Abstract
Despite the widespread occurrence of intracellular crystalline inclusions in unicellular eukaryotes, scant attention has been paid to their composition, functions, and evolutionary origins. Using Raman microscopy, we examined >200 species from all major eukaryotic supergroups. We detected cellular crystalline inclusions in 77% species out of which 80% is composed of purines, such as anhydrous guanine (62%), guanine monohydrate (2%), uric acid (12%) and xanthine (4%). Our findings shifts the paradigm assuming predominance of calcite and oxalates. Purine crystals emerge in microorganisms in all habitats, e.g., in freshwater algae, endosymbionts of reef-building corals, deadly parasites, anaerobes in termite guts, or slime molds. Hence, purine biocrystallization is a general and ancestral eukaryotic process likely present in the last eukaryotic common ancestor (LECA) and here we propose two proteins omnipresent in eukaryotes that are likely in charge of their metabolism: hypoxanthine-guanine phosphoribosyl transferase and equilibrative nucleoside transporter. Purine crystalline inclusions are multifunctional structures representing high-capacity and rapid-turnover reserves of nitrogen and optically active elements, e.g., used in light sensing. Thus, we anticipate our work to be a starting point for further studies spanning from cell biology to global ecology, with potential applications in biotechnologies, bio-optics, or in human medicine.
Similar content being viewed by others
Crystalline inclusions, conspicuous in many unicellular eukaryotes (protists), have attracted the attention of scientists since the emergence of microscopy. After Charles Darwin documented the crystal-like particles in flagellates [1], Haeckel coined the term “biocrystal” for calcite (CaCO3), celestite (SrSO4), silica (SiO2), and oxalate in protists [2]. Biocrystals or biogenically originated crystalline inclusions of either organic or inorganic chemical nature, are typically formed inside vacuoles and grow into shells and scales on cell surfaces, e.g., calcite scales of coccolithophores that take part in the global carbon cycle [3]. On the other hand, rarely reported purine inclusions of guanine, xanthine, hypoxanthine, or uric acid are often overlooked [4,5,6,7,8]. The recent resurgence of research on purine inclusions has shown rapid uptake kinetics of different nitrogen compounds that are converted into massive guanine nitrogen stores with sufficient capacity, in some cases, to support three consecutive cell cycles [8]. As a fundamental biogenic element, nitrogen represents a great share of biotic elemental stoichiometry, from cellular to global scales, impacting Earth’s climate [9]. Biocrystalized guanine polarizes and reflects light in many animals, e.g., fish with the opalescent guanine crystals in the iridocytes of their scales resembling glittery camouflage, or as an adaptation for vision in scallops, deep-sea fishes, and arthropods that exhibit guanine-reflective retinal tapeta in their eyes [10,11,12] with analogous functions in alveolates [5, 13, 14].
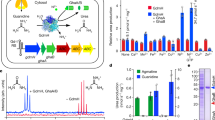
Using Raman microscopy, we have screened all major eukaryotic groups, >200 species, most of them for the first time, searching for birefringent (light-polarizing) crystalline inclusions (for Raman spectra, see Figs. S1–S5). They are wobbling by Brownian motion inside vacuoles (Movies S1–S8). We detected common biocrystals in accordance with previously described crystalline inclusions, such as calcite, oxalate, celestite, and baryte [3], together with other birefringent structures (i.e., starch, chrysolaminarin, strontianite, and newly observed crystals of sterols and carotenoids). However, apart from these, we found a surprisingly broad occurrence of purines (>80% of examined species containing crystals), particularly crystalline anhydrous guanine (62%), uric acid (12%), xanthine (4%) and guanine monohydrate (2%), (Fig. 1; Table S1; Supplementary Text). We found anhydrous guanine crystals as dominant type in model and biotechnologically important species. It commonly occurs in cosmopolitan marine and freshwater algae—including bloom-causing dinoflagellates, in the endosymbionts of corals—crucial for the maintenance of entire coral reef ecosystems, in unicellular parasites of warm-blooded animals and in cellulose-digesting anaerobic symbionts of termites, or in slime molds. Additionally, we found uric acid in cryptophytes, diatoms, zygnematophytes, and klebsormidiophytes. We identified xanthine crystals in Amoebozoa and in biotechnologically important microalgae (e.g., Chlorella and Isochrysis). We also discovered pure guanine monohydrate in marine diplonemids or its admixture to uric acid in green algae. Particular triggers for purine inclusion formation are unknown, but they are often produced after transfer to fresh growth media, media containing surplus sources of nitrogen, and under stress conditions [4, 6]. The lack of purine inclusions in some strains (Table S1) does not exclude the ability to form biocrystals because the induction of crystal formation may occur only under specific conditions. As previously reported, purine crystals may act as nitrogen storage for microalgae in which they are formed in a type of luxury uptake resulting in net removal of nitrogen from the medium [8].
