Abstract
Penicillium and Bactrocera dorsalis (oriental fruit fly, Hendel) are major pathogens and pests of citrus fruits, as both of them can cause detrimental losses in citrus production. However, their interaction in the cohabitation of citrus fruits remains elusive. In this study, we revealed a mutualistic relationship between Penicillium and B. dorsalis. We found that insect behaviors can facilitate the entry of fungal pathogens into fruits, and fungal pathogens promote the fitness of insects in return. More specifically, Penicillium could take advantage of the openings left by ovipositors of flies, and adult flies contaminated with Penicillium could spread the fungus to new sites. Moreover, the volatile emissions from fungi could attract gravid flies to the infected site for egg laying. The fungus and B. dorsalis were able to establish mutual interaction, as revealed by the presence of Penicillium DNA in intestinal tracts of flies throughout all larval stages. The fungal partner seemed to promote the emergence rate and shorten the emergence duration of the flies by providing pyridoxine, one of the B group vitamins. Different from previously reported scenarios of strong avoidance of Drosophila and attraction of Aedes aegypti toward Penicillium, our findings unveil a hitherto new paradigm of the mutualism between Penicillium and B. dorsalis, by which both insect and fungus earn benefits to facilitate their propagation.
Similar content being viewed by others
Introduction
Volatile organic compounds (VOCs) are implicated in the interactions between insects and microorganisms [1, 2]. Insects recognize and discriminate among different environmental microorganisms based on their VOC profiles, thereby maintaining specific preferences toward beneficial microorganisms which could either provide nutritional benefits or aid in the detoxification and digestion of food [3, 4]. Accordingly, the VOCs produced by microorganisms can manipulate the behavior of insect vector to facilitate their dissemination [5,6,7]. Such mutual co-adaptations between insects and environmental microorganisms are best exemplified by the ubiquitous interactions between yeast and Drosophila melanogaster [8, 9]. Gravid flies are attracted by yeast-infected fruits, and their progeny could benefit from the high nutrition condition due to fermentation by yeast [10]. Moreover, as most yeasts do not have active spore dispersal mechanisms, fly vectors play indispensable roles in the propagation of fungi, which favors mutualism between fly and yeast [11,12,13].
Many insects mainly feed and oviposit on decaying fruits that are inhabited by microorganisms. The Penicillium genus ubiquitously present on fruit and soil surfaces could be found in both urban and natural landscapes [3, 14]. Penicillium is known to emit off-flavors containing geosmin, which repels Drosophila and might serve as a signal for the presence of toxic fungi [15]. However, geosmin is not repellent but functions as an oviposition attractant for mosquitos [16]. Insects from the same order respond differently toward Penicillium. The complex interplay between insects and fungi could be modulated by the secondary metabolites produced by fungi and the physiological responses of insects, and the ecological relationship is determined by whether co-habitation brings about mutual benefits or disadvantages [1, 7].
Bactrocera dorsalis (Hendel), oriental fruit fly, belongs to Diptera and is one of the most prevalent insect pests in fruit cultivation in both tropical and subtropical regions. It is considered to be a highly invasive and destructive agricultural pest due to its strong dispersal, adaptive capacity [17] and broad host range (46 plant families and more than 250 types of fruits and vegetables, especially citrus fruits) [18, 19]. B. dorsalis infestation causes ~10% yearly economic losses of citrus fruits due to oviposition beneath the peel of citrus fruits and pulp feeding by the larvae [20, 21]. In addition, it was reasoned that most green molds (such as Penicillium) could take advantage of the openings created by the insect ovipositor to penetrate the fruits and infect the plants, resulting in additional crop damage [22]. Understanding the interaction between Penicillium and B. dorsalis may provide insights into the biological pest and pathogen control in citrus fruits.
In this study, we revealed a mutually beneficial relationship between Penicillium and B. dorsalis on citrus fruits. In contrast to both mosquito and fruit fly, our results showed that gravid female B. dorsalis preferred to oviposit on the Penicillium-inoculated substrate, but non-gravid flies showed avoidance. We further demonstrated that the ingestion of fungus by larvae could promote the emergence of flies by providing the B group vitamin, pyridoxine. In return, oviposition behavior of B. dorsalis could facilitate the colonization of fungus in the injured fruits, and adults could serve as potential vectors for the transmission of Penicillium to new sites. Collectively, the mutually beneficial relationship between Penicillium and B. dorsalis could facilitate their propagation on citrus fruits.
Materials and methods
Insects and microorganisms
Bactrocera dorsalis was obtained from a laboratory-reared stock colony (Key Laboratory of Pesticide and Chemical Biology, South China Agricultural University, Guangzhou, China) and maintained at 28 °C with 70% relative humidity and a 14:10 h (L:D) photoperiod. Adult flies were reared on artificial diets consisting of yeast extract (25%) and sugar (75%). Adult flies could get full access to distilled water placed in a bottle (9.5 cm × 6 cm × 7 cm) with cotton covered. Larval diets consisted of banana (31.8%), corn (31.8%), yeast extract (3.2%), sugar (3.2%), sodium benzoate (0.1%), fiber (3.2%), hydrochloric acid (0.2%) and distilled water (26.5%).
Penicillium citrinum was purchased from Guangdong Microbial Culture Collection Center (GDMCC 3.458). Penicillium italicum and Penicillium expansum were obtained from the China General Microbiological Culture Collection Center (CGMCC 3.7045 and CGMCC 3.15687).
Chemical reagents
Geosmin was purchased from Enrenstorfer at a 10−4 dilution in methanol. Linalool, 3-carene and D-limonene of the highest purity (generally >98%) were purchased from J&K Scientific Ltd. α-Pinene and myrcene were purchased from Shanghai Macklin Biochemical Co. and were diluted in ethanol before use. 13C3-Thiamin, 13C415N2-riboflavin, 2H4-nicotinic acid, 13C4-pyridoxine, 13C5-biotin, and 13C5-folic acid were purchased from Sigma-Aldrich Trading Co., Ltd.
Behavioral assays
To examine the responses of the flies under different states toward Penicillium strains, a trap assay was performed in a testing chamber containing two traps made from transparent plastic vials (100 ml) with micropipette tips inserted into a hole at the top of each vial. The control vial contained sterile citrus or medium (– Penicillium), while the treated vial contained Penicillium-colonized citrus or medium (+ Penicillium). These two vials were placed diagonally in the testing chamber. The Penicillium-colonized citrus or medium was prepared by inoculating citrus (Citrus reticulata) or medium (potato dextrose agar medium, PDA) with 10 μl of Penicillium suspension at a concentration of 8 × 104 conidia/ml. Seven days post-inoculation, the Penicillium-colonized citrus or medium was then subjected to examination for the appearance of typical mold lesions. To examine the responses of flies toward different chemicals, trap assay was performed. The control vial contained filter paper (1 cm × 3 cm) perfumed with solvent, while the treated vial contained filter paper perfumed with chemical (geosmin/methanol; linalool, 3-carene and D-limonene/ethanol). The flies under different states (gravid females or non-gravid females or males, 20 days post-emergence) were tested separately. Gravid female flies were prepared by using sexually mature female flies mated with male flies, while non-gravid sibling females were separated from male flies on the 3rd day post-emergence to prevent copulation. For one-time examination, fifteen flies were released into one chamber, and the number of flies inside each vial was counted after 2 h. The attraction index (A.I.)a was calculated as (# treatment – # control)/15, where # treatment indicates the number of flies in the Penicillium-colonized vial, and # control indicates the number of flies in the control vial. All the experiments were performed at the same time of the day and carried out in a climate chamber (28 °C with 70% relative humidity and a 14:10 h (L:D) photoperiod).
