Abstract
Arsenic pollution is a widespread threat to marine life, but the ongoing rise pCO2 levels is predicted to decrease bio-toxicity of arsenic. However, the effects of arsenic toxicity on marine primary producers under elevated pCO2 are not well characterized. Here, we studied the effects of arsenic toxicity in three globally distributed diatom species (Phaeodactylum tricornutum, Thalassiosira pseudonana, and Chaetoceros mulleri) after short-term acclimation (ST, 30 days), medium-term exposure (MT, 750 days), and long-term (LT, 1460 days) selection under ambient (400 µatm) and elevated (1000 and 2000 µatm) pCO2. We found that elevated pCO2 alleviated arsenic toxicity even after short acclimation times but the magnitude of the response decreased after mid and long-term adaptation. When fed with these elevated pCO2 selected diatoms, the scallop Patinopecten yessoensis had significantly lower arsenic content (3.26–52.83%). Transcriptomic and biochemical analysis indicated that the diatoms rapidly developed arsenic detoxification strategies, which included upregulation of transporters associated with shuttling harmful compounds out of the cell to reduce arsenic accumulation, and upregulation of proteins involved in synthesizing glutathione (GSH) to chelate intracellular arsenic to reduce arsenic toxicity. Thus, our results will expand our knowledge to fully understand the ecological risk of trace metal pollution under increasing human activity induced ocean acidification.
Similar content being viewed by others
Introduction
Average seawater pH has dropped by 0.1 U in the last two centuries, and a further decrease of 0.3 U has been predicted for the end of the century [1]. This decrease in pH has been termed ocean acidification (OA). The bioavailability of heavy metals and accumulation thereof in the food chain via primary producers hinges on water pH [2, 3]. With rapid global urbanization and industrialization, anthropogenic pollutants ultimately lead to increasing heavy metal contaminations in estuarine and coastal environments [4, 5]. China alone release over 30,000 tons of heavy metals into the sea annually, with detrimental repercussions for coastal ecosystems [5, 6]. The metalloid arsenic (As) is one of the most toxic trace elements [7]. While inorganic arsenic concentrations are relative stable in the open oceans (ranging from 12.9 to 15.7 nmol L−1), levels can reach up to 10.24 µmol L−1 in highly polluted coastal waters at Kuala Langat [8,9,10]. There, high arsenic concentrations are a threat to marine life [5]. Phytoplankton are primary accumulators of arsenic in aquatic environments playing a significant role in arsenic biogeochemical cycles (Fig. S1) [10, 11].
Biological mechanisms to mitigate the hazardous effects of arsenic compounds evolved billions of years ago, and remain widespread in unicellular organisms [12]. Under oxidative conditions, inorganic arsenate (AsV) is the most mobile and most common arsenic species, and its entry into cell arises from competitively impairing PO43− uptake transporters due to ionic similarities [13, 14]. Under reducing conditions, arsenite (AsIII) is the most common arsenic species. AsIII is taken up via membrane proteins belonging to the aquaporin family [13, 15]. While similarities between these transporters and silicate uptake transporters have been reported in rice, it is unclear whether these structural similarities exist in silicifying phytoplankton [16]. If so, silicifying diatoms would experience elevated exposure to arsenite compared to plankton without silicate transporters.
Diatoms contribute up to approximately 40% of global primary productivity and are the foundation of coastal food webs [17]. Their responses to elevated pCO2, have been studied in the laboratory and in mesocosm settings [18,19,20,21,22]. In a complex environment where multiple drivers interact, evolutionary responses are highly likely to differ from those found as a result of selection under elevated pCO2 alone [1]. The more ecologically likely scenario is that anthropogenic activities (e.g., mining), release arsenic into natural waters, typically following sudden bursts rather than a gradual release throughout time [5]. Therefore, algae may evolve under high pCO2 for hundreds of generations until they experience acute arsenic toxicity.
Here, we tested whether three globally distributed diatoms (Phaeodactylum tricornutum, Thalassiosira pseudonana, and Chaetoceros mulleri) selected under elevated pCO2 (1000 and 2000 µatm) reacted to arsenic exposure differently than samples evolved under ambient pCO2 (400 µatm). We hypothesized that the effects of elevated pCO2 on these diatoms would further alleviate arsenic toxicity, and examined this question on physiological (a few generations) and evolutionary time scales (up to 1500 generations). We describe the physiological consequences and examine biochemical and transcriptional responses in arsenic-related pathways, in order to understand the mechanisms of the mitigating effects of carbon enrichment on arsenic toxicity.
Material and methods
Microalgae culture conditions
To assess the effect of elevated pCO2 and associated lower pH on the eco-toxicity of arsenic to coastal diatoms, we obtained three diatom species Phaeodactylum tricornutum, Thalassiosira pseudonana, and Chaetoceros mulleri from the Yellow Sea Fisheries Research Institute Microalgae Culture Center of National Marine Genetic Resource Center (http://marine.fishinfo.cn/). The stocks of these species were isolated from the Yellow Sea, Rongcheng, Shandong Province, China and had been in laboratory culture for ~10 years. They were chosen for their ecological importance, as all three are fundamental players at the foundation of coastal food webs. The diatoms P. tricornutum and T. pseudonana are globally distributed and typical model species [23,24,25]. C. mulleri is a dominant diatom species in Chinese coastal waters and plays a vital role in the marine food chain [26, 27]. In the laboratory, cultures were inoculated at identical densities of 8 × 104 cells mL−1 and maintained in semicontinuous batch culture in sterile seawater enriched with f/2 medium (with 100 μM N, 6 μM P, and 100 μM Si) at 20 ± 1 °C. Irradiance was 120 μmol photons m−2 s−1 in a 12 h/12 h light–dark cycle (light on at 8:00 a.m. and off at 20:00 p.m.).
