Abstract
Introduction
Spinal hemangiomas are benign vascular tumors that most commonly originate from the osseous structures of the spinal column. Epidural spinal hemangiomas without osseous involvement are uncommon and are classified as pure epidural spinal hemangiomas. Extraosseous spinal epidural cavernous hemangiomas are rarely described and among available reports; most patients present with slowly progressive neurological symptoms. Herein, we present a novel case of acute neurological dysfunction from a pure spinal epidural hemangioma that was managed through surgical resection with good neurological recovery at follow-up.
Case presentation
A 45-year-old previously healthy man presented to the emergency room with sudden inability to ambulate and was found to have bilateral lower extremity weakness. Magnetic resonance imaging of the spine demonstrated an epidural mass extending out of the right T5/6 neural foramen. The mass enhanced heterogeneously, and the preoperative diagnosis favored an atypical schwannoma. The lesion was surgically removed en-bloc through a midline posterior decompression with instrumentation. Histopathologic examination confirmed cavernous hemangioma pathology. Within 6 weeks of the surgical intervention, the patient had regained full sensorimotor function and these effects were durable through long term follow-up.
Discussion
Pure spinal epidural hemangiomas are rare and generally have an insidious clinical course. This case report highlights that these uncommon lesions may present with substantial and acute neurological dysfunction requiring urgent neurosurgical intervention. This should prompt clinicians to consider cavernous hemangioma in the differential diagnosis of patients presenting with acute neurological deterioration and an epidural spinal tumor.
Similar content being viewed by others
Introduction
Cavernous hemangiomas are vascular lesions of the microcirculation that may be distributed throughout the central nervous system among other body systems. Within the nervous system, these lesions are typically encountered in the intracranial compartment, though they do comprise ~4% of spinal epidural tumors [1, 2]. Spinal cavernous hemangiomas are most commonly found arising from vertebrae and they have a predilection to involve the ventral epidural space [2]. Purely extraosseous spinal hemangiomas are a rare subset and are termed pure spinal hemangiomas. There are limited series of individual cases detailing the presentation, diagnosis, and management of pure spinal epidural cavernous hemangiomas; within these reports acute neurological presentation is not commonly described [2,3,4,5,6,7,8]. Current epidemiological estimates are limited to case reports and case series; in available literature these lesions are described in the thoracic spine in over 60% of cases followed by both cervical and lumbosacral regions [1,2,3,4,5,6,7,8]. The typical symptom trajectory from available literature appears to favor a chronic course with low risk of acute neurological deterioration [2, 8, 9]. Given the increased utilization of spinal magnetic resonance imaging (MRI), the frequency of encountering incidental and symptomatic spinal hemangiomas will increase and therefore an understanding of the natural history and spectrum of presentations is paramount to effective patient counseling. This report contributes to our understanding of spinal cavernous hemangioma presentation and management. Herein, we report an unusual case of acute onset neurological dysfunction secondary to a pure epidural spinal hemangioma at the T5/6 level as well as the surgical approach employed and results of long-term follow-up.
Case presentation
A 45-year-old previously healthy man presented to the emergency department reporting the sudden onset of radiating right-sided back pain and bilateral lower extremity weakness with inability to ambulate. The neurological examination revealed an inability to overcome gravity in the testable myotomes of the right lower extremity and only antigravity strength in the myotomes of the left lower extremity consistent with an ASIA C cord injury. Sensory testing revealed bilaterally diminished sharp touch and light touch sensation in the lower extremities and right flank as well as a loss of proprioceptive sensation at the great toe and ankles bilaterally.
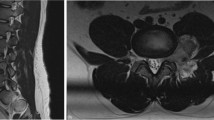
Urgent MRI of the spine showed an extradural mass located eccentric to the right in the epidural space extending laterally out of the right T5/6 neural foramen. The lesion occupied the majority of the spinal canal at this level and was associated with significant cord compression. The mass was heterogeneously T1-hypo-intense, T2-hyperintense, and enhanced heterogeneously with the administration of gadolinium. Despite severe compression of the thoracic spinal cord, there was no spinal cord T2-signal change or myelomalacia (Fig. 1). The imaging appearance was favored to represent atypical schwannoma because the lesion appeared to exit through the neural foramen and was located in the epidural space without evidence of invasion into the adjacent osseous structures.