For the oviposition assay, 50 gravid female flies were released into the testing chamber containing two traps made from transparent plastic vials (100 ml) with holes on the side wall of each vial to allow oviposition. Oviposition was allowed for 24 h, and the number of eggs laid in each vial was counted. The control vial contained sterile citrus or medium or filter paper perfumed with solvent, while the treated vial contained Penicillium-colonized citrus or medium or filter paper perfumed with chemical. Oviposition index (O.I.) was calculated as follows: (# treatment − # control)/(# treatment + # control), where # treatment indicates the number of eggs laid in the Penicillium-colonized or chemical-perfumed vial and # control indicates the number of eggs laid in the control vial.
Volatile collection and detection
The testing samples included three Penicillium strains (including Penicillium expansum, Penicillium citrinum, and Penicillium italicum) inoculated on standard PDA medium, with blank control PDA medium serving as a control. For sample preparation, 10 μl of Penicillium suspension at a concentration of 8 × 104 conidia/ml was added to standard PDA medium. Seven days post-inoculation, the sample was placed into a 2 l air-Tedlar bag, respectively, and connected to a tube with 3 mg Tenax TA + 3 mg activated carbon powder and stuffed with quartz wool for 24 h as previously described [23]. Immediately after collection, collected volatiles were eluted with 200 μl hexane from Tenax TA. A gas chromatograph mass spectrometer (GC-MS, Agilent, China) fitted with an HP-5MS column (30 m × 0.25 mm × 0.25 μm) was used to separate and analyze the extracted volatiles. Helium was kept as a carrier gas at a constant flow rate of 1 ml/min. Split GC-inlet mode (5:1) was used to inject the volatiles at ~250 °C for 5 min. The GC oven temperature program was initially set at 40 °C (5 min) and increased to 280 °C at a rate of 5 °C per min. The transfer line temperature was maintained at 250 °C. The mass spectrometer was operated in electron impact mode with electron energy of ~70 eV. Compounds were putatively identified by comparisons of their spectra and retention times with mass spectral data from the National Institute of Standards and Technology (NIST 2.0) library.
Electroantennogram (EAG) measurements
For EAG recording, the antennae of adult flies (non-gravid female, gravid female or male flies) were cut off at the base of the head. The antenna was connected by two recording electrodes. A filter paper strip containing testing solution was allowed to evaporate for 10 min and then was inserted into a Pasteur pipette, which was connected to a rubber tube with a continuous flow rate of 4 ml/s. The stimulus controller transported the volatiles to the antenna for stimulation with 0.5 s pulse duration. Each component condition was tested by at least three antennae from different flies. The amplitude of the EAG response was recorded and digitized with EAG-adapted software. The protocol for EAG was followed as previously described [24].
RNA interference
To investigate the functional roles of odorant receptors (Ors) in chemical perception, RNA interference (RNAi) was performed to silence specific genes. dsRNAs targeting different genes were designed (Supplementary Table S1) and synthesized by the T7 RiboMAX Express RNAi System (P1700, Promega, US). The synthesized dsRNAs were purified using an RNeasy MinElute Cleanup Kit (74204, Qiagen, Germany). The size of the dsRNA product was confirmed by electrophoresis on a 1.5% agarose gel, and the final concentration of dsRNA was adjusted to 3.5 μg/μl. One microgram of dsRNA was injected into the abdomen of flies (gravid or non-gravid females, 18 days post-emergence), and control flies were injected with equivalent volumes of DEPC water or dsGFP. After injection, all flies were then reared under standard rearing conditions (28 °C with 70% relative humidity in a 14:10 h (L:D) photoperiod) without any fungus. Two days post-injection, the expression level of the target gene was examined by quantitative real-time PCR (qRT-PCR). Trap assays or oviposition preference assays were performed after the confirmation of gene knockdown to examine the roles of Ors in the perception of chemicals.
RNA extraction and qRT-PCR
Total RNA was extracted using the Eastep Super total RNA isolation kit (LS1040, Promega, USA) based on the instructions of the manufacturer. The concentration of isolated RNA was measured by a Nanodrop spectrophotometer (Thermo Fisher Scientific, USA). One microgram of total RNA was reverse-transcribed to first-strand cDNA by M-MLV reverse transcriptase (AG11711, AG, China) with oligo (dT)18 as the primer. qRT-PCR analysis was performed using SYBR green dye (AG11701, AG, China) on the iCycler iQ Real-Time PCR Detection System (Bio-Rad). Two reference genes (Tubulin and EF1a) were used to normalize the expression levels. The primers used in qPCR analysis are listed in Supplementary Table S1. Relative gene expression data were analyzed using the 2−△△CT method as previously described [25].
Vitamin measurement
Vitamin measurement was performed as previously described with minor modifications [26]. Gravid female flies were allowed to lay eggs on substrate with or without P. citrinum. After eggs hatched, the larvae were provided larval diets and were able to get access to the medium inoculated with P. citrinum (control flies were able to get access to diet and blank medium only). The medium with or without P. citrinum were renewed every day. One hundred milligrams of larvae (2 days post-hatch) developed from eggs laid on substrate with or without P. citrinum were homogenized with 0.5 ml water-acetonitrile-methanol (1:2:2, v/v/v) and 100 μl internal standard solution (20 mg/l of 13C3-thiamin, 13C415N2-riboflavin, 2H4-nicotinic acid, 13C4-pyridoxine, 13C5-biotin, and 13C5-folic acid). After centrifugation, the supernatant was collected and dried under nitrogen gas. The residue was re-dissolved in 100 μl water and centrifuged at 14,000 × g, and the supernatant was then injected for high performance liquid chromatography-mass spectrometry (HPLC-MS) analysis. The separation was performed on a UPLC system (Agilent 1290 Infinity UHPLC) equipped with a C-18 column (Waters, HSS T3 1.7 μm, 2.1 mm × 100 mm column) at 40 °C under a gradient of 0.1% formic acid and 2.5 mM ammonium formate in water and acetonitrile at a flow rate of 0.2 ml/min. The gradient schedule was 0% methanol at the starting point for 2 min, 0–50%, for 2–3 min; 50–100%, for 3–4.5 min; 100%, for 4.5–5.5 min; 100–0%, for 5.5–6 min; 100%, for 6–11 min. Before injecting the next sample, the column was equilibrated with the initial mobile phase for 11 min. The flow rate was constant at 0.3 ml/min and the column temperature was set at 45 °C. A 5500 QTRAP (AB SCIEX) was used in positive switch mode. The ESI source conditions were as follows: source temperature: 550 °C; ion source gas 1 (Gas 1): 55; ion source gas 2 (Gas 2): 55; curtain gas (CUR): 40; and ion spray voltage floating, + 4500 V. Full scan mass spectra were acquired in the range of 100 to 500 m/z.