Experiment overview
We conducted four sets of experiments across different spatial and temporal scales (see Supplementary Fig. S2 for flowchart indicating how these experiments are connected). One, we assayed the diatoms’ responses to ambient and elevated pCO2 with and without addition of arsenic at three time scales (short, medium, and long-term exposure). Two, we carried out a full reciprocal transplant experiment to be able to calculate the magnitude of the evolutionary responses to elevated pCO2, and to test whether these explained some of the variations observed in the responses to high arsenic. Three, we transferred the long-term selected strains into an outdoor culture system in Sanggou Bay, located on the northwestern coast of the Yellow Sea, China (37°01′–37°09′N, 122°24′–122°35′E) for 14 days using 10 L tanks under natural temperature and light. This set up was used to determine whether the results of the responses to arsenic after long-term selection under elevated pCO2 obtained in the laboratory were comparable to those occurring under more natural temperature and light conditions. Subsequently, we fed the scallop Patinopecten yessoensis with ambient or elevated pCO2 selected diatoms which had also been exposed to arsenic, to examine possible food chain effects. The fourth and final set of experiments aimed to gain a more mechanistic understanding of how elevated pCO2 influences arsenic detoxification strategies in diatoms. We examined the expression of candidate target genes through transcriptome sequencing and Real-Time quantitative Polymerase Chain Reaction (RT-qPCR) in P. tricornutum.
pCO2 selection experiment
Cell culture
To investigate the responses of the three diatoms to elevated pCO2, three pCO2 levels were set up (Table S1). These were 400, 1000, and 2000 μatm, according to pCO2 levels projected under the Intergovernmental Panel on Climate Change (IPCC) representative concentration pathway 8.5 scenario. For each species, three independent replicate cultures were maintained at ambient and elevated pCO2 levels, realized by equilibrating the culture medium overnight prior to inoculations. Three biological replicates are sufficient here, as pilot studies showed large effect sizes of pCO2—adding more replicates would not significantly increase power in this case. The medium was bubbled either with air (~400 μatm) or air/CO2 mixed gas in a programmed CO2 chamber (HP1000G-D, Ruihua, China) to achieve the desired pCO2 levels. Sample cultures under ambient and elevated pCO2 were maintained in tightly closed polycarbonate bottles that were completely filled with culture medium, without any gas head space, to prevent gas exchange. Cultures were shaken three times every day to avoid settling and biofilm formation. The triplicate cultures at each pCO2 were transferred into fresh media every 4–6 days (when cultures had reached the exponential growth phase, Fig. S3). Roughly 8 × 104 cells mL−1 were transferred into fresh medium to maintain stable carbonate chemistry (pH variations < ±0.05). The pH in each culture medium was measured before and after each transfer using a pH meter (Orion ROSS, Fisher Scientific Instruments) that was calibrated with National Bureau of Standards buffers. Temperature, salinity, and total alkalinity (TA) were also measured periodically (but not at every transfer due to logistical restrictions) throughout the experiment. Other carbonate system parameters (e.g., DIC) were calculated with the CO2SYS package using pH and TA (Table S1) [28].
Selection experiment and arsenic assays
The conditions described above were maintained until the selection experiment was terminated after 1460 days (4 years). During the selection period, samples were inspected regularly under the microscope to monitor bacterial abundance via staining with SYBR gold and enumerating with flow cytometry (FACS Calibur, BD, USA)). The proportion of bacteria remained stable at low quantities: co-occurring bacteria contributed <1% of total biomass throughout. Flow cytometry and microscopy were also used to test for changes in cell size (on average a slight decrease in cell size, as expected), and gamete formation (very rare). To study whether exposure to elevated pCO2 has an impact on how phytoplankton deal with arsenic compounds, we exposed aliquots of all samples to arsenite (AsIII) or arsenate (AsV) for 96 h after 30 days (short term, or ST) of acclimation, or 750 days (medium-term or MT) and 1460 days (long-term or LT) of selection. We examined whether arsenic eco-toxicity, as well as bioaccumulation and speciation within the cells differed (i), between three different species of diatoms evolving under ambient or elevated pCO2, and (ii), between diatoms that had evolved under elevated pCO2 on different time-scales.
During the arsenic assays, culture medium without additional arsenic was used as a control, where the background natural arsenic level was at 0.03–0.05 μmol L−1. Media with addition of NaAsO2 or Na2HAsO4·7H2O were used in the 96 h arsenite (AsIII) or arsenate (AsV) treatments, respectively. Two levels of AsIII/AsV concentration were selected and used in arsenic assays. The low arsenic treatment was set as 0.5 μmol L−1 AsIII/AsV which did not have any negative effect on algal growth of all experimental diatoms. The high concentrations of AsIII and AsV were added according to the IC50, 96 h of each diatom species as established during the pilot studies. When IC50, 96 h was more than 30 μm L−1 (much higher than known naturally occurring concentrations) [8,9,10], it was set as the highest concentration.
Under arsenic exposure, growth rate over the course of 96 h was calculated as follows:
where N1 and N0 represent cell concentrations at times t1 and t0, respectively. At the end of the ST, MT, and LT experiment, two samples of 30 mL aliquots from each replicate were harvested by centrifugation and washed twice using Milli-Q water and an ice-cold phosphate buffer to remove the arsenic weekly adsorbed on algal surface [29]. Algal cells were used to estimate intracellular arsenic concentration ([As]intra) and arsenic speciation within cells (see Supplementary Methods for arsenic determination [30,31,32]). All arsenic samples were determined within 24 h to avoid arsenic transformation.
Additionally, 4 mL aliquots of the samples were collected after the end of the incubation period to determine net photosynthesis (NP, μmol O2 cell−1 h–1) at 20 ± 1 °C under irradiance of 120 μmol photons m−2 s−1 using an oxygen electrode (Unisense). After dark acclimation for 1 h, dark respiration (DR, μmol O2 cell–1 h–1) was also monitored. The variation of oxygen concentration was obtained using 4 ml respiration chambers fitted with microprobes, glass-coated stir bars, Clark-type OX-MR oxygen microsensors and a PA 2000 picoammeter. Data were logged using MIcOx2.6 data acquisition software (Microrespiration system, Unisense). The carbon-use efficiency (CUE) was calculated as (see e.g., Schaum et al. [33]):
While CO2 may not have been the only carbon source available in culture, changes in CUE still yield crucial information on the relative importance of two metabolic fluxes, oxygen consumption and evolution.
To study whether elevated pCO2 changed valence state of arsenite (AsIII) or arsenate (AsV) in culture medium, we used the same experimental set up as arsenic assays but without algal inoculation in the different pCO2 adjusted culture medium. Two levels of arsenic concentration (0.5 μmol L−1 for low arsenic concentration and 25 μmol L−1 for high arsenic concentration) were supplemented in culture medium. At time points of 0 h, 3 h, 6 h, 12 h, 24 h, 48 h, 72 h, and 96 h, 10 ml culture medium were collected from three independent samples and used for arsenic determination within 6 h. Both total arsenic concentration and its speciation were determined and analysed. Since cultures were maintained in tightly closed polycarbonate bottles that were completely filled with culture medium without any gas exchange, we found that there were little variations of arsenic speciation in culture medium. In the AsV assay, speciation and concentration of AsV remained constant and stable under different pCO2 along with different sampling times (Table S2). In AsIII assay, <5% of AsIII transformed into AsV by the end of experiment. But there was not significant variation among different pCO2 treatment (Table S3).