The occurrence of anhydrous guanine (cyan), guanine monohydrate (violet), uric acid (yellow) and xanthine (orange) are illustrated in the evolutionary scheme as pie charts and in Raman maps (A–Q) together with the Raman spectra (R). Ratio of species positively tested for purine inclusions out of the total number of screened samples are expressed for each taxonomic category. Lineages highlighted in gray possess purine inclusions already reported elsewhere. A Eimeria maxima, (B) Glenodinium foliaceum, (C) Paramecium sp., (D) Eutreptiella gymnastica, (E) Naegleria gruberi, (F) Tribonema aequale, (G) Schizochytrium sp., (H) Tetraselmis subcordiformis, (I) Pediastrum duplex, (J) Gefionella okellyi, (K) Nannochloropsis oculata, (L) Bigelowiella natans, (M) Isochrysis sp., (N) Chlorella vulgaris, (O) Cryptomonas sp., (P) Klebsormidium flaccidum, (Q) Penium margaritaceum. Scale bars: 5 µm (A–D, I, Q), 1 µm (E–H).
Our results suggest that purine crystals might have been present in the last eukaryotic common ancestor (LECA), becoming the first type of biocrystals in eukaryotes contingent on the emergence of cell compartmentalization in early eukaryotes. We employed comparative transcriptomics and genomics to identify candidate proteins of ancient pathways responsible for purine crystal formation (PCF). Firstly, we proved the key enzyme of the salvage pathway—hypoxanthine-guanine phosphoribosyl transferase (HGPT) to be omnipresent among eukaryotes (Fig. 2, Fig. S8)—it might be responsible for purine release from its derivatives during PCF or its reusage after crystal degradation. Then, we focused on the transporters of nucleobases (i.e., purines), nucleosides and nucleotides that must be delivered to the vesicles where crystals are formed (Fig. 2). Despite the purine transporters were considered omnipresent among eukaryotes [15], our exhaustive homolog search and subsequent phylogenetic analyses challenged the ubiquity of the three of them (Supplementary Text). Nucleobase-cation symporter 1 exists in several paralogs in eukaryotes, at least three of them emerged by relatively recent horizontal gene transfer from eubacteria (Figs. S8, S9). Nucleobase-ascorbate transporter emerged independently four times in eukaryotes (Fig. S10). Only the AzgA could be possibly present in the LECA (Fig. 2, Fig. S11), although distribution of both its eukaryotic paralogs is rather limited. Finally, the solo-purine transporters are absent in some of the purine crystal-forming groups (Heterolobosea, Ciliophora, and Apicomplexa) indicating that they do not participate in PCF.