Pyridoxine measurement
A microbiological assay was used for pyridoxine quantification in flies using Saccharomyces cerevisiae Meyen ATCC 9080 (Beijing Landbridge Technology Limited, Beijing, China) according to the published protocol [27]. Briefly, 20 larvae (2 days post-hatch) developing from eggs laid on substrate with or without Penicillium were collected, weighed and stored in liquid nitrogen. The samples were then homogenized in citrate buffer (with 2.1% citric acid and 7.16% disodium hydrogen phosphate dodecahydrate) and incubated in sulfuric acid at 95 °C for 30 min. Then, the samples were sterilized by a 0.2 µm nylon 66 membrane, mixed with pyridoxine Y medium (Beijing Landbridge Technology Limited, Beijing, China) and incubated with S. cerevisiae for 24 h at 37 °C. A background control consisting of citrate buffer only was subjected to the same procedures. A standard concentration of pyridoxine dissolved in citrate buffer was mixed with S. cerevisiae culture to create a standard curve. The growth of S. cerevisiae with different samples was measured using a microplate reader (Synergy H1, BioTek) at 440 nm. The OD value was subtracted from the background control, and the relative concentration of pyridoxine in each sample was quantified using the standard curve and normalized to the weight of the larvae.
Performance of fly progeny
Gravid female flies were allowed to lay eggs on a substrate with or without P. citrinum. After the eggs hatched, the larvae were provided by larval diets and were able to get access for the medium inoculated with P. citrinum (control flies were able to get access for diet and blank medium only). The medium with or without P. citrinum was renewed every day. Two days post-hatch, the larvae were transferred to a new chamber without P. citrinum, but with continuous larval diet supply. The performances of these developing larvae were monitored. For each treatment, the following parameters were recorded: egg phase, % hatching, total larval duration, pupal stage duration, adult emergence percentage, and emergence duration. The experiment was performed under controlled conditions: temperature 28 ± 2 °C, relative humidity 70 ± 10% and a 14:10 h (L:D) photoperiod.
To investigate the role of pyridoxine in fly development, newly hatched larvae (1 day post-emergence) were fed pyridoxine supplemented artificial diets (with 0.1 ng/ml pyridoxine) or control diets (with the same amount of double distilled water) after the hatching of eggs laid under normal conditions without any mold present. The pyridoxine supplemented diet was renewed every day until the pupal stage. The performance of developing flies (total larval duration, pupal stage duration, adult emergence percentage, and emergence duration) was monitored daily.
Fluorescence in situ hybridization (FISH)
Gravid female flies were allowed to oviposit in vials with or without P. citrinum. Newly hatched larvae could get full access to the artificial diet, and the treated larvae were also fed P. citrinum. Two days post-hatch, the flies were transferred to new chambers without P. citrinum. Flies at different developmental stages were collected and dissected. Digestive tracts from flies at different developmental stages were then incubated in saline buffer. The fixation was performed in PBS with 4% paraform and HEPES (700 μl PBS + 200 μl paraform + 100 μl HEPES) for 1.5 h. After fixation, the samples were washed three times with PBS. The hybridization was performed in hybridization buffer (20 mM Tris-HCl [pH 8.0], 0.9 M NaCl, 0.01% sodium dodecyl sulfate, 30% formamide) containing 2.5 ng/μl fluorescent probes (100 Um, Sangon, China) with incubation for 12 h at 37 °C. After washing, the tissues were again incubated at room temperature for 10 min in Hoechst and PBT (1:1000). The stained samples were whole mounted and viewed under a confocal microscope (Nikon A1 plus).
Fruit sample preparation
After being superficially disinfected with 0.025% sodium hypochlorite and then rinsed with sterile distilled water, mature and intact citrus fruits were obtained. The mechanically injured fruit was prepared by puncturing ten times on different sides of the fruit with a 1 ml syringe to a depth of 1 cm (~0.8 mm in outer diameter) to pierce the rinsed fruit skin and enter the pulp. To prepare the ovipositor-infested fruits, mature and mated females were released into containers with fruits. The oviposition behavior was monitored and recorded when the female was depositing eggs (at which time she was quiescent and exhibited full ovipositor extrusion) into a fruit. After ten times, the ovipositor-infested fruit was obtained. We collected volatiles from three groups of citrus, intact, mechanically damaged, and fly-ovipositor-infested, as previously described immediately.
Effect of compounds identified in injured citrus fruits on fungal growth
The citrus fruits, including intact (control), mechanically damaged and fly-ovipositor-infested citrus, were inoculated with 1 ml of three strains of Penicillium-conidial suspension (8 × 104 conidia/ml), respectively. The inoculated fruits were then placed in sterile plastic cans (9 cm diameter × 8 cm deep) covered with thin transparent plastic film at 20 ± 2 °C and 16 h of illumination per day for 7 days. The mycelial mat was gently scraped from the citrus by disposable blades, and the dry weight of mycelia was determined after being dried at 50 °C for 24 h.
Standard chemicals (α-pinene and myrcene) were purchased, and the effects of these chemicals on the growth of three strains of Penicillium were assessed, while the control group was treated with equal amounts of ethanol. For the chemical-mediated growth test, we used 90 mm Petri dishes that were divided into two equal parts. Penicillium was inoculated on one side of the Petri dish (10 μl of Penicillium suspension at a concentration of 8 × 104 conidia/ml), and chemical solution (5 × 10–5 dilution) was added on the opposite side of the Petri dish. On the 7th day post-inoculation, the mycelial mat was gently scraped from the agar by disposable blades, and its dry weight was determined. Pictures were randomly taken from the area of the mycelial mat at a distance of 1 cm and under microscope.
Bioassays of conidial transmission of Penicillium by contaminated adult flies
The bioassay was performed as described in a previous study [28]. After inoculation with Penicillium-conidial suspension, PDA medium was then incubated at 20 ± 2 °C for 7 days until the appearance of typical mold lesions. Adult flies were introduced into the isolated plastic chamber (30 cm length × 30 cm width × 30 cm height) containing the resulting inoculated medium or control medium without fungal inoculation. The flies were left in the chamber for 24 h before the conidial transmission assay. Ten flies (female:male = 1:1) in contact with inoculated medium were introduced into a plastic can (9 cm diameter × 8 cm deep) containing sterilized food and freshly made medium or citrus (superficially disinfected with 0.025% sodium hypochlorite beforehand). The introduced flies were left in the cans for another 24 h. The control group was set up in the cans with sterilized PDA medium without Penicillium inoculation, and then the non-contaminated flies were introduced to a plastic can containing sterilized food and freshly made medium or citrus. In addition, a plastic can without flies was set up to exclude the possibility of air contamination. All cans were then incubated at 20 ± 2 °C and 16 h of illumination per day until the appearance of typical Penicillium lesions. The dry weight of typical lesions was determined and measured.