Reciprocal transplant experiments using long-term selected samples
After having been grown under ambient pCO2 (400 μatm) and elevated pCO2 (1000 μatm or 2000 μatm) for 1460 days, all lineages were transferred into 800 mL of fresh media in a full reciprocal transplant at pCO2 of 400, 1000, and 2000 μatm. After 10 days in semicontinuous batch culture (corresponding to 13, 11, and 6 generations for P. tricornutum, T. pseudonana, and C. mulleri, respectively), all samples were cultured in triplicate with additive exposure to high AsIII or AsV for another 96 h. Controls did not receive any arsenic treatment. Cell growth and intracellular arsenic accumulation were determined using the same methods as described above.
Outdoor experiment using long-term selected populations
Aliquots of the long-term selected populations were transferred from the laboratory to an outdoor culture system consisting of 10 L tanks with 8 L sterilized seawater at their respective pCO2 (Fig. S4). The respective pCO2 levels of 400, 1000, and 2000 μatm were established by bubbling the medium with air or air/CO2 premixed gas using a CO2 Enrichlor (CE-100B; Wuhan Ruihua Instrument & 25 Equipment Ltd). After 10 days in semicontinuous batch culture (Corresponding to 15, 13, and 8 generations for P. tricornutum, T. pseudonana, and C. mulleri, respectively) at each pCO2 under natural temperature and light, all samples apart from the controls were exposed to arsenic for 96 h, as described above. Variations of temperature, irradiance and carbonate chemistry in the tanks were monitored throughout (Figs. S4, S5). Growth rates over the course of the experiment and intracellular arsenic concentration at the end of experiment were determined as described above. Additionally, 4 mL aliquots of the samples were collected after the end of the incubation period to determine net photosynthesis (NP, μmol cell−1 h–1), dark respiration (DR, μmol cell−1 h−1), and CUE, following the same protocol as carried out in the laboratory. Another two 300 mL aliquots were collected and centrifuged, and used for determination of the activity of chloroplast ATP synthase and mitochondrial ATP synthase using Enzyme Activity Assay kits (Comin Biotechnology, http://www.cominbio.com).
Feeding experiments in outdoor culture system
To further access the effect of elevated pCO2 on arsenic transfer across trophic levels, feeding experiments were set up using the scallop Patinopecten yessoensis and diatoms selected under ambient and elevated pCO2 with or without arsenic exposure in the outdoor culture system for 96 h. Cultures from each treatment were centrifuged at 12,000 × g for 10 min at room temperature and inoculated into 8 L of seawater at a final concentration of 2 × 105 cell mL−1. P. yessoensis (average tissue dry weight of 0.58 ± 0.05) were collected from Sanggou Bay (37°01′–37°09′N, 122°24′–122°35′E), northwest coast of the Yellow Sea, China. After they had been carefully cleaned of epibionts, they were incubated in the 10 L tanks with air-bubbling for 3 h at 20 ± 1 °C. At the end of the experiment, scallops were collected and cleaned with sterile seawater. Soft tissue including the adductor muscle and the gonads were shelled, peeled, and rinsed with Milli-Q water. After vacuum freeze-drying, the soft tissue was powdered in a blender and stored at −80 °C for arsenic determination [34].
Molecular responses involved in arsenic metabolic pathways in the diatom P. tricornutum
At the end of the long-term selection experiment and the arsenic assays, 250 mL samples of P. tricornutum (as a representative species) for each biological replicate and treatment were harvested during early exponential phase, centrifuged at 4 °C, frozen in liquid nitrogen and stored at −80 °C for RNA extraction, transcriptional sequencing, and gene RT-qPCR. Another five 100 mL aliquots were collected and centrifuged, and used for determination of the activity of gamma glutamylcysteine synthetase (γ-ECs) and glutamine synthase (GS), and phytochelatin synthase (PCs), chloroplast ATP synthase and mitochondrial ATP synthase using Enzyme Activity Assay kits (Comin Biotechnology, http://www.cominbio.com).
Statistical analysis
All analyses and all preparations for graphic presentation were carried out in the R environment [35] (Supplementary Dataset S1). Throughout, we report the summary F statistics in the text. Tables detailing on how models were built, selected, and analysed can be found in the Supplementary Material. Pairwise contrasts have been submitted as separate files.
Effects of elevated pCO2 on diatom growth on different time scales in the laboratory
To determine any short-term species-specific responses of how the three diatom species reacted to high pCO2, we constructed a simple mixed model within the “lme” package (version 3.1-137), where the magnitude of the response to elevated pCO2 was the response variable, and pCO2 and diatom species were the explaining variables. Biological replicate nested within species within treatment was used as random factor. The most complex model contained pCO2 levels and species in full interaction. Models were then simplified and compared via the dredge function within the ‘MuMIn’ package (version 1.42-1). When candidate models deviated from the most parsimonious model (that with the lowest Akaike information criterion score) by less than two AICc units, parameters were averaged across those candidate models (Fig. 1, Tables S4–S7).
Values are displayed as means and ±1 SEM of triplicate cultures and that have the same letter are not significantly different (p > 0.05). Individual samples are superimposed (as shapes) on the means. Blue for 400 µatm-selected lineages, green for 1000 µatm-selected lineages, and red for 2000 µatm-selected lineages. The opacity of each symbol indicates the length of the experiment, with the palest shade for the shortest, and the darkest shade for the longest duration of the experiment.
Effects of arsenic on specific growth rate under ambient pCO2, and after ST, MT, and LT culture under elevated pCO2
To compare whether exposure to arsenic at the end of the (ST, MT, or LT) experiment had an impact on growth rate that depended on the species, pCO2 level, or length of the experiment, we examined differences in growth rate between isolates from the different treatments using a nonlinear mixed effects model, with fixed and random factors chosen as described above, and model selection again based on AICc values via the dredge function (Fig. 1, Tables S8, S9, Dataset S2).
Intracellular arsenic concentrations under ambient pCO2, and after ST, MT, and LT culture under elevated pCO2
We examined the impact of species, pCO2 level, and length of the experiment on intracellular arsenic concentrations by first developing a global mixed model, where intracellular arsenic concentration was the response variable, and species, pCO2 level, arsenic treatment, and duration of experiment (i.e., ST, MT, and LT) were the fixed factors. The global model considered all fixed factors in full interaction. Biological replicates were nested into treatments as random factors. Model fitting and selection started with the most complex (i.e., the global) model. Model selection was carried out as described above (Fig. 2, Tables S10, S11, Dataset S3).