A Examples of analyzed protein families of nucleobases, nucleosides and nucleotides transporters: CNT concentrative nucleoside transporter, ENT equilibrative nucleoside transporter, NAT nucleobase-ascrobate transporter, NCS1 nucleobase-cation symporter 1, SLC solute carrier family, VNUT vesicular nucleotide transporter. Metabolic enzyme HGPT hypoxanthine-guanine phosphoribosyl transferase is located in the cytoplasm and possibly inside of the crystal containing vesicles (? in yellow). ENT and HGPT protein families show possibly general distribution among eukaryotes (Fig. S8, Table S3). There may be more unkown tranporters and enzymes or crystallization nuclei involved (???). B Summary table of phylogenetic distribution of the purine transporters in relation to the purine crystals occurrence in tested phylogenetic lines: NCS1, NAT, AzgA, and the metabolic enzyme of salvage pathway—HGPT. There are notions on horizontal gene transfer (HGT) in two cases. In the case of AzgA we anticipate a possible origin in last eukaryotic common ancestor (LECA).
The exact distribution, function, and localization of nucleotide transporters (e.g., vesicular nucleotides transporter) cannot be reliably predicted in silico without further biochemical studies [16]. Our analyses of nucleoside transporters showed that the concentrative nucleoside transporters (CNT) occur only infrequently (Table S2). However, the equilibrative nucleoside transporters (ENT) are omnipresent in eukaryotes (Table S3) becoming the most promising target for further studies on nucleotide/nucleoside/nucleobase transporters involved in PCF. Members of the ENT family are specific for nucleosides and nucleobases, operating in a bidirectional mode or as cation symporters, with different localization in the plasma membrane or in intracellular vesicles [17]. The metabolism and transport of nucleobases, nucleosides and nucleotides is essential for all organisms and hence transporters involved in PCF may play multiple roles in the cell and are retained even when the lineage has lost the ability to form purine crystals. Thus, extensive biochemical and proteomic studies have to be employed to answer this question in future.
Due to low-solubility and high-capacity, purine inclusions might have emerged as an adaptation to nitrogen detoxification, protection against exposure to high levels of ammonia or nitrates, utilizing vacuoles as a versatile sequestration space. Simultaneously, as nitrogen-rich storage, they became a competitive advantage during nitrogen fluctuations. Currently, the nitrogen-rich microbes might be of use in biofertilizers. The value of algae-based food supplements may be limited by the medical issues associated with regular intake of purines, e.g., hyperuricemia manifesting as gouty arthritis [18]. Conversely, unicellular model organisms can help to understand the biocrystallization in the gouty joints yielding in its potential treatment, which is currently missing [18]. The exceptional optical activity of purine crystals can be exploited in bio-optics [10]. In conclusion, purine biocrystallization is a general and an ancestral eukaryotic process operating by an as-yet-unknown mechanism bringing enough material for future studies spanning from cell biology to global ecology.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files.
References
Darwin C. Journal of Researches Into the Geology and Natural History of the Varoius Countries Visited by HMS Beagle, Under the Command of Captain Fitzroy from 1832 to 1836 by Charles Darwin. Colburn, London; 1840.
Haeckel E. Kristallseelen: Studien über das anorganische Leben. Leipzig: Alfred Kröner Verlag; 1917.
Raven JA, Knoll AH. Non-skeletal biomineralization by eukaryotes: matters of moment and gravity. Geomicrobiol J. 2010;27:572–84.
Creutz CE, Mohanty S, Defalco T, Kretsinger RH. Purine composition of crystalline cytoplasmic inclusions of Paramecium tetraurelia. Protist. 2002;153:39–45.
Jantschke A, Pinkas I, Hirsch A, Elad N, Schertel A, Addadi L, et al. Anhydrous β-guanine crystals in a marine dinoflagellate: Structure and suggested function. J Struct Biol. 2019;207:12–20.
Moudříková Š, Nedbal L, Solovchenko A, Mojzeš P. Raman microscopy shows that nitrogen-rich cellular inclusions in microalgae are microcrystalline guanine. Algal Res. 2017;23:216–22.
Roush AH. Crystallization of purines in the vacuole of Candida utilis. Nature. 1961;190:449.
Mojzeš P, Gao L, Ismagulova T, Pilátová J, Moudříková Š, Gorelová O, et al. Guanine, a high-capacity and rapid-turnover nitrogen reserve in microalgal cells. Proc Natl Acad Sci. 2020;117:32722–30.