Tent assays
A bioassay of conidial transmission was also performed in a tent (40 cm × 40 cm × 60 cm, white fine polyester mesh cover) with one citrus plant inside (Citrus reticulata). Fifty eggs were evenly introduced onto five citrus fruits, which were inoculated with 1 ml of P. citrinum-conidial suspension (5.0 × 106 conidial/ml) beforehand, while citrus fruits from the control group received equal amounts of water and the same amount of eggs. Sterile sand was placed underneath the citrus plant to allow pupation. After 35 days, when they were sexually mature and able to oviposit (typical P. citrinum lesions could be observed on the 5th day post-inoculation), these emerging adult flies were transferred into a new tent with a citrus plant inside. The number of infected citrus carrying P. citrinum conidia was counted, and the dry weight of typical lesions was determined and measured on the 7th day after transfer. The assay was repeated for three times.
Statistical analyses
For the EAG recordings and trap assays to examine the responses of B. dorsalis toward mixed chemicals, including D-limonene, linalool, 3-carene and geosmin, statistical differences were evaluated using one-way ANOVA at a significance level of 0.05 followed by LSD post hoc tests. For the attraction index, OI, gene expression level comparison, pyridoxine titer, and fungal dry weight, statistical comparisons were based on Students’s t test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
Substrate inoculated with Penicillium citrinum attracted gravid female Bactrocera dorsalis for oviposition
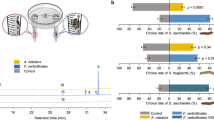
Fungal VOCs play important roles in insect-fungus interactions and can influence insect behaviors by acting as attractants or repellents [1, 29]. We therefore first examined the behavioral response and oviposition preference of B. dorsalis toward odors from a typical pathogenic fungus of citrus fruits, P. citrinum (Fig. 1a). We found non-gravid females and males (20 days post-emergence) showed strong preferences toward sterile citrus over P. citrinum-colonized citrus in trap assays (Fig. 1b and Supplementary Fig. 1a). This result was consistent with a previous study by Stensmyr et al. [15], which demonstrated that geosmin (a volatile compound from Penicillium) elicited innate avoidance in flies. However, we also noted gravid female B. dorsalis preferred the vial containing citrus infected with P. citrinum (Fig. 1b and Supplementary Fig. 1a). We next questioned whether gravid B. dorsalis also preferred citrus infected with P. citrinum as an oviposition site. Unsurprisingly, the oviposition preference assay demonstrated that gravid female flies tended to lay more eggs in the vial containing P. citrinum-infected citrus (Fig. 1c). To exclude the odor of citrus, we repeated the same tests of behavioral responses and oviposition preference using an alternative behavioral paradigm by presenting P. citrinum on PDA medium. Again, we observed that male and non-gravid female flies showed avoidance to the vial containing P. citrinum (Fig. 1d and Supplementary Fig. 1b), but gravid females preferred to choose and lay more eggs in the vial containing medium with P. citrinum (Fig. 1d, e and Supplementary Fig. 1b). Together, these data suggest that P. citrinum differentially affects the behavioral responses and oviposition preference of B. dorsalis. Since flies in these experiments were raised on artificial diet and had no prior access to Penicillium, we conclude that such behavior toward P. citrinum is innate.
a Schematic illustration of behavioral assays, including trap assay (left) and oviposition preference assay (right). b Trap assay of adult B. dorsalis in a chamber with vial containing sterile citrus on one side (control) and vial containing P. citrinum-colonized citrus (treated) on the diagonal side (n = 8–9 independent experiments, 15 flies were analyzed in each experiment). The flies under different states (gravid females, non-gravid females, males) were examined separately, and the respective attraction index (A.I.) was calculated. c Oviposition preference assay between citrus and citrus infected with P. citrinum. Egg number was counted and oviposition index (O.I.) was calculated (n = 9 independent experiments, 50 gravid female flies were analyzed in each experiment). d Trap assay of B. dorsalis between sterile medium (control) and P. citrinum-colonized medium (n = 7–8 independent experiments). e Oviposition preference assay between medium and medium inoculated with P. citrinum (n = 6 independent experiments). Values are the means (±SEs) of replicates. Statistical comparisons were based on Students’s t test. The level of significance for the results was set at **p < 0.01, ****p < 0.0001.
Three Penicillium strains produce geosmin as a repellent for non-gravid flies
To further determine whether other strains of Penicillium elicit similar behavioral responses in B. dorsalis, we performed trap assays using three strains of Penicillium (including Penicillium expansum, Penicillium citrinum, and Penicillium italicum) and found that the medium inoculated with those three Penicillium strains all caused significant avoidance in non-gravid female and male B. dorsalis, but triggered strong attraction of gravid females (Fig. 2a and Supplementary Fig. 2a–c). As geosmin was identified from Penicillium as a potent repellent to many insects [15, 30] and to confirm if geosmin was present on these Penicillium strains, we performed GC-MS on samples of three Penicillium strains inoculated on standard PDA medium, respectively. Blank PDA medium was served as a control to remove the odors appeared in the blank medium. We analyzed the chromatograms of hexane extracts by comparison of mass spectra with those found in the NIST spectral library (Fig. 2b and Supplementary Table S2). As revealed by GC-MS profiles, geosmin (peak 30) was identified in all tested Penicillium strains (Fig. 2b and Supplementary Table S2). A plausible assumption would be that geosmin signals the presence of Penicillium and acts as a repellent to non-gravid B. dorsalis, akin to its function in a previous report [15]. We next performed the same trap assay by using 10 μl geosmin (10−5 dilution, the concentration that appeared in a natural Penicillium strain [31] and within the receptive range of insect [16]) on filter paper (1 cm × 3 cm). Consistent with the previous study [15], the substrate without geosmin attracted more non-gravid and male flies, while gravid female flies did not exhibit such avoidance to geosmin (Fig. 2c and Supplementary Fig. 2d). Gravid female flies also yielded weaker electrophysiological responses to geosmin than male and non-gravid sibling females (Fig. 2d and Supplementary Fig. 3), indicating attenuated geosmin sensing.
