49]. Previous studies have found that plasticity may facilitate evolution by maintaining a large population size, with great genetic and mutational variance [50,51,52]. Phenotypic acclimation of DA production and adaptation of transcriptomic expression under long-term warming indicates that P. multiseries had a capacity to adapt to rising temperatures. This suggests that future ocean warming may increase DA production of P. multiseries, and could enhance trophic transfer of toxins to food webs in the marine ecosystem. However, caution is needed when linking P. multiseries growth and DA production potential with global change since not all strains produce the toxin, and some strains produce more than others [4].
Physiological differences between short-term and long-term acclimation
Studies have shown that different timescales of acclimation to a perturbation can result in different physiological responses, and acclimation to multiple drivers or extreme changes may be slower [53, 54]. During the 800-day long-term acclimation experiment, physilogical traits including cell size and cell quotas of POC, PON, BSi were measured, and compared the differences between short-term and long-term acclimation. Cell size is an important master trait affecting nutrient acquisition, sinking velocity and the trophic role of plankton [55, 56]. Cell length in P. multiseries decreased significantly under high temperatures for all cells after short-term acclimation under all pCO2 levels, but showed no significant differences among treatments after long-term acclimation. Therefore, high DA concentrations under long-term acclimation of high temperature and/or elevated pCO2 cannot be atttributed to physiological changes in cell size, but rather to plastic acclimation. These findings are similar to a previous study by Bates et al., who reported that P. multiseries underwent progressive cell reduction by 11.4% in length after long-term culture [4]. It should be noted that along with reductions in cell size, cellular DA concentration decreased gradually after 800-day acclimation (data not shown) regardless of whether they were grown at low or high temperature or pCO2. Therefore, we terminated the long-term acclimation expeirments at 800 days and conducted short-term shift experiments after 30 days acclimation. Possible reasons for this may be that laboratory constant conditions are very different from natural dynamic environments [4, 57, 58]. Zooplankton grazers, UVB exposure, and nutrient limitation can also induce and increase DA production in Pseudo-nitzschia, and thus long-term, semi-continuous maintenance of the cultures in the laboratory without these factors could explain gradual loss or decline of DA metabolic capacity [4, 58].
Particulate organic carbon (POC) and (PON) content as well as POC/PON ratios are important predictors of nutritional quality to higher trophic levels [59]. Similar to elevated POC quotas under high pCO2 [12, 20], POC content of P. multiseries increased linearlly under medium and high pCO2 at all acclimation temperature gradients. However, after long-term warming acclimation (25 °C and 30 °C) there were no significant differences among different treatments. In contrast to the effects of increasing pCO2, short-term warming treatments resulted in linear decreases of POC and PON at all three pCO2 levels, but there were no signicant differences in PON content or POC/PON among treatments after long-term acclimation. Stable POC/PON suggests the maintenance of one aspect of nutritional quality owing to adaptive evolution under high temperature or pCO2 individually or in combination [46]. Our results also support previous studies showing that ocean acidification significantly reduced BSi levels in both short-term and long-term acclimation experiments [12, 60]. Decreasing BSi quotas and increasing or stable POC quotas resulted in lower Si/C ratios under medium and high pCO2 at all selective temperatures. As with other diatoms, the reduced BSi quotas and Si/C of P. multiseries under ocean acidification may have significant impacts on global carbon and silicon cycles by altering the vertical flux of these elements to the deep ocean [60].
Ecological implications
With increasing discovery and reporting of new species of Pseudo-nitzschia, the known distribution of this genus has expanded globally [4,5,6]. Although apparent trends are hard to distinguish from increased observational efforts, the extended distributions and increased number of toxigenic species worldwide has important consequences for ecosystem health and human seafood safety [1, 2, 4, 7]. Our work suggests that global warming associated with increasing CO2 emissions could be one of the most important environmental drivers influencing growth rate and DA production of P. multiseries. However, experiments considering a more complex matrix of changing enviromental variables under more realistic conditions may be necessary to determine the full range of evolutionary responses of these toxin producing organisms to an altered future ocean. Kelly et al. indicated that UVB exposure, warming, and urea availability can synergistically enhance cell-specific toxin production, while at the same time bloom formation may be inhibited by increased exposure to UVB [58]. Despite these necessary qualifications, our results provide new insights into the DA exposure potential for ocean ecosystems and harvested marine resources under continuing climate change conditions.
Data availability
Transcriptome sequencing data are available at the National Center for Biotechnology Information (NCBI) with the accession number PRJNA686847.
References
Cavicchioli R, Ripple WJ, Timmis KN, Azam F, Bakken LR, Baylis M, et al. Scientists’ warning to humanity: microorganisms and climate change. Nat Rev Microbiol. 2019;17:569–86.