A, B Preoperative T2 and T1 weighted MRI images. Heterogeneous T1- hypo-intense and T2 hyperintense mass lesion is located primarily in the right epidural space. There is severe compression and leftward deviation of the thoracic cord opposite T5-T6 without significant cord edema or myelomalacia. C, D Preoperative Gadolinium-DTPA administration on T1 weighted image. The lesion demonstrated a heterogeneous enhancement pattern. E 6-month post-operative T2 weighted imaging demonstrating gross-total resection of the lesion and complete radiographic resolution of cord compression.
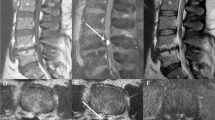
The patient was taken urgently for surgical intervention. T5/T6 laminectomies were performed revealing a large hemorrhagic appearing tumor in the epidural space causing significant leftward spinal cord deviation (Fig. 2). There was no epidural hematoma in tumor surroundings. A right sided facetectomy at T5 and T6 was performed to further expose the lateral foraminal component of the tumor. The tumor was mobilized and it appeared adherent to the right T5 nerve root. Given our presumptive diagnosis of an atypical schwannoma, we sectioned T5 nerve root medially to mobilize the tumor, however, it became apparent that the mass was simply adherent, rather than arising from, the right T5 nerve root. The tumor was resected en-bloc and intraoperative ultrasound was used to confirm there was no intradural component or remaining residual. Instrumentation was performed from T4–7.
A, B A large and hemorrhagic appearing tumor in the extradural space on the right side and causing significant left ward spinal cord deviation. T5 / T6 facetectomy was performed to expose the lateral foraminal component of the lesion. C Tumor appeared to be arising from T5 nerve root (white arrow). D, E, F Feeding and draining vessels (white arrows) were coagulated. En bloc tumor resection was achieved by sectioning T5 nerve root.
Microscopic sections showed an encapsulated mass made of densely packed blood vessels of variable size, ranging from capillaries to several hundred μm in diameter, without intervening tissue (Fig. 3A, B). Areas of recognizable streaming or palisading patterns were not identified. By immunohistochemistry there were no Sox 10 positive cells. CD 34 immunostaining highlighted the dense network of blood vessels within the lesion (Fig. 3C). The final result of histopathologic examination was cavernous hemangioma.
A, B Hematoxylin and eosin staining at low and high power demonstrated an encapsulated mass made up of densely packed blood vessels of variable size. There were no regions showing palisading architecture. C CD34 immunostaining highlights the dense network of blood vessels occupying the entire space.
On post-operative day 1, he had regained substantial strength in the lower extremities. Myotomal testing revealed full antigravity function in all myotomes and the ability to overcome light resistance. However, significant proprioceptive deficits limiting balance persisted. The remainder of his post-operative course was unremarkable, and he was discharged to an inpatient rehabilitation facility. Upon assessment in our ambulatory clinic 6 weeks following surgery he was living independently at home and had no residual lower extremity sensorimotor deficits. MRI of the spine at 6 months demonstrated gross total resection of the lesion. Follow-up data is available 1-year post-surgery at which point he had stable imaging without neurological deficits and intact sphincter function.
Discussion
Pure extraosseous spinal epidural hemangiomas are rarely described in the literature and most commonly present with insidious onset of neurological signs and symptoms [2,3,4,5,6,7,8]. The largest series to date described by Zhang et al. reported that the clinical course was uniformly chronic in all 23 of their cases with average duration over 26 months [8]. Hemosiderin staining on MRI at different chronological ages has been associated with these lesions and suggests intermittent microhemorrhage as the mechanism for this chronic stepwise phenotype. In other series, acute clinical presentation with Frankel grade A injuries have been rarely described, but despite early surgery, did not make significant functional improvement [2]. Neurological improvement following early surgery for epidural cavernous hemangioma hemorrhage has been described in children [10]. Acute symptom onset may be caused by intralesional hemorrhage, epidural hematoma, venous thrombotic occlusion or stagnation of the draining vein with congestive myelopathy [2, 10,11,12]. Though our patient had no inciting history, acute bleeding can be triggered by trauma, heavy exercise, and coagulopathy. We speculate that a spontaneous intra-tumoral hemorrhage might have been the inciting event leading to the sudden neurological deficits. Intra-tumoral bleeding likely caused acute volumetric expansion and acute symptomatic cord compression [11, 13].