a Attraction index (A.I.) of flies from trap assays between medium and medium inoculated with three Penicillium strains, respectively, (n = 6 independent experiments, 15 flies were analyzed in each experiment). The flies under different states (gravid females, non-gravid females, males, 20 days post-emergence) were examined separately, and the respective A.I. was calculated. b Representative gas chromatogram traces of head space emitted by three strains of Penicillium. Supplementary Table S2 summarized the identifications of numbered peaks in the GC-MS chromatograms by comparison of their spectra and retention times with mass spectral data from the National Institute of Standards and Technology (NIST 2.0) library. Geosmin, linalool, 3-carene and D-limonene were further confirmed by comparing against synthetic standards. c A.I. of gravid, non-gravid female and male flies from trap assays between control solvent methanol and geosmin (10 μl of 10−5 dilution). d EAG measurement of flies in response to geosmin (10−5 dilution). EAG amplitude data were summarized in d and examples of responding curves in EAGs measurement were shown in Supplementary Fig. 3 (n = 3 independent experiments, 3 flies were analyzed in each experiment). e Expression levels of geosmin-responsive Ors in gravid and non-gravid females. f A.I. of DEPC water-, dsGFP- and dsRNA-injected non-gravid female flies from trap assays between solvent and geosmin. g A.I. of DEPC water-, dsGFP- and dsRNA-injected gravid female flies from trap assays between solvent and geosmin (n = 6 independent experiments). Statistical comparisons were compared individual treatment with control based on Students’s t test, respectively. The levels of significance for the results were set at *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In Drosophila melanogaster, geosmin activates a single class of sensory neurons expressing the olfactory receptor Or56a [15], we therefore hypothesized that geosmin perception in B. dorsalis is mediated by the same receptor. We first analyzed the expressions of three Or56a orthologs in B. dorsalis (Or7, Or21 and Or25) [32] and found that the expressions of Or7 and Or21 were significantly decreased in gravid females compared to those in non-gravid females (Fig. 2e). To validate their roles in geosmin sensing, we silenced these genes in non-gravid females, which largely abrogated the avoidance toward geosmin (Fig. 2f and Supplementary Figs. 2e and 4). In gravid females, knockdown of these genes did not affect the behaviors of females toward geosmin (Fig. 2g and Supplementary Figs. 2f and 4). Thus, we conclude that Or7, Or21 and Or25 are required for geosmin sensing and aversion in the non-gravid B. dorsalis, and the down-regulations of these Ors may account for the diminished aversive responses toward geosmin in gravid females.
Oviposition on substrate with Penicillium enhances the progeny fitness of B. dorsalis
We next tested whether other Penicillium strains also attracted oviposition of B. dorsalis and determined whether the Or-mediated olfactory pathway governed the oviposition attraction to Penicillium strains. We found that dsGFP-injected gravid females tended to lay more eggs in substrate inoculated with different strains of Penicillium, and such preference was abrogated in dsOrco-injected gravid female flies (Fig. 3a). These results imply the dependence of oviposition preference on the olfactory system. Given the vital roles of microbial volatiles in the oviposition preference of flies, we screened volatile profiles of Penicillium strains and identified VOCs commonly produced by three Penicillium strains. Linalool, 3-carene, D-limonene and 6-Pentyl-2H-pyran-2-one were identified in the samples of these fungal strains (peaks 4, 5, 13, 32) (Fig. 2b and Supplementary Table S2). We then investigated the roles of these compounds in oviposition preference using concentrations that matched those used in previous studies (10 μl of 10−5 dilution on 1 cm × 3 cm filter paper) [33, 34]. The four-odor mixture induced the strongest oviposition preference in gravid females, and linalool, 3-carene and D-limonene alone or the two-odor mixture elicited relatively weaker oviposition preference. However 6-Pentyl-2H-pyran-2-one was unable to attract gravid flies for oviposition (Fig. 3b). Further oviposition preference assays indicated that gravid flies showed oviposition preferences toward these three active compounds in dose-dependent manners (Fig. 3c).
a Oviposition preference assays of dsGFP- or dsOrco-injected gravid female B. dorsalis between substrate with or without three trains of Penicillium, respectively. Egg number was counted and oviposition index (O.I.) was calculated (n = 6 independent experiments, 50 gravid female flies were analyzed in each experiment). b Oviposition preference assays of gravid females to various combinations of four identified volatiles from three trains of Penicillium (10 μl of odorants in 10−5 dilution) (n = 6 independent experiments, 50 gravid female flies were analyzed in each experiment). c Oviposition preference assays between different concentrations of chemicals and solvent control (n = 10 independent experiments). d Attraction index (A.I.) of flies from trap assay between identified compounds (10 μl of odorants in 10−5 dilution) and solvent (n = 6 independent experiments, 15 flies were analyzed in each experiment). The flies under different states (gravid females, non-gravid females, males, 20 days post-emergence) were examined separately, and the respective A.I. was calculated. e EAG measurements of flies in response to different chemicals. EAG amplitude data were summarized in e and examples of responding curves in EAGs measurement were shown in Supplementary Fig. 5 (n = 3 independent experiments, 3 flies were analyzed in each experiment). Statistical comparisons in d and e were based on One-way ANOVA, different letters indicated significant difference, p < 0.05. f Comparisons of performance between flies developed from eggs laid on substrate with and without Penicillium citrinum. After gravid flies laid eggs on substrate with or without P. citrinum, the hatch rate, pupation rate and emergence rate was calculated (upper) and the egg phase, larval duration, pupation period, and emergence period was monitored (bottom) (n = 3 independent experiments, 20 flies were analyzed in each experiment). g Pyridoxine titer in larvae developed from eggs laid on substrate with or without P. citrinum (n = 6 independent experiments, 20 larvae were analyzed in each experiment). h Comparisons of performance between flies with pyridoxine supplement in larval diet and flies raised in control diet (without pyridoxine supplement). The pupation rate and emergence rate were calculated (upper) and the larval duration, pupation period, and emergence period was measured (bottom) (n = 3 independent experiments, 20 flies were analyzed in each experiment). Values are the means (±SEs) of replicates. Statistical comparisons were based on Students’s t test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
To further explore the perception of odors emitted by Penicillium strains in B. dorsalis, we conducted a series of trap assays and EAG measurements with these odors. Linalool, 3-carene or D-limonene alone was sufficient to elicit attraction in gravid female, non-gravid female and male flies (Fig. 3d). However, the addition of geosmin strongly inhibited the attraction toward these odors in non-gravid and male flies, while the preference of gravid females remained unchanged (Fig. 3d). EAG measurements revealed that gravid flies yielded strong electrophysiological responses to linalool, 3-carene, D-limonene and mixed odors with or without geosmin and exhibited significantly higher responses to these odors (linalool, 3-carene and D-limonene) than non-gravid female and male flies (Fig. 3e and Supplementary Fig. 5). Although we could not exclude the possible roles of other volatile and non-volatile compounds, these results indicated that these chemicals at least partially mediate the oviposition preference toward Penicillium strains in gravid B. dorsalis.
Since non-gravid B. dorsalis showed avoidance toward Penicillium, we then hypothesized that oviposition on Penicillium-containing substrates might confer developmental advantages to the progeny. After gravid females laid eggs on substrate with or without P. citrinum, we monitored the development of these flies, including the larval (pupal) growth rate and developmental duration. We found that the hatch rate, pupation rate, egg duration, larval duration, and pupation period were not significantly affected by the presence of P. citrinum, while the emergence period was significantly shorter and the emergence rate was significantly higher than those of emerging flies developing from eggs laid on the substrate without P. citrinum (Fig. 3f). Many associations between insects and microorganisms are nutrition based, enabling those insects, especially their larvae, to survive under nutrient-poor conditions [35, 36]. As most insects lack the genetic capacity to synthesize B vitamins from precursors in central metabolism [37], we hypothesized that P. citrinum probably produced certain B vitamins to support the development of B. dorsalis. We then measured the contents of B vitamins by HPLC-MS, and found that the concentrations of thiamin, riboflavin, nicotinic acid, biotin, and folic acid were comparable between larval samples developing in substrates with or without P. citrinum, while there was a significantly higher level of pyridoxine in larval sample with P. citrinum inoculated (Supplementary Fig. 6). The microbiological assay further confirmed that the newly hatched and mature larvae developing from eggs laid on P. citrinum-inoculated substrate exhibited higher titers of pyridoxine than the control (Fig. 3g). Furthermore, supplementation with pyridoxine (0.1 ng/ml) recapitulated the scenario of fly development on the substrate with P. citrinum (Fig. 3h). We therefore conclude that after female lay eggs on substrates infected with P. citrinum, the enhanced emergence rate of the offspring may result from the higher level of pyridoxine.