Myers SS, Smith MR, Guth S, Golden CD, Vaitla B, Mueller ND, et al. Climate Change and Global Food Systems: Potential Impacts on Food Security and Undernutrition. Annu Rev Pub Health. 2017;38:259–77.
Brown AR, Lilley M, Shutler J, Lowe C, Artioli Y, Torres R, et al. Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Rev Aquac. 2020;12:1663–88.
Bates SS, Hubbard KA, Lundholm N, Montresor M, Leaw CP. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae. 2018;79:3–43.
Silver MW, Bargu S, Coale SL. Toxic diatoms and domoic acid in natural and iron enriched waters of the oceanic pacific. Proc Natl Acad Sci. 2010;107:20762–67.
Trick CG, Bill BD, Cochlan WP, Wells ML, Trainer VL, Pickell LD. Iron enrichment stimulates toxic diatom production in high-nitrate, low-chlorophyll areas. Proc Natl Acad Sci. 2010;107:5887–92.
Hallegraeff G, Enevoldsen H, Zingone A. Global harmful algal bloom status reporting. Harmful Algae. 2021;102:101992.
McKibben SM, Peterson W, Wood AM, Trainer VL, Hunter M, White AE. Climatic regulation of the neurotoxin domoic acid. Proc Natl Acad Sci. 2017;114:239–44.
Clark S, Hubbard KA, Ralston DK, McGillicuddy DJ, Stocke C, Alexander MA, et al. Projected effects of climate change on Pseudo-nitzschia bloom dynamics in the Gulf of Maine. J Mar Syst. 2022;230:103737.
Trainer VL, Kudela RM, Hunter MV, Adams NG, McCabe RM. Climate extreme seeds a new domoic ccid hotspot on the US West Coast. Front Clim. 2020;2:1–11.
Hinder SL, Hays GC, Edwards M, Roberts EC, Walne AW, Gravenor MB. Changes in marine dinoflagellate and diatom abundance under climate change. Nat Clim Change. 2012;2:271–75.
Sun J, Hutchins DA, Feng Y, Seubert EL, Caron DA, Fu FX. Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries. Limnol Oceanogr. 2011;56:829–40.
Zhu Z, Qu P, Fu F, Tennenbaum N, Tatters AO, Hutchins DA. Understanding the blob bloom: warming increases toxicity and abundance of the harmful bloom diatom Pseudo-nitzschia in California coastal waters. Harmful Algae. 2017;67:36–43.
Radan RL, Cochlan WP. Differential toxin response of Pseudo-nitzschia multiseries as a function of nitrogen speciation in batch and continuous cultures, and during a natural assemblage experiment. Harmful Algae. 2018;73:12–29.
Wingert CJ, Cochlan WP. Effects of ocean acidification on the growth, photosynthetic performance, and domoic acid production of the diatom Pseudo-nitzschia australis from the California Current System. Harmful Algae. 2021;107:102030.
Auro ME, Cochlan WP. Nitrogen utilization and toxin production by two diatoms of the Pseudo-nitzschia pseudodelicatissima complex: P. cuspidate and P. fryxelliana. J Phycol. 2013;49:156–69.
Lundholm N, Clarke A, Ellegaard M. A 100-year record of changing Pseudo-nitzschia species in a sill-fjord in Denmark related to nitrogen loading and temperature. Harmful Algae. 2010;9:449–57.
Ryan JP, Kudela RM, Birch JM, Blum M, Bower HA, Chavez FP, et al. Causality of an extreme harmful algal bloom in Monterey Bay, California, during the 2014–2016 northeast Pacific warm anomaly. Geophys Res Lett. 2017;44:5571–79.
McCabe RM, Hickey BM, Kudela RM, Lefebvre KA, Adams NG, Bill BD, et al. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys Res Lett. 2016;43:10,366–76.
Tatters AO, Fu FX, Hutchins DA. High CO2 and silicate limitation synergistically increase the toxicity of Pseudo-nitzschia fraudulenta. PLoS One. 2012;7:e32116.
Lundholm N, Hansen PJ, Kotaki Y. Effect of pH on growth and domoic acid production by potentially toxic diatoms of the genera Pseudo-nitzschia and Nitzschia. Mar Ecol Prog Ser. 2004;273:1–15.
Trimborn S, Lundholm N, Thoms S, Richter KW, Krock B, Hansen P, et al. Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: the effect of pH-induced changes in seawater carbonate chemistry. Physiol Plant. 2008;133:92–105.
Brunson JK, McKinnie SMK, Chekan JR, McCrow JP, Miles ZD, Bertrand EM, et al. Biosynthesis of the neurotoxin domoic acid in a bloom-forming diatom. Science. 2018;361:1356–58.