There are several characteristic imaging features that help to distinguish epidural cavernous hemangiomas from other lesions. In this particular case, we considered a hemorrhagic schwannoma based on initial imaging that showed avid contrast enhancement along with the foraminal course. Schwannomas demonstrate less avid enhancement than hemangiomas and both can appear with cystic lesions as well as involve the neural foramen [2]. Schwannoma can be associated with neural foramina expansion while cavernous hemangiomas would not. Lee et al. correlated specific imaging findings with histopathological features and identified four groups of spinal cavernous hemangiomas: arteriovenous type with organized hematoma, venous type and cavernous types with or without hematoma [14]. Hemangiomas can extend to multiple levels and may exhibit a peripheral rim of low signal intensity on T1 and T2 images can suggest old hemorrhage products [2, 14, 15]. Angiolipoma and lymphoma were not considered based on lack of hyperintense T1-weighted signal and absence of T2-weighted isointensity, respectively. Our case exhibited typical imaging feature of epidural cavernous hemangioma including hyperintense signal on T2-weighted images and a homogeneous isointense signal on T1-weighted images with homogenous contrast enhancement [1, 2, 14, 15]. Digital subtraction angiography (DSA) and trans-arterial embolization for purely epidural cavernomas is not useful in because they are not typically visible [10, 16, 17]. In the case of significant flow voids on initial MRI, further DSA or MR angiography is suggested to identify possible vascular lesions.
Pre-operative neurologic condition and radiographic findings appear to be important as prognostic factors for patients with acute onset neurological symptoms. Complete surgical resection of spinal epidural cavernous hemangioma is considered the optimal treatment when feasible and in our case was associated with durable disease control at 1-year follow-up [2, 3, 8,9,10]. Given the limited understanding of disease natural history, there is no available guideline for the role of radiation for residual disease. Sohn et al. describe the use of radiotherapy at 32 Gy in four fractions for thoracic epidural cavernous hemangioma [18]. Given the vascularity of the lesion, we employed an en bloc microsurgical approach to limit intra-operative blood loss—a strategy that has previously been described [9, 12, 13]. Further studies are required to determine the optimal surgical timing; in our case a young patient presented with acute incomplete neurological deficits along with no evidence of myelomalacia on imaging and was therefore taken for emergent resection. The neurological outcome described in this report was favorable. Furthermore, we felt instrumentation was indicated given the requirement to perform a full facetectomy to unroof the foramen to access the lateral foraminal component and achieve gross total resection.
A pure extraosseous epidural cavernous hemangioma is rare tumor. Radiographic findings help to distinguish differential diagnosis. Preoperative patient condition and radiographic findings may influence postoperative neurological prognosis. The best treatment for this lesion is total resection when possible and early decompression in setting of significant acute decline. Tumor resection with instrumentation is useful if access to the neural foramen and facetectomy is required. This case adds to the body of literature describing pure epidural spinal cavernous hemangioma natural history, management and outcomes.
References
Feng J, Xu YK, Li L, Yang RM, Ye XH, Zhang N, et al. MRI diagnosis and preoperative evaluation for pure epidural cavernous hemangiomas. Neuroradiology. 2009;51:741–7.
Li TY, Xu YL, Yang J, Wang J, Wang GH. Primary spinal epidural cavernous hemangioma: clinical features and surgical outcome in 14 cases. J Neurosurg Spine. 2015;22:39–46.
Esene IN, Adhour AM, Marvin E, Nosseir M, Fayed ZY, Seoud K, et al. Pure spinal epidural cavernous hemangioma: a case series of seven cases. J Craniovertebr Junction Spine. 2016;7:176–83.
