Abstract
Introduction
Pregnancies are rare in patients with severely disabilitating spinal cord injuries (SCI) but increasing alongside social awareness concerning reproductive equality. Physicians should be aware of several potential complications during pregnancy and delivery, particularly autonomic dysreflexia.
Case presentation
We report a successful pregnancy of a 32-year-old woman with a severe SCI at the C2 level (C1-4 ASIA Impairment Scale grade A) and total dependency on home invasive mechanical ventilation (HIMV), an extremely rare treatment. An elective cesarean section was chosen as the delivery mode at 34 + 0 weeks of gestation. Both the mother and the child recovered well.
Discussion
Severe spinal cord injury and dependency on mechanical ventilation are not absolute contraindications for pregnancy. With careful planning, pregnancy is possible also for patients with the most severe forms of SCI. Adequate pain relief during cesarean delivery is required despite complete spinal cord injury in order to avoid excessive hemodynamic responses and spinal reflexes. A multidisciplinary team is needed to ensure safe pregnancy and delivery of these high-risk pregnancies.
Similar content being viewed by others
Introduction
Pregnancy after traumatic spinal cord injury (SCI) is less frequent compared to healthy women, but possible, as fertility is usually unaffected [1,2,3]. Existing literature on pregnancy outcomes is limited mainly to small patient series with a varying level and severity of the injury [2, 4,5,6,7,8,9,10], while higher maternal motor score is associated with higher likelihood for pregnancy [1]. In view of increasing social awareness of equality among SCI patients, clinicians may be faced with questions relating to the safety and feasibility of pregnancy even in patients suffering from the most severe forms of SCI.
Case presentation
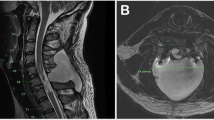
Our 32-year-old patient was hit by a car as a 9-year-old and has a traumatic atlanto-occipital dislocation with C2 spinal cord and medulla injury, resulting in a complete loss of movement and sensation below the trauma level (C1-4 ASIA Impairment Scale Grade A). She moves with an electronic wheelchair, is tracheostomized, and totally dependent on her pressure-controlled ventilator (Trilogy100®). She lives as a home-invasive-mechanically-ventilated (HIMV)-patient with a 24-h nursing team. She has marked scoliosis, hypothyreosis and a neurogenic bladder which has required multiple operations due to bladder distension-induced headaches reflecting autonomic dysreflexia [11].
During a follow-up visit at the pulmonary clinic, she informs the staff about her spontaneous pregnancy. Baclofen, tizanidine, and tolterodine were gradually stopped due to potential adverse fetal effects and thyroxin doses were elevated. Frequent follow-ups on mechanical ventilation with an overnight transcutaneous oximetry-capnographies (Sentec®) were organized and the settings of the ventilator were titrated as the pregnancy continued.
She was monitored in the obstetric department of a tertiary hospital. The first and second trimester abnormality scans were normal at 13 + 0 and 20 + 0 weeks of gestation, respectively. Fetal growth was linear at 25 + 0, 30 + 0, and 32 + 1 weeks. Vascular flows were normal without indication of placental insufficiency or fetal distress. At 25 + 0 weeks, a prophylactic low-molecular-weight heparin (dalteparin 2500 IU 1 × 1) was started. Her recurrent urinary tract infections were treated with cephalexin, pivmesilliname, and metenamine. At 25 weeks, her hemoglobin was