Values are displayed as means and ±1 SEM of triplicate cultures and that have the same letter are not significantly different (p > 0.05). Individual samples are superimposed (as shapes) on the means. Blue for 400 µatm-selected lineages, green for 1000 µatm-selected lineages, and red for 2000 µatm-selected lineages. The opacity indicates the length of the experiment, with the palest shade for the shortest, and the darkest shade for the longest duration of the experiment.
Reciprocal transplant data analysis
To analyse the correlated responses of elevated pCO2-evolved samples (e.g., LT 1000 µatm evolved samples measured at 400 µatm compared to LT 400 µatm evolved samples measured at 400 µatm), we first calculated all growth rates and intracellular arsenic concentrations respectively as relative to the non-arsenic “control” measured and evolved at 400 µatm. These values (i.e., either relative growth or intracellular arsenic concentrations) are our response variables. Species, pCO2 level, arsenic treatment, and duration of experiment (i.e., ST, MT, and LT), were fixed factors in full interaction. Nested biological replicates were used as random factors. Model selection and post hoc analyses then proceeded as described above.
Details on the analyses of the shape of the curve of growth rate as a function of assay pCO2 can be found in the supporting information (Fig. 3, Tables S12–S15, Dataset S4).
Analysis of changes in metabolic traits in response to elevated pCO2 and arsenic treatments
In order to examine how metabolic traits changed in response to elevated pCO2, and the interaction of these responses under the addition of arsenic exposure in the laboratory and in outdoor mesococms, all phenotype data (i.e., gross and net photosynthesis, respiration, and carbon-use efficiency) were normalized to the respective “control” phenotype data, that is samples measured and evolved at 400 µatm pCO2 with no added arsenic. Building and selecting the models then proceeded via the same packages and criteria as described above (Fig. 4, Tables S16, S17, Dataset S5).
All values are calculated as relative to trait values of 400 µatm pCO2 selected samples assayed at 400 µatm pCO2. Data points above the dashed line thus indicate upregulation of CUE relative to of 400 µatm pCO2 selected samples assayed at 400 µatm pCO2. Values are displayed as means and ±1 SEM of triplicate cultures and that have the same letter are not significantly different (p > 0.05). Individual samples are superimposed on the means. Blue for 400 µatm-selected lineages, green for 1000 µatm-selected lineages, and red for 2000 µatm-selected lineages. Shapes denote the diatom species, with squares for P. tricornutum, circles for T. pseudonana, and triangles for C. muelleri.
Results and discussion
Consistent with previous findings on AsV in sediments [36], we confirmed that elevated pCO2 neither changed the valence state of arsenate (AsV), nor altered arsenate concentration in the seawater culture medium without diatoms (Table S2). In the AsIII assay, although <5% of AsIII transformed into AsV by the end of the experiment, there was no significant variation among different pCO2 treatments (Table S3). Therefore, any reduction in intracellular arsenic or increased resilience of marine diatoms under elevated pCO2 to arsenic exposure reported here must be biologically mediated.
Growth rate responses to arsenic exposure are modulated by the pCO2 selection regime
All diatom lineages from elevated-pCO2 regimes had higher growth rates than lineages from the ambient-pCO2 treatment (400 μatm) (Fig. 1, effect of pCO2, F2,208 = 652.05, p < 0.0001, details in Tables S4, S5 and Dataset S2). These findings are in line with previous studies indicating that selection under elevated pCO2 can lead to increased growth rates in diatom species [19, 37, 38]. As the experiment continued, the initial effect of elevated pCO2 leveled out and resulted in slightly lower growth rates at LT (long-term) than at MT (mid-term) or ST (short-term) (effect of time, F2,6 = 14.39, p < 0.01, details in Tables S6, S7). As growth rates also decreased to the same amount in the 400 µatm-selected cultures, this is likely an effect induced by adaptation to the laboratory setting, and potentially exacerbated by fast growth not always being the best strategy in a nutrient replete environment [39].
When samples were assayed under exposure to high levels of AsIII or AsV, arsenic species affected growth rate in interaction with pCO2 (effect of arsenic treatment × pCO2, F4,208 = 119.01, p < 0.0001, details in Tables S8, S9)—samples that had grown under elevated pCO2 were less sensitive to arsenic. While the strength of the response varied between species, the general direction did not, pointing towards the response being conserved at least within these diatoms from coastal waters. Growth rates were reduced significantly under high AsIII or AsV compared to the respective control (p < 0.05, pairwise comparison LSD’s post hoc test), owing to the intrinsic toxicity of arsenic compounds [7]. However, for samples evolved under elevated pCO2, the drop in growth rate relative to the same lineage assayed under control conditions (i.e., no arsenic added) was less pronounced as the selection pCO2 increased. Under high AsIII or AsV, growth rates were significantly ameliorated when samples had spent at least 30 days (~30 generations) at elevated pCO2.
Previous studies have shown that co-occurring bacteria can take up and metabolize arsenic, and even grow on arsenic as a substrate [40,41,42]. It is therefore possible that growth of the bacterial part of the holo-biont was also affected by arsenic addition and equally that bacterial growth or their ability to reduce arsenate may have influenced the results found in the focal diatom species to some degree. Although we took great care to avoid bacterial contamination of the cultures, axenic cultures could not be maintained during the length of experiment, as is common when phytoplankton are separated from their bacterial co-habitants [43]. However, via regular determination under the microscope and flow cytometry, we found that co-occurring bacteria contributed less than 1% of total biomass throughout, with no systematic variation across different treatments, suggesting that effects of co-occurring bacteria metabolism was small and similar across treatments.
Our findings add to the growing body of evidence that adaptive evolution of marine diatoms under elevated pCO2 would increase their resilience to further environmental deterioration [1, 20]. But can the observed changes in growth rate be explained mechanistically by the increased resilience of marine diatoms to arsenic exposure?