The persistence of P. citrinum in intestinal tracts from the larval to pupal stage
To examine the presence of P. citrinum in intestinal tracts of B. dorsalis, we performed FISH using a probe targeting P. citrinum DNA. The results showed that specific staining signals of P. citrinum could be observed in intestinal tracts of larvae (Fig. 4a). Quantifications by qRT-PCR revealed that the amount of P. citrinum DNA in day one larvae from the substrate with P. citrinum was 97.30 times greater than that in the control (larvae developing from eggs on substrate without P. citrinum) (Fig. 4a). P. citrinum persisted in intestinal tracts throughout larval stages (135.28 times greater than that in the control in 7-d-larvae) (Fig. 4b), with a reduced level in pupae (5.32 times greater than the control level) (Fig. 4c). However, P. citrinum seemed to be eliminated from the intestine of the adult stages (Fig. 4d, e). These results indicate that P. citrinum could be ingested by the larvae of B. dorsalis and persisted till the pupal stage.
a–f Identification and quantification of P. citrinum by FISH (upper) and qRT-PCR (below) in intestinal tracts of different stages of Bactrocera dorsalis. The bars in 10× and 60× graphs represent 500 μm and 50 μm, respectively. The white boxes indicated the area with fungal persistence. Values are the means (±SEs) of five replicates. Statistical comparisons were based on Students’s t test, *p < 0.05.
B. dorsalis facilitates fungal dissemination in citrus fruits
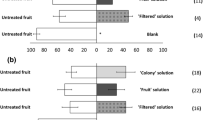
Since gravid flies tended to oviposit on citrus fruits containing Penicillium, we proposed that ovipositor puncturing in citrus fruits by gravid flies might facilitate the infection of the fungus. We then compared the growth of three Penicillium strains on intact citrus fruits or fruits injured by mechanical damage or ovipositor-infested. The results showed that injury caused by both mechanically damaged and ovipositor significantly promoted the growth of three strains of Penicillium observed on the 7th day post-inoculation (Fig. 5a and Supplementary Fig. 7a). We then compared the changes in VOC emissions from injured and intact citrus fruits. Among various compounds identified in the citrus VOC profiles, α-pinene and myrcene (peaks 16 and 19) were present only in injured fruits, and a previous study also indicated the possible roles of these compounds in stimulating fungal growth (Fig. 5b and Supplementary Table S3) [14]. Indeed, the addition of α-pinene and myrcene (5 × 10−5 dilution) to the culture plate significantly promoted the growth of the mycelia of Penicillium with more fungal biomass detected than in culture on plates without these compounds (dry weight was evaluated on the 7th day post-inoculation) (Fig. 5c, d and Supplementary Fig. 7b, c).
a The effects of injury caused by syringe or ovipositor on fungal growth. Images showing the typical lesions of Penicillium citrinum on fruits on the 7th day post-inoculation. The column bar represented the comparisons of the dry weight of P. citrinum on the 7th day post-inoculation (n = 3 independent experiments, 3 samples were analyzed in each experiment). b Representative gas chromatogram traces of volatiles emitted by ovipositor-infested, mechanically damaged or control intact citrus fruits. Supplementary Table S3 summarized the identifications of numbered peaks in the GC-MS chromatograms. α-pinene and myrcene were further confirmed by comparing mass spectra against synthetic standards. Effects of α-pinene (c) and myrcene (d) on fungal mycelial growth (Top: Low-magnification images from day 1–7. Bottom: High-magnification images of samples from day 7, scale bar: 100 μm). The column bar represented the comparisons of the dry weight of P. citrinum on day 7 (n = 6 independent experiments, 3 samples were analyzed in each experiment). e, f Modality of conidial transmission of P. citrinum to intact citrus or sterilized medium by contaminated adult flies. Images showed the typical lesions of P. citrinum infection on fruits and the mycelial growth in medium, respectively. The column bar represented the comparison of the dry weight of P. citrinum on the 5th day post-transfer (n = 3 or 6 independent experiments, 3 samples were analyzed in each experiment). g Tent assay. Schematic illustration of tent assay, and the infected citrus and the fungal dry weight from control (inoculated with water) and P. citrinum + (inoculated with P. citrinum) was recorded at the 7th day post-transferring (n = 3 independent experiments). Values are the means (±SEs) of replicates. Statistical comparisons were based on Students’s t test between two groups.
We also hypothesized that flies contaminated with Penicillium might facilitate fungal dissemination. Control adult flies or flies contaminated with Penicillium were introduced into containers with fresh medium or intact and sterile citrus. Contaminated flies resulted in significant fungal infection on citrus (5 days post-transfer) (Fig. 5e and Supplementary Fig. 7d) and in the medium (5 days post-transfer) (Fig. 5f and Supplementary Fig. 7e), and no symptoms of Penicillium infection on citrus fruits or signs of medium colonization were observed in the groups with uncontaminated flies and no flies. In addition, we also found that the flies emerging from eggs on the infected fruits could carry Penicillium spores to a new citrus plant and resulted in significantly more infected citrus fruits and more fungal biomass in tent assays (Fig. 5g). Collectively, these results indicate that the oviposition behaviors of B. dorsalis could facilitate the colonization of fungus in injured fruits and that adults could serve as potential vectors for the transmission of Penicillium on citrus fruits.
Discussion
In this study, we demonstrate how a fungal pathogen manipulates the oviposition behavior of B. dorsalis to facilitate its dissemination, and that in return, the progeny of insects gain nutritional benefits. The interaction of insect and fungus begins with the release of fungal VOCs to attract gravid flies for oviposition. The fungus takes advantage of fruit injury from ovipositor for its infection, and provides essential nutrients (pyridoxine) to support larval development. The flies contaminated with Penicillium might also facilitate fungal propagation.
In contrast to the strong avoidance of Drosophila [15] and the favorable attraction of Aedes aegypti [16] toward Penicillium, we reported a scenario where gravid B. dorsalis showed a tendency to lay eggs on sites containing Penicillium, while avoided Penicillium-infected sites at other adult stages. The selection of oviposition site by a gravid female is based on oviposition stimulants or attractants [38]. We identified VOCs from Penicillium, including linalool, 3-carene and D-limonene, which served as efficient oviposition attractants for gravid female B. dorsalis. In addition, the expression levels of geosmin-responsive Ors were reduced in gravid female flies compared with those in non-gravid females. These results indicate that both the suppression of geosmin sensing in gravid females and the production of oviposition attractants by Penicillium might contribute to oviposition preference toward Penicillium-inoculated sites in gravid flies, which may be mediated by integrated olfactory recognition [7, 39, 40]. The specific neural circuit underlying oviposition site determination needs to be further elucidated.