Boissonneault KR, Henningsen BM, Bates SS, Robertson DL, Milton S, Pelletier J, et al. Gene expression studies for the analysis of domoic acid production in the marine diatom Pseudo-nitzschia multiseries. BMC Mole Biol. 2013;14:1–19.
Pierrot DE, Lewis E, Wallace DWR MS Excel program developed for CO2 system calculations. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of. Energy, Oak Ridge, TN. 2006; Retrieved from https://doi.org/10.3334/CDIAC/otg.CO2SYS_XLS_CDIAC105a.
Brzezinski MA, Nelson DM. The annual silica cycle in the Sargasso Sea near Bermuda. Deep-Sea Res Pt I Oceanogr Res Papers. 1995;42:1215–37.
Schlüter L, Lohbeck KT, Gutowska MA, Gröger JP, Riebesell U, Reusch TBH. Adaptation of a globally important coccolithophore to ocean warming and acidification. Nat Clim Change. 2014;4:1024–30.
Schaum CE, Barton S, Bestion E, Buckling A, Garcia-Carreras B, Lopez P, et al. Adaptation of phytoplankton to a decade of experimental warming linked to increased photosynthesis. Nat Ecol Evol. 2017;1:0094.
Wang Z, Maucher-Fuquay J, Fire SE, Mikulski CM, Haynes B, Doucette GJ, et al. Optimization of solid-phase extraction and liquid chromatography–tandem mass spectrometry for the determination of domoic acid in seawater, phytoplankton, and mammalian fluids and tissues. Anal Chim Acta. 2012;715:71–9.
Brandenburg KM, Velthuis M, Van de Waal DB. Meta-analysis reveals enhanced growth of marine harmful algae from temperate regions with warming and elevated CO2 levels. Glob Change Biol. 2019;25:2607–18.
Wohlrab S, John U, Klemm K, Rberlein T, Grivogiannis AMF, Krock B, et al. Ocean acidification increases domoic acid contents during a spring to summer succession of coastal phytoplankton. Harmful Algae. 2020;92:101697.
Zhong J, Guo Y, Liang Z, Huang Q, Lu H, Pan J, et al. Adaptation of a marine diatom to ocean acidification and warming reveals constraints and trade-offs. Sci Total Environ. 2021;771:145167.
Trainer VL, Bates SS, Lundholm N, Thessen AE, Cochlan WP, Adams NG, et al. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae. 2012;14:271–300.
Zhu Z, Qu P, Gale J, Fu F, Hutchins DA. Individual and interactive effects of warming and CO2 on Pseudo-nitzschia subcurvata and Phaeocystis antarctica, two dominant phytoplankton from the Ross Sea, Antarctica. Biogeosciences. 2017;14:5281–95.
Hutchins DA, Walworth NG, Webb EA, Saito MA, Moran D, McIlvin MR, et al. Irreversibly increased N2 fixation in Trichodesmium experimentally adapted to high CO2. Nat Commun. 2015;6:8155.
Walworth NG, Lee MD, Fu FX, Hutchins DA, Webb EA. Molecular and physiological evidence of genetic assimilation to high CO2 in the marine nitrogen fixer Trichodesmium. P Natl Acad Sci. 2016;113:E7367–74.
Schaum CE, Buckling A, Smirnoff N, Studholme DJ, Yvon-Durocher G. Environmental fluctuations accelerate molecular evolution of thermal tolerance in a marine diatom. Nat Commun. 2018;9:1719.
Hutchins DA, Capone DG. The ocean nitrogen cycle: New developments and global change. Nat Rev Microbiol. 2022;20:401–14.
Xu D, Tong S, Wang B, Zhang X, Wang W, Zhang X, et al. Ocean acidification stimulation of phytoplankton growth depends on the extent of departure from the optimal growth temperature. Mar Pollut Bull. 2022;177:113510.
Hennon GMM, Sefbom J, Schaum E, Dyhrman ST, Godhe A Studying the acclimation and adaptation of HAB species to changing environmental conditions. In: Wells ML, et al. (eds.). GlobalHAB. 2021. Guidelines for the Study of Climate Change Effects on HABs. Paris: UNESCO-IOC/SCOR, 2021. pp 64–78.
Collins S, Bell G. Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature. 2004;431:566–9.
Kremp A, Godhe A, Egardt J, Dupont S, Suikkanen S, Casabianca S, et al. Intraspecific variability in the response of bloom-forming marine microalgae to changed climate conditions. Ecol Evol. 2012;2:1195–207.