Intracellular arsenic content decreases in elevated pCO2-selected samples
Studies have documented that elevated pCO2 does not only directly affect primary producers, but also changes the distribution, speciation and bioavailability of organic and inorganic trace metals and will therefore modify their interaction with organisms [44]. Bautista-Chamizo et al. [45] for instance, demonstrated that lower pH increased zinc (Zn) toxicity in the marine microalga Pleurochrysis roscoffensis. Zhang et al. [46] found that OA reduced cadmium (Cd) accumulation and thus its toxicity in the diatom P. tricornutum. However, no study is available on the effect of elevated pCO2 on the fate of arsenic in diatoms. Here, we measured intracellular arsenic (Fig. 2) as well as its speciation (Fig. S6) to test whether samples evolved under elevated pCO2 accumulated the same amount of intracellular arsenic but showed reduced sensitivity, or were able to reduce the amount of intracellular arsenic. Samples cultured and assayed under elevated pCO2 (1000 and 2000 μatm) consistently had reduced intracellular arsenic concentrations ([As]intra) (Fig. 2, effect of pCO2, F2,358 = 288.41, p < 0.0001, details Tables S10, S11, Dataset S3). As the selection experiment proceeded, the magnitude of the response of [As]intra to elevated pCO2 decreased (Fig. 2, effect of time, F2,6 = 27.10, p < 0.0001, Tables S10, S11). Again, this is largely attributable to evolutionary responses under laboratory conditions, as the same trend was found in the control samples. The amount of intracellular arsenic did not scale with cell diameter or volume, both of which decreased slightly throughout the experiment (Fig. S7). Four arsenic forms including AsIII, AsV, MMA, and DMA were found within algal cells, which accounted for >90% of total arsenic (Fig. S6). With pCO2 increasing from 400 to 1000 and 2000 μatm (across treatments), the proportion of the less toxic organic DMA significantly increased (p < 0.05, pairwise comparison LSD’s post hoc test) across three diatom species tested here.
Poor growth of high pCO2 adapted cells at ambient pCO2
To determine whether samples had adapted to the high pCO2 conditions, we measured growth rates of long-term selected samples from the 400, 1000, and 2000 µatm pCO2 treatments in a reciprocal transplant assay (10 days) at the three pCO2 levels used throughout the experiments (400, 1000 and 2000 μatm). Here, growth rates increased with pCO2 regardless of the evolutionary history of the samples, the diatom species used, or whether or not arsenic compounds had been added (Fig. 3, effect of assay pCO2, F2,208 = 277.51, p < 0.0001, see also Tables S12, S13, Dataset S4). However, the addition of arsenic modulated the shape of the curve (Fig. 3, Tables S14, S15).
For ambient pCO2 lineages, our data show that mitigating effects of elevated pCO2 occur on assay time-scales as short as 10 days (corresponding to 13, 11, and 6 generations for P. tricornutum, T. pseudonana, and C. mulleri, respectively), i.e., on time-scales short enough that they do not necessarily require evolutionary responses [1]. Samples from the long-term treatment at elevated pCO2 performed poorly upon being transplanted back into ambient pCO2 conditions, with slower growth than ambient selected lineages at ambient pCO2. This pattern is indicative of and typical for evolutionary responses in a high pCO2 world and has been described for numerous green algae, spanning those with and without carbon concentrating mechanisms [47, 48]. Previous studies suggest that the long-term selected lineages under elevated pCO2 had less efficient carbon concentrating mechanisms (either through evolutionary or regulatory processes), or a higher per-cell requirement for inorganic carbon [47]. Either scenario—decreased affinity for inorganic carbon or higher demand of carbon to maintain cellular function—resulted in decreased growth, which may easily be exacerbated in a genuinely stressful (i.e., high arsenic) environment, relative to a merely sub-ideal (i.e., ambient pCO2) environment [47, 49]. In line with these findings, algal growth was the lowest under high AsIII exposure in high pCO2-selected samples transplanted back into ambient pCO2.
The underlying mechanism for the improved resilience of diatoms under elevated pCO2 may stem from the reduced energy requirement of carbon concentration mechanisms (CCMs) under elevated pCO2 [50]. Here, we provided further evidence that the increased resilience of diatoms under elevated pCO2 was linked to changes in metabolic traits (Figs. 4, S8–S11), e.g., stronger upregulation of photosynthesis than respiration resulting in higher carbon-use efficiency (Fig. S11). The high activity of both chloroplast and mitochondrial ATP synthase (Fig. S12) under elevated pCO2 may be one way in which ATP-demanding processes within the chloroplast can be sustained to support the higher rates of photosynthesis.
Effects of pCO2 and arsenic on carbon-use efficiency
Most of what is known about phytoplankton responses to OA has been obtained from relatively short-term, and hand full of longer term, laboratory experiments which usually use simplified versions of the natural environments such as ideal light and temperature levels [51]. Although these studies can pin point potential responses to changing seawater carbonate chemistry per se, the results are difficult to extrapolate to natural, dynamic environments. More recent studies consider mesocosms to be a powerful tool to maintain a relatively complex community, which take into account more aspects of “the real world” [52, 53]. To assess the responses of long-term high-pCO2 selected diatoms to arsenic in a more natural setting, we set up outdoor experiments. The results obtained in the outdoor experiments were comparable with those obtained at the end of the laboratory selection experiments (Figs. S13, S14). That is, the responses to pCO2 selection were maintained when samples were transplanted into an environment that was more variable with regards to light and temperature, and growth was faster in elevated pCO2 selected samples than in ambient pCO2 selected samples (Fig. S13a). This difference in growth rates was underpinned by a higher carbon-use efficiency (CUE) in elevated pCO2 evolved samples (Fig. 4, Tables S16, S17, Dataset S5), brought about by an increase in gross photosynthesis relative to dark respiration (Fig. S14). As in the laboratory experiment, intracellular arsenic concentration decreased with elevated pCO2 (Fig. S13b).
Transfer of arsenic from diatom to higher trophic level
Arsenic contamination in marine ecosystems can cause harmful impacts on human health as arsenic can accumulate through the food chain [54]. A wealth of studies has shown that arsenic can be transferred from phytoplankton to zooplankton, bivalves, or fish [55]. Although it has been shown that OA would impact the bioavailability and speciation of heavy metals in aquatic ecosystem, few studies focused on the trophic transfer of arsenic contamination. To test whether the altered arsenic accumulation in diatoms would have impacts on trophic transfer, we fed the scallop P. yessoensis with the diatoms which had been selected under ambient or elevated pCO2 and pre-exposed with different levels of arsenic. We found that the toxic arsenic could be transferred up in food chain from diatoms to scallops: When fed with the elevated pCO2 selected diatoms, the scallops fed on high pCO2 diatoms had significantly lower (3.26–52.83%) arsenic content than those fed on ambient pCO2 diatoms (Fig. 5a). There were significant relationships between the intracellular arsenic concentration in P. tricornutum (R2 = 0.89, p < 0.05), T. pseudonana (R2 = 0.92, p < 0.05), or C. mulleri (R2 = 0.76, p < 0.05) and its tissue content in P. yessoensis, respectively (Fig. 5b). We speculate that the altered arsenic transfer across trophic levels would affect arsenic biogeochemical cycles in marine ecosystem in a high pCO2 world [54].
a Changes in arsenic concentration in scallop of P. yessoensis fed with P. tricornutum, T. pseudonana, and C. mulleri. b Linear regression analysis between arsenic concentration in diatom and that in P. yessoensis. It showed that arsenic could be transferred from diatom to its predator of P. yessoensis. Colored symbols show the average arsenic concentration of ten biological replicates (±SE) fed with diatoms and that have the same letter are not significantly different (p > 0.05).