We propose that oviposition preference could be attributed to pregnancy-induced “prenatal care behavior” to entail fitness benefits for offspring development in the habitat [41]. The commonly described maternal behaviors, such as feeding and aggression against intruders, serve the purpose of nurturing and protecting newborns. For example, female D. melanogaster can readily turn on and off their attraction to acetic acid depending on the egg-laying needs [42]. Females also tend to oviposit on plant species better supporting the growth of their offspring [43], as insect larvae can only exploit limited resources for their growth and development [41]. In our study, the high level of fungal DNA in the intestinal tracts of larvae and pupae could be explained by the consumption of fungus, as newly hatched larvae often ingest a variety of microorganisms present in food resources or the surrounding environment. As a result, the developing larvae maintained a higher level of B vitamin (pyridoxine) possibly from their microbial partner Penicillium, which enhanced the emergence rate and shortened the emergence duration. The acquisition of B vitamins via diet is crucial for insects because they lack the genetic capacity to synthesize these compounds in their central metabolism [37]. Most microorganisms, including Penicillium [44, 45], can de novo synthesize B vitamins and consequently benefit insects feeding on or interacting with microorganisms [46, 47]. It has been well documented that mutualistic microorganisms could provide essential nutrients to hosts or modify the availability of specific nutrients in the diet [27, 48]. Our results also highlight the beneficial role of Penicillium in supporting larval development of B. dorsalis, possibly by producing pyridoxine via direct synthesis or by modifying the dietary components.
Our data also showed that the VOCs released by Penicillium could mediate the oviposition preference of gravid female flies, which potentially facilitates the fungal infection as dormant Penicillium spores could germinate rapidly and colonize the injured fruits [14]. α-Pinene and myrcene produced by injured citrus are also able to stimulate the germination and growth of Penicillium. These data support the notion that the oviposition behaviors of gravid female flies assist in fungal infection in citrus fruits. Moreover, the results of tent assays indicate that the emerging flies of eggs on infected fruits could serve as transmission vectors for Penicillium, possibly through the fungal conidia adhering to the mouthparts or abdominal tip of contaminated flies. As a consequence, the emerging flies from infected fruits increase the likelihood of fungal dissemination.
In summary, our study revealed the beneficial interplay between Penicillium and B. dorsalis. Penicillium produces VOCs to attract gravid flies to lay eggs, which could facilitate the infection and dispersal of fungus on citrus fruits. The fungus rewards developing larvae with pyridoxine supplementation to enhance their emergence. This interaction brings about benefits for both partners and stabilizes the fungus-insect mutualistic relationship. Importantly, our findings also resolve the puzzle of why cleaning up rotting and damaged fruits could alleviate yield loss, as the presence of damaged fruit facilitates the mutual interaction and spread of these pests and pathogens.
Data availability
All relevant data supporting the findings of this study are included within the article and its Supplementary Information files.
References
Franco FP, Túler AC, Gallan DZ, Gonçalves FG, Favaris AP, Peñaflor MFGV, et al. Fungal phytopathogen modulates plant and insect responses to promote its dissemination. ISME J. 2021;15:3522–33.
Huang H, Ren L, Li H, Schmidt A, Gershenzon J, Lu Y, et al. The nesting preference of an invasive ant is associated with the cues produced by actinobacteria in soil. PLoS Pathog. 2020;16:e1008800.
Angleró-Rodríguez YI, Blumberg BJ, Dong Y, Sandiford SL, Pike A, Clayton AM, et al. A natural Anopheles-associated Penicillium chrysogenum enhances mosquito susceptibility to Plasmodium infection. Sci Rep. 2016;6:34084.
Davis TS, Landolt PJ. A survey of insect assemblages responding to volatiles from a ubiquitous fungus in an agricultural landscape. J Chem Ecol. 2013;39:860–8.
Flury P, Vesga P, Dominguez-Ferreras A, Tinguely C, Ullrich CI, Kleespies RG, et al. Persistence of root-colonizing Pseudomonas protegens in herbivorous insects throughout different developmental stages and dispersal to new host plants. ISME J. 2018;13:860–72.
Kandasamy D, Gershenzon J, Andersson MN, Hammerbacher A. Volatile organic compounds influence the interaction of the Eurasian spruce bark beetle (Ips typographus) with its fungal symbionts. ISME J. 2019;13:1788–800.
Keesey IW, Koerte S, Khallaf MA, Retzke T, Guillou A, Grosse-Wilde E, et al. Pathogenic bacteria enhance dispersal through alteration of Drosophila social communication. Nat Commun. 2017;8:265.
Paul GB, Gerhard F, Elżbieta R, Alexandra S, Arne H, Sébastien L, et al. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct Ecol. 2012;26:1365–2435.
Ganter PF. Yeast and invertebrate associations. In: Gábor P, Carlos R, editors. Biodiversity and ecophysiology of yeasts. Berlin, Heidelberg: Springer; 2006. pp 303–70.
Anagnostou C, Legrand EA, Rohlfs M. Friendly food for fitter flies?—Influence of dietary microbial species on food choice and parasitoid resistance in Drosophila. Oikos. 2010;119:533–41.
Günther CS, Knight SJ, Jones R, Goddard MR. Are Drosophila preferences for yeasts stable or contextual? Ecol Evol. 2019;9:8075–86.
Luo Y, Johnson JC, Chakraborty TS, Piontkowski A, Gendron CM, Pletcher SD. Yeast volatiles double starvation survival in Drosophila. Sci Adv. 2021;7:eabf8896.
Fogleman S. Coadaptation of Drosophila and yeasts in their natural habitat. J Chem Ecol. 1986;12:1037–55.
Droby S, Eick A, Macarisin D, Cohen L, Rafael G, Stange R, et al. Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol Tec. 2008;49:386–96.
Stensmyr MC, Dweck HK, Farhan A, Ibba I, Strutz A, Mukunda L, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–57.
Melo N, Wolff GH, Costa-da-Silva AL, Arribas R, Triana MF, Gugger M, et al. Geosmin attracts Aedes aegypti mosquitoes to oviposition sites. Curr Biol. 2020;30:127–34.
Wei DD, He W, Lang N, Miao ZQ, Xiao LF, Dou W, et al. Recent research status of Bactrocera dorsalis: Insights from resistance mechanisms and population structure. Arch Insect Biochem. 2019;102:e21601.
Han P, Wang X, Niu CY, Dong YC, Zhu JQ, Desneux N. Population dynamics, phenology, and overwintering of Bactrocera dorsalis (Diptera: Tephritidae) in Hubei Province, China. J Pest Sci. 2011;84:289–95.
Duyck PF, David P, Quilici S. A review of relationships between interspecific competition and invasions in fruit flies (Diptera: Tephritidae). Ecol Entomol. 2004;29:511–20.