Sterner RW, Elser JJ. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Encyclopedia of Ecology, Five-Volume Set. Princeton, New Jersey, USA: Princeton University Press; 2002.
Tadepalli S, Slocik JM, Gupta MK, Naik RR, Singamaneni S. Bio-optics and bio-inspired optical materials. Chem Rev. 2017;117:12705–63.
Palmer BA, Taylor GJ, Brumfeld V, Gur D, Shemesh M, Elad N, et al. The image-forming mirror in the eye of the scallop. Science. 2017;358:1172–5.
Wagner A, Wen Q, Pinsk N, Palmer BA. Functional molecular crystals in biology. Isr J Chem. 2021;61:668–78.
Kuhlmann HW, Bräucker R, Schepers AG. Phototaxis in Porpostoma notatum, a marine scuticociliate with a composed crystalline organelle. Eur J Protistol. 1997;33:295–304.
Yamashita H, Kobiyama A, Koike K. Do uric acid deposits in zooxanthellae function as eye-spots? PLoS ONE. 2009;4:1–9.
Kourkoulou A, Pittis AA, Diallinas G. Evolution of substrate specificity in the nucleobase-ascorbate transporter (NAT) protein family. Micro Cell. 2018;5:280–92.
Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105:5683–6.
Boswell-Casteel RC, Hays FA. Equilibrative nucleoside transporters—a review. Nucleosides Nucleotides Nucleic Acids. 2017;36:7–30.
Bove M, Cicero AFG, Veronesi M, Borghi C. An evidence-based review on urate-lowering treatments: implications for optimal treatment of chronic hyperuricemia. Vasc Health Risk Manag. 2017;13:23–8.
Acknowledgements
We express our gratitude to Lukáš Falteisek, Richard Dorrell, Jan Petrášek, Stanislav Volsobě, Kateřina Schwarzerová and Jana Krtková for constructive discussions. English has been kindly corrected by William Bourland. Furthermore, we thank to Dovilė Barcytė, William Bourland, Antonio Calado, Dora Čertnerová, Yana Eglit, Ivan Fiala, Martina Hálová, Miroslav Hyliš, Dagmar Jirsová, Petr Kaštánek, Viktorie Kolátková, Alena Kubátová, Alexander Kudryavtsev, Frederik Leliaert, Julius Lukeš, Jan Mach, Joost Mansour, Jan Mourek, Yvonne Němcová, Fabrice Not, Vladimír Scholtz, Alastair Simpson, Pavel Škaloud, Jan Šťastný, Róbert Šuťák, Daria Tashyreva, Dana Savická, Jan Šobotník, Zdeněk Verner, Jan Votýpka for kindly providing cultures and taxonomic identifications.
Funding
Financial support from the Czech Science Foundation (grants 17–06264 S, 19–19297 S, 20-16549Y, 21-03224S, and 21-26115 S); Grant Agency of Charles University (grant 796217), Charles University Research Center program No. 204069, European Regional Development Fund and the state budget of the Czech Republic, projects no. CZ.1.05/4.1.00/16.0340, CZ.1.05/4.1.00/16.0347, CZ.2.16/3.1.00/21515 and CZ.02.1.01/16_019/0000759, LM2018129.
Author information
Authors and Affiliations
Contributions
JP conceived the study, handled the cell cultures, performed the Raman measurements and data processing, prepared the graphics and videos and wrote the paper; TP and MO performed phylogenetic analyses and profiling; PM conceived the study and corrected the paper; IČ provided the cell cultures and corrected the paper. All authors discussed and approved the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pilátová, J., Pánek, T., Oborník, M. et al. Revisiting biocrystallization: purine crystalline inclusions are widespread in eukaryotes. ISME J 16, 2290–2294 (2022). https://doi.org/10.1038/s41396-022-01264-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-022-01264-1
This article is cited by
-
Guanine crystal formation by bacteria
BMC Biology (2023)
-
Macromolecular sheets direct the morphology and orientation of plate-like biogenic guanine crystals
Nature Communications (2023)