Effects of elevated pCO2 and arsenic exposure are reflected on the transcription level of P. tricornutum
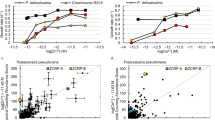
Toxicological transcriptomics provides an important tool to explore the molecular mechanisms of organisms’ responses to environmental change [56]. When the coastal diatom P. tricornutum was exposed to high AsIII (at IC50, 96 h concentration of 22.75 μmol L−1) or AsV (at IC50, 96 h concentration of 30 μmol L−1) individually using ambient (400 μatm, ambient pCO2, AC) and elevated pCO2 (1000 μatm, elevated pCO2, EC) selected P. tricornutum, significant differences were found in the transcriptomics, yielding a total of 2189 DEGs (Fig. S15a) and obtaining eight clusters (Fig. S15b). Based on the gene expression profile (Fig. 6a, d, and confirmed by RT-qPCR in Fig. S16, Tables S18, S19) and physiological response (Fig. 6b, e), we hypothesized that the elevated pCO2 selected P. tricornutum expressed a specific arsenic detoxification strategy (Fig. 6c, f). As suggested in Fig. 6a (exposed to AsIII) and Fig. 6d (exposed to AsV), AsIII efflux transporters (ACR3 and ArsB) associated with ArsA, an ATPase, were significantly up-regulated under elevated pCO2, potentially to purge harmful AsIII from the cell [13]. As a result, arsenic efflux rate was increased under elevated pCO2 in both AsIII and AsV exposure scenarios (Fig. S17). Inside the cells, under AsIII exposure, gamma glutamylcysteine synthetase (γ-ECs) and glutamine synthase (GS), and phytochelatin synthase (PCs) appeared to be more stimulated under elevated than ambient pCO2 in protein activity (Fig. 6b, e) to synthesize glutathione (GSH) and phytochelatins (PCn) to chelate intracellular arsenic and thus reduce toxicity. Studies have provided strong evidences that the complexation of intracellular arsenite by glutathione (GSH) and phytochelatins (PCn) is an important mechanism of As detoxification in plants and microbes [14, 57]. Then, GSH may form a complex with AsIII as (GSH)3-AsIII, and PCn could chelate with MMA or DMA as AsIII-PCn, which can then be sequestrated in the vacuole via MPR and HMT1, respectively [14]. Phytochelatin synthase (PCs) was downregulated either in gene expression or protein activity when comparing ambient and elevated pCO2 evolved samples under AsV exposure. We further speculate that GSH complexation might be the main detoxification way under AsV exposure. The downregulated CCM and increased ATPase activity in the chloroplasts for photophosphorylation and mitochondria for oxidative phosphorylation under elevated CO2 would be a potential pathway to produce more ATP in order to meet the energy requirement to detoxify arsenic exposure stress under elevated carbon (Fig. S12).
Variation of gene expression (a, d), enzyme activity (b, f), and metabolic pathway (c, d) under high AsIII (a, b, c) or AsV (d, e, f) treatment by comparison between ambient (AC, 400 μatm) and elevated pCO2 (EC, 1000 μatm) selected samples. NIP aquaporin-like protein, SIT silicon transporter, PHT phosphate transporter, ACR3 arsenical resistance protein, ArsA arsenical pump ATPase, ArsB arsenic efflux transporter, ArsH organoarsenical oxidase, ArsM AsIII S-adenosylmethionine (SAM) methyltransferases, MRP multidrug resistance-associated protein, ABC superfamily, HMT1 heavy Metal Tolerance 1, ABC superfamily, CA carbonic anhydrase involved in CCM, γ-ECs gamma glutamylcysteine synthetase, GS glutamine synthetase, PCs phytochelatin synthase. The red and green symbols represent up and downregulated genes or processes, respectively. *(p value < 0.05) represent significant differences between ambient (400 μatm) or elevated (1000 μatm) pCO2 selected samples.
Diatoms are unique amongst the phytoplankton in their silica biomineralization to produce silica cell walls [58] and act as an effective pH buffer for the activity of the extracellular carbonic anhydrase [59]. A previous study showed that OA diminished diatom silica production via increasing silica efflux from cell in the Southern Ocean. Due to structural similarities between arsenite and silicate, they could share the same SIT transporter to transport As and Si [14, 16]. Although there were none consistent variation trends for the SIT expression under different pCO2 via transcriptome sequencing (downregulated under high pCO2) and RT-qPCR (none-significant variation), we speculate that the OA affect both silicate and arsenic metabolism together.
Conclusion
Our results provide both quantitative and mechanistic insights of the pathways that may allow diatoms selected under elevated pCO2 to cope with a sudden, drastic drop in environmental quality, specifically, with a sudden increase in inorganic arsenic compounds. The extent to which carbon enrichment helped to counteract toxic effects of arsenic compounds depended on the pCO2 level (1000 or 2000 µatm), the arsenic compound (arsenite or arsenate), the duration of the experiment (short term, mid-term, or long-term), and to a much smaller degree, the diatom species.
Responses of phytoplankton to OA depend on the timescale of the experiment relative to the organism’s generation time [60, 61]. Microorganisms with a high standing genetic diversity, short generation times, and large population size have a high potential to rapidly evolve in a changing or changed environment [61,62,63]. As the stock cultures of experimental diatom were not clonal, responses to environmental conditions likely represent a mixture of rapid within-species sorting and de novo mutation. However, gamete formation was very rare during our selection period. We found that although elevated pCO2 initially leads to faster growth rates and reduced arsenic accumulation after short-term acclimation, the magnitude of the response decreased with time. Such differences between short-term and long-term responses may arise from counter gradient variation whereby rapid changes in physiological traits under short-term acclimation are not attributed to genetic change and long-term evolution could minimize or oppose phenotypic change to the varied environmental gradient [33, 64,65,66].
Transcriptomic and chemical data show that the long-term selected diatoms under elevated pCO2 were able to respond to arsenic exposure via a specific arsenic detoxification strategy, indicating phenotypic trait responses to OA resulting from genetic influences [64]. These changes in arsenic metabolism in a primary producer as well as its trophic transfer in food chain (Fig. 5) may impact marine biogeochemical cycling of arsenic under elevated pCO2. While these responses are potentially conserved within diatoms from coastal areas, care must now be taken to extrapolate these findings to other phytoplankton, e.g., those without CCMs or silicon transporters, and those that do not usually find themselves in nutrient-rich and potentially highly contaminated coastal regions.
References
Valenzuela JJ, de Lomana ALG, Lee A, Armbrust EV, Orellana MV, Baliga NS. Ocean acidification conditions increase resilience of marine diatoms. Nat Commun. 2018;9:2328.
Ivanina AV, Sokolova IM. Interactive effects of metal pollution and ocean acidification on physiology of marine organisms. Curr Zool. 2015;61:653–68.
Stockdale A, Tipping E, Lofts S, Mortimer RJ. The effect of ocean acidification on organic and inorganic of trace metals. Environ Sci Technol. 2016;50:1906–13.
Murcott S. Arsenic contamination in the world. London, New York: IWA publishing; 2012.
Pan K, Wang WX. Trace metal contamination in estuarine and coastal environments in China. Sci Total Environ. 2012;421:3–16.
NBSC. National Bureau of Statistics of China. Beijing: China Statistical Yearbook; 2001–2009.
Nordstrom DK. Worldwide occurrences of arsenic in ground water. Science. 2002;296:2143–5.
Cutter GA, Cutter LS, Featherstone AM, Lohrenz SE. Antimony and arsenic biogeochemistry in the western Atlantic Ocean. Deep-Sea Res Pt II. 2001;48:2895–915.
Yunus SM, Hamzah Z, Wood AKH, Saat A. Natural radionuclides and heavy metals pollution in seawater at kuala langat coastal area. Malays J Anal Sci. 2015;19:766–74.
Sanders JG. Role of marine phytoplankton in determining the chemical speciation and biogeochemical cycling of arsenic. Can J Fish Aquat Sci. 1983;40:192–6.
Wang Y, Zhang C, Zheng Y, Ge Y. Phytochelatin synthesis in Dunaliella salina induced by arsenite and arsenate under various phosphate regimes. Ecotox Environ Safe. 2017;136:150–60.
Fru EC, Arvestål E, Callac N, El Albani A, Kilias S, Argyraki A, et al. Arsenic stress after the Proterozoic glaciations. Sci Rep. 2015;5:17789.
Saunders JK, Rocap G. Genomic potential for arsenic efflux and methylation varies among global Prochlorococcus populations. ISME J. 2016;10:197–209.
Zhao FJ, Ma JF, Meharg AA, McGrath SP. Arsenic uptake and metabolism in plants. N Phytol. 2009;181:777–94.
Ye J, Rensing C, Rosen BP, Zhu YG. Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. 2012;17:155–62.
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, et al. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA. 2008;105:9931–5.
Nelson DM, Tréguer P, Brzezinski MA, Leynaert A, Quéguiner B. Production and dissolution of biogenic silica in the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob Biogeochem Cycles. 1995;9:359.
Crawfurd KJ, Raven JA, Wheeler GL, Baxter EJ, Joint I. The response of Thalassiosira pseudonana to long-term exposure to increased CO2 and decreased pH. PloS ONE. 2011;6:e26695.
Wu Y, Campbell DA, Irwin AJ, Suggett DJ, Finkel ZV. Ocean acidification enhances the growth rate of larger diatoms. Limnol Oceanogr. 2014;59:1027–34.
Domingues RB, Guerra CC, Barbosa AB, Brotas V, Galvão HM. Effects of ultraviolet radiation and CO2 increase on winter phytoplankton assemblages in a temperate coastal lagoon. J Plankton Res. 2014;36:672–84.
Heydarizadeh P, Boureba W, Zahedi M, Huang B, Moreau B, Lukomska E, et al. Response of CO2-starved diatom Phaeodactylum tricornutum to light intensity transition. Philos Trans R Soc B. 2017;372:20160396.
Heydarizadeh P, Veidl B, Huang B, Lukomska E, Wielgosz-Collin G, Couzinet-Mossion A, et al. Carbon orientation in the diatom Phaeodactylum tricornutum: the effects of carbon limitation and photon flux density. Front Plant Sci. 2019;10:471.
Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam N, et al. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science. 2004;459:79–86.
Armbrust EV. The life of diatoms in the world’s oceans. Nature. 2009;459:185–92.
Rastogi A, Vieira FRJ, Deton-Cabanillas A, Veluchamy A, Cantrel C, Wang G, et al. A genomics approach reveals the global genetic polymorphism, structure, and functional diversity of ten accessions of the marine model diatom Phaeodactylum tricornutum. ISME J. 2020;14:347–63.
Liang C, Zhang Y, Wang L, Shi L, Xu D, Zhang X, et al. Features of metabolic regulation revealed by transcriptomic adaptions driven by long‐term elevated pCO2 in Chaetoceros muelleri. Phycol Res. 2020;68:236–48.
Uddin S, Bebhehani M, Al-Musallam L, Kumar VV, Sajid S. Po uptake in microalgae at different seawater pH: An experimental study simulating ocean acidification. Mar Pollut Bull. 2020;151:110844.
Pierrot D, Lewis E, Wallace D. MS Excel program developed for CO2 system calculations ORNL/CDIAC-105a. Oak Ridge, Tennessee: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy; 2006.
Karadjova IB, Slaveykova VI, Tsalev DL. The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater. Aquat Toxicol. 2008;87:264e271.
Hirata S, Toshimitsu H. Determination of arsenic species and arsenosugars in marine samples by HPLC-ICP-MS. Appl Organomet Chem. 2007;21:447–54.
Yan Y, Ye J, Xue XM, Zhu YG. Arsenic demethylation by a C·As lyase in cyanobacterium nostoc sp.PCC 7120. Environ Sci Technol. 2015;49:14350–8.
Guo YQ, Xue XM, Yan Y, Zhu YG, Yang GD, Ye J, et al. Arsenic methylation by an arsenite s-adenosylmethionine methyltransferase from spirulina platensis. J Environ Sci. 2016;49:162–8.
Schaum CE, Buckling A, Smirnoff N, Studholme DJ, Yvon-Durocher G. Environmental fluctuations accelerate molecular evolution of thermal tolerance in a marine diatom. Nat Commun. 2018;9:1719.
Li P, Pan Y, Fang Y, Du M, Pei F, Shen F, et al. Concentrations and health risks of inorganic arsenic and methylmercury in shellfish from typical coastal cities in China: a simultaneous analytical method study. Food Chem. 2019;278:587–92.
R Core Development Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. https://www.R-project.org/.
de Orte MR, Sarmiento AM, Basallote MD, Rodríguez-Romero A, Riba I. Effects on the mobility of metals from acidification caused by possible CO2 leakage from sub-seabed geological formations. Sci Total Environ. 2014;470:356–63.
Qu P, Fu FX, Hutchins DA. Responses of the large centric diatom Coscinodiscus sp to interactions between warming, elevated CO2, and nitrate availability. Limnol Oceanogr. 2018;63:1407–24.
Zhu Z, Qu P, Gale J, Fu FX, Hutchins DA. Individual and interactive effects of warming and CO2 on Pseudo-nitzschia subcurvata and Phaeocystis antarctica, two dominant phytoplankton from the Ross Sea, Antarctica. Biogeosciences. 2017;14:1–15.
Collins S. Growth rate evolution in improved environments under Prodigal Son dynamics. Evol Appl. 2016;9:1179–88.
Amin SA, Hmelo LR, Van Tol HM, Durham BP, Carlson LT, Heal K, et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature. 2015;522:98–101.
Amin SA, Parker MS, Armbrust EV. Interactions between diatoms and bacteria. Microbiol Mol Biol Rev. 2012;76:667–84.
Zhu YG, Xue XM, Kappler A, Rosen BP, Meharg AA. Linking genes to microbial biogeochemical cycling: lessons from arsenic. Environ Sci Technol. 2017;51:7326–39.
Lefebvre SC, Benner I, Stillman JH, Parker AE, Drake MK, Rossignol PE, et al. Nitrogen source and pCO2 synergistically affect carbon allocation, growth and morphology of the coccolithophore Emiliania huxleyi: potential implications of ocean acidification for the carbon cycle. Glob Change Biol. 2012;18:493–503.
MillerO FJ, Woosley R, Ditrolio B, Waters J. Effect of ocean acidification on the speciation of metals in seawater. Oceanography. 2009;22:72–85.
Bautista-Chamizo E, De Orte MR, DelValls TA, Riba I. Simulating CO2 leakages from CCS to determine Zn toxicity using the marine microalgae Pleurochrysis roscoffensis. Chemosphere. 2016;144:955–65.
Zhang XS, Xu D, Huang SJ, Wang SH, Han WT, Liang CW, et al. The effect of elevated pCO2 on cadmium resistance of a globally important diatom. J Hazard Mater. 2020;396:122749.
Collins S, Bell G. Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature. 2004;431:566–9.
Schaum CE, Collins S. Plasticity predicts evolution in a marine alga. Proc Biol Sci. 2014;281:20141486.
Spalding MH, Van K, Wang Y, Nakamura Y. Acclimation of Chlamydomonas to changing carbon availability. Funct Plant Biol. 2002;29:221–30.
Raven JA, Giordano M, Beardall J, Maberly SC. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth Res. 2011;109:281–96.
IGBP, IOC, SCOR. Ocean acidification summary for policymakers—third symposium on the ocean in a high-CO2 world. Stockholm, Sweden: International Geosphere-Biosphere Programme; 2013.
Liu NN, Tong SY, Yi XQ, Li Y, Li ZZ, Miao HB, et al. Carbon assimilation and losses during an ocean acidification mesocosm experiment, with special reference to algal blooms. Mar Environ Res. 2017;129:229–35.
D’Amario B, Pérez C, Grelaud M, Pitta P, Krasakopoulou E, Ziveri P. Coccolithophore community response to ocean acidification and warming in the Eastern Mediterranean Sea: results from a mesocosm experiment. Sci Rep. 2020;10:1–14.
Hussain MM, Wang J, Bibi I, Shahid M, Niazi NK, Iqbal J, et al. Arsenic speciation and biotransformation pathways in the aquatic ecosystem: the significance of algae. J Hazard Mater. 2020;403:124027.
Huang JH. Arsenic trophodynamics along the food chains/webs of different ecosystems: a review. Chem Ecol. 2016;32:803–28.
Heijne WH, Kienhuis AS, Van Ommen B, Stierum RH, Groten JP. Systems toxicology: applications of toxicogenomics, transcriptomics, proteomics and metabolomics in toxicology. Expert Rev Proteomic. 2005;2:767–80.
Tsai SL, Singh S, Chen W. Arsenic metabolism by microbes in nature and the impact on arsenic remediation. Curr Opin Biotech. 2009;20:659–67.
Petrou K, Baker KG, Nielsen DA, Hancock AM, Schulz GK, Davidson AT. Acidification diminishes diatom silica production in the Southern Ocean. Nat Clim Change. 2019;9:781–6.
Milligan AJ, Morel FM. A proton buffering role for silica in diatoms. Science. 2002;297:1848–50.
Collins S, Rost B, Rynearson TA. Evolutionary potential of marine phytoplankton under ocean acidification. Evol Appl. 2014;7:140–55.
Reusch TBH, Boyd PW. Experimental evolution meets marine phytoplankton. Evolution. 2013;67:1849–59.
Hutchins DA, Fu FX. Microorganisms and ocean global change. Nat Microbiol. 2017;2:17508.
Lohbeck KT, Riebesell U, Reusch TBH. Adaptive evolution of a key phytoplankton species to ocean acidification. Nat Geosci. 2012;5:346–51.
Conover DO, Schultz ET. Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol Evol. 1995;10:248–52.
Griffin KL, Anderson OR, Gastrich MD, Lewis JD, Lin G, Schuster W, et al. Plant growth in elevated CO2 alters mitochondrial number and chloroplast fine structure. Proc Natl Acad Sci USA. 2001;98:2473–8.
Sültemeyer D. Carbonic anhydrase in eukaryotic algae: characterization, regulation, and possible function during photosynthesis. Can J Bot. 1998;76:962–72.
Acknowledgements
This work was supported by National key research and development program of China (2018YFD0900703), National Natural Science Foundation of China (41976110); Central Public-interest Scientific Institution Basal Research Fund, YSFRI, CAFS (20603022019006, 2020TD27); Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (NO. 2018SDKJ0406-3); Major Scientific and Technological Innovation Project of Shandong Provincial Key Research and Development Program (2019JZZY020706); Financial Fund of the Ministry of Agriculture and Rural Affairs, P.R. of China (NFZX2018); China Agriculture Research System (CARS-50); Taishan Scholars Funding and Talent Projects of Distinguished Scientific Scholars in Agriculture; Shandong Provincial Natural Science Foundation, China (ZR2017MD025); U.S. National Science Foundation grants (OCE 1638804, OCE 1538525).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, D., Schaum, CE., Li, B. et al. Acclimation and adaptation to elevated pCO2 increase arsenic resilience in marine diatoms. ISME J 15, 1599–1613 (2021). https://doi.org/10.1038/s41396-020-00873-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-020-00873-y
This article is cited by
-
Phytoplankton community shift in response to experimental Cu addition at the elevated CO2 levels (Arabian Sea, winter monsoon)
Environmental Science and Pollution Research (2023)