Wen T, Zheng L, Dong S, Gong Z, Sang M, Long X, et al. Rapid detection and classification of citrus fruits infestation by Bactrocera dorsalis (Hendel) based on electronic nose. Postharvest Biol Tec. 2019;147:156–65.
Li X, Yang H, Wang T, Wang J, Wei H. Life history and adult dynamics of Bactrocera dorsalis in the citrus orchard of Nanchang, a subtropical area from China: implications for a control timeline. ScienceAsia. 2019;45:212–20.
Chalupowicz D, Veltman B, Droby S, Eltzov E. Evaluating the use of biosensors for monitoring of Penicillium digitatum infection in citrus fruit. Sens Actuat B-Chem. 2020;311:127896.
Turlings TC, Lengwiler UB, Bernasconi ML, Wechsler D. Timing of induced volatile emissions in maize seedlings. Planta. 1998;207:146–52.
Wang B, Dong W, Li H, D’Onofrio C, Bai P, Chen R, et al. Molecular basis of (E)-β-farnesene-mediated aphid location in the predator Eupeodes corollae. Curr Biol. 2022;32:951–62.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–8.
Cellar NA, De Nison JE, Seipelt CT, Twohig M, Burgess JA. Title of subordinate document. In: Dramatic improvements in assay reproducibility for water-soluble vitamins using ACQUITY UPLC and the Ultra-Sensitive Xevo TQ-S Mass Spectrometer. 2013. https://www.waters.com/webassets/cms/library/docs/720004690en.pdf.
Ren FR, Sun X, Wang TY, Yan JY, Yao YL, Li CQ, et al. Pantothenate mediates the coordination of whitefly and symbiont fitness. ISME J. 2021;15:1655–67.
Batta YA. Quantitative postharvest contamination and transmission of Penicillium expansum (Link) conidia to nectarine and pear fruit by Drosophila melanogaster (Meig.) adults. Postharvest Biol Tec. 2006;40:190–6.
Rohlfs M. Clash of kingdoms or why Drosophila larvae positively respond to fungal competitors. Front Zool. 2005;2:2.
Becher PG, Bengtsson M, Hansson BS, Witzgall P. Flying the fly: long-range flight behavior of Drosophila melanogaster to attractive odors. J Chem Ecol. 2010;36:599–607.
Dionigi C, Ahten T, Wartelle L. Effects of several metals on spore, biomass, and geosmin production by Streptomyces tendae and Penicillium expansum. J Ind Microbiol Biot. 1996;17:84–88.
Jin S, Zhou X, Gu F, Zhong G, Yi X. Olfactory plasticity: variation in the expression of chemosensory receptors in Bactrocera dorsalis in different physiological states. Front Physiol. 2017;8:672.
Li H, Ren L, Xie M, Gao Y, He M, Hassan B, et al. Egg-surface bacteria are indirectly associated with oviposition aversion in Bactrocera dorsalis. Curr Biol. 2020;30:4432–40.
Liu Y, Cui Z, Si P, Liu Y, Zhou Q, Wang G. Characterization of a specific odorant receptor for linalool in the Chinese citrus fly Bactrocera minax (Diptera: Tephritidae). Insect Biochem Molec. 2020;122:103389.
Ju JF, Bing XL, Zhao DS, Guo Y, Hong XY. Wolbachia supplement biotin and riboflavin to enhance reproduction in planthoppers. ISME J. 2019;14:1–12.
Liu F, Wickham JD, Cao Q, Lu M, Sun J. An invasive beetle–fungus complex is maintained by fungal nutritional-compensation mediated by bacterial volatiles. ISME J. 2020;14:2829–42.
Douglas AE. The B vitamin nutrition of insects: the contributions of diet, microbiome and horizontally acquired genes. Curr Opin Insect Sci. 2017;23:65–69.
Honda K, Ômura H, Hayashi N, Abe F, Yamauchi T. Conduritols as oviposition stimulants for the danaid butterfly, Parantica sita, identified from a host plant, Marsdenia tomentosa. J Chem Ecol. 2004;30:2285–96.
Soldano A, Alpizar YA, Boonen B, Franco L, Lopez-Requena A, Liu G, et al. Gustatory-mediated avoidance of bacterial lipopolysaccharides via TRPA1 activation in Drosophila. Elife. 2016;5:e13133.
Hussain A, Üçpunar HK, Zhang M, Loschek LF, Grunwald Kadow IC. Neuropeptides modulate female chemosensory processing upon mating in Drosophila. PLoS Biol. 2016;14:e1002455.
Stötefeld L, Holighaus G, Schütz S, Rohlfs M. Volatile-mediated location of mutualist host and toxic non-host microfungi by Drosophila larvae. Chemoecology. 2015;5:271–83.
Gou B, Liu Y, Guntur A, Stern U, Yang HC. Mechanosensitive neurons on the internal reproductive tract contribute to egg-laying-induced acetic acid attraction in Drosophila. Cell Rep. 2014;9:522–30.
Mezzera C, Brotas M, Gaspar M, Pavlou HJ, Goodwin SF, Vasconcelos ML. Ovipositor extrusion promotes the transition from courtship to copulation and signals female acceptance in Drosophila melanogaster. Curr Biol. 2020;30:3736–48.
Teimoori-Boghsani Y, Ganjeali A, Cernava T, Müller H, Asili J, Berg G. Endophytic fungi of native Salvia abrotanoides plants reveal high taxonomic diversity and unique profiles of secondary metabolites. Front Microbiol. 2020;10:3013–20.
Holden JT, Furman C, Snell EE. D-alanine and the vitamin B6 content of microorganisms. J Biol Chem. 1949;178:789–97.
Michalkova V, Benoit JB, Weiss BL, Attardo GM, Aksoy S. Vitamin B6 generated by obligate symbionts is critical for maintaining proline homeostasis and fecundity in tsetse flies. Appl Environ Micro. 2014;80:5844–53.
Ren FR, Sun X, Wang TY, Yao YL, Huang YZ, Zhang X, et al. Biotin provisioning by horizontally transferred genes from bacteria confers animal fitness benefits. ISME J. 2020;14:2542–53.
Salem H, Bauer E, Strauss AS, Vogel H, Marz M, Kaltenpoth M. Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc Biol Sci. 2014;281:20141838.
Acknowledgements
This work was supported by the grants from The National Key Research and Development Program of China (No. 2019YFD1002102), National Natural Science Foundation of China (No. 32072460), Guangdong Province Natural Science Foundation (No. 2019A1515012201) and Guangdong Special Branch Plan for Young Talent with Scientific and Technological Innovation (2019TQ05N158).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: XY, TZ, and GZ. Performed the experiments: FG, SJ, YC, and SA. Wrote the paper: XY and XX.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gu, F., Ai, S., Chen, Y. et al. Mutualism promotes insect fitness by fungal nutrient compensation and facilitates fungus propagation by mediating insect oviposition preference. ISME J 16, 1831–1842 (2022). https://doi.org/10.1038/s41396-022-01237-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-022-01237-4