Zhong W, Huang S, Chen H, Sun H, Cai B, Liu Y, et al. Pure spinal epidural cavernous hemangioma. Acta Neurochir. 2012;154:739–45.
Jang D, Kim C, Lee SJ, Ryu YJ, Kim J. Pure spinal epidural cavernous hemangioma with intralesional hemorrhage: a rare cause of thoracic myelopathy. Korean J Spine. 2014;11:85–8.
Jo BJ, Lee SH, Chung SE, Paeng SS, Kim HS, Yoo SW, et al. Pure epidural cavernous hemangioma of the cervical spine that presented with an acute sensory deficit caused by hemorrhage. Yonsei Med J. 2006;47:877–80.
Cossandi C, Fanti A, Gerosa A, Crobeddu E, Forgnone S, Magrassi L, et al. Rare case of dumbbell-shaped spinal cavernous hemangioma and literature review. World Neurosurg. 2018;120:181–4.
Zhang L, Qiao G, Shang A, Yu X. Clinical features and long-term surgical outcomes of pure spinal epidural cavernous hemangioma-report of 23 cases. Acta Neurochir. 2020;162:2915–21.
Khalatbari MR, Abbassioun K, Amirjmshidi A. Solitary spinal epidural cavernous angioma: report of nine surgically treated cases and review of the literature. Eur Spine J. 2013;22:542–7.
Sarikaya-Seiwert S, Gierga K, Wessalowski R, Steiger HJ, Hänggi D. Solitary spinal epidural cavernous angiomas in children presenting with acute neurological symptoms caused by hemorrhage. Report of 2 cases. J Neurosurg Pediatr. 2010;5:89–93.
Floeth F, Riemenschneider M, Herdmann J. Intralesional hemorrhage and thrombosis without rupture in a pure spinal epidural cavernous angioma: a rare cause of acute lumbal radiculopathy. Eur Spine J. 2010;19:S193–6.
Meng Y, Shamji MF. Solitary spinal epidural cavernous haemangiomas as a rare cause of myelopathy. BMJ Case Rep. 2015;2015:bcr2015211644.
Caruso G, Galarza M, Borghesi I, Pozzati E, Vitale M. Acute presentation of spinal epidural cavernous angiomas: case report. Neurosurgery. 2007;60:E575–E576.
Lee JW, Cho EY, Hong SH, Chung HW, Kim JH, Chang KH, et al. Spinal epidural hemangiomas: various types of MR imaging features with histopathologic correlation. Am J Neuroradiol. 2007;28:1242–8.
Nagi S, Megdiche H, Bouzaidi K, Haouet S, Khouja N, Douira W, et al. Imaging features of spinal epidural cavernous malformations. J Neuroradiol. 2004;31:208–13.
Hasan A, Guiot MC, Torres C, Marcoux J. A case of a spinal epidural capillary hemangioma: case report. Neurosurgery. 2011;68:E850–E853.
Rahman A, Hoque SU, Bhandari PB, Abu Obaida AS. Spinal extradural cavernous haemangioma in an elderly man. BMJ Case Rep. 2012;2012:bcr2012006453.
Sohn MJ, Lee DJ, Jeon SR, Khang SK. Spinal radiosurgical treatment for thoracic epidural cavernous hemangioma presenting as radiculomyelopathy: technical case report. Neurosurgery. 2009;64:E1202–E1203.
Author information
Authors and Affiliations
Contributions
KE contributed to the literature review and manuscript preparation. ARM contributed to the literature review and manuscript preparation and editing. AKM contributed to the literature review and manuscript preparation and editing. EMH contributed to manuscript preparation and editing. DGM prepared the microscopic slide images and contributed to manuscript editing. YN contributed to the literature review and manuscript editing. JRW contributed to manuscript editing. CDW contributed to manuscript preparation and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval was not required for this study given its case report nature.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Eguchi, K., Malhotra, A.R., Malhotra, A.K. et al. Pure extraosseous spinal epidural cavernous hemangioma presenting with acute paraplegia: a case report. Spinal Cord Ser Cases 8, 63 (2022). https://doi.org/10.1038/s41394-022-00531-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-022-00531-9