Tatters AO, Schnetzer A, Fu F, Lie AY, Caron DA, Hutchins DA. Short‐versus long‐term responses to changing CO2 in a coastal dinoflagellate bloom: Implications for interspecific competitive interactions and community structure. Evolution. 2013;67:1879–91.
Schaum CE, Collins S. Plasticity predicts evolution in a marine alga. P Roy Soc B-Biol Sci. 2014;281:20141486.
Moran XAG, Lopez-Urrutia Á, Calvo-Díaz A, Li WKW. Increasing importance of small phytoplankton in a warmer ocean. Glob Change Biol. 2010;16:1137–44.
Thomas MK, Kremer CT, Klausmeier CA, Litchman EA. Global pattern of thermal adaptation in marine phytoplankton. Science. 2012;338:1085–88.
Toseland ADSJ, Daines SJ, Clark JR, Kirkham A, Strauss J, Uhlig C, et al. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat Clim Change. 2013;3:979–84.
Collins S. Many Possible Worlds: Expanding the Ecological Scenarios in Experimental Evolution. Evol Biol. 2011;38:3–14.
Qu PP, Fu F, Wang XW, Kling JD, Elghazzawy M, Huh M, et al. Two co‐dominant nitrogen‐fixing cyanobacteria demonstrate distinct acclimation and adaptation responses to cope with ocean warming. Env Microbiol Rep. 2022;14:203–17.
Lande R. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J Evol Biol. 2009;22:1435–46.
Draghi J, Whitlock MC. Phenotypic plasticity facilitates mutational variance, genetic variance, and evolvability along the major axis of environmental variation. Evolution 2012;66:2891–902.
Collins S, Rost B, Rynearson TA. Evolutionary potential of marine phytoplankton under ocean acidification. Evol Appl. 2014;7:140–55.
Kim H, Spivack AJ, Menden-Deuer S. pH alters the swimming behaviors of the raphidophyte Heterosigma akashiwo: Implications for bloom formation in an acidified ocean. Harmful Algae. 2013;26:1–11.
Hennon GMM, Quay P, Morales RL, Swanson LM, Armbrust EV. Acclimation conditions modify physiological response of the diatom Thalassiosira pseudonana to elevated CO2 concentrations in a nitrate-limited chemostat. J Phycol. 2014;50:243–53.
Daufresne M, Lengfellner K, Sommer U. Global warming benefits the small in aquatic ecosystems. Proc Natl Acad Sci. 2009;106:12788–93.
Atkinson D, Ciotti BJ, Montagnes DJS. Protists decrease in size linearly with temperature: ca. 2.5% °C-1. Proc R Soc Lond B 2003;270:2605–11.
Tong S, Gao K, Hutchins DA. Adaptive evolution in the coccolithophore Gephyrocapsa oceanica following 1,000 generations of selection under elevated CO2. Glob Chang Biol 2018;24:3055–64.
Kelly KJ, Fu FX, Jiang X, Li H, Xu D, Yang N, et al. Interactions between ultraviolet B radiation, warming, and changing nitrogen source may reduce the accumulation of toxic Pseudo-nitzschia multiseries biomass in future coastal oceans. Front Mar Sci. 2021;8:433.
Sterner R, Elser, J Ecological stoichiometry. In: Levin SA, et al. (eds) The Princeton Guide to Ecology. Princeton Univ. Press, 2009. pp 376–85.
Petrou K, Baker KG, Nielsen DA, Hancock AM, Schulz KG, Davidson AT. Acidification diminishes diatom silica production in the Southern Ocean. Nat Clim Change 2019;9:781–86.
Acknowledgements
This work was supported by Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (No.2021QNLM050103-1), National Natural Science Foundation of China (41976110; 32072329; 32000404); the Young Taishan Scholars Program to DX, Taishan Scholars Funding; Central Public-interest Scientific Institution Basal Research Fund, CAFS (NO. 2020TD27, 20603022021019); Natural Science Foundation of Shandong Province (ZR2021MD075); Laoshan Laboratory (LSKJ202203204); China Agriculture Research System (CARS-50); U.S. California Urban Ocean Sea Grant and National Science Foundation grants (OCE 1638804, OCE 1538525, OCE 2149837, OCE 2120619).
Author information
Authors and Affiliations
Contributions
NY designed the project with contributions from DX. NY, DX, GCZ, ZW, TJ, KS, XF, CB, DAH, and ZJT analyzed the data. DX, NY, GB, ZW, and DAH wrote the manuscript; all authors contributed to editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, D., Zheng, G., Brennan, G. et al. Plastic responses lead to increased neurotoxin production in the diatom Pseudo-nitzschia under ocean warming and acidification. ISME J 17, 525–536 (2023). https://doi.org/10.1038/s41396-023-01370-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-023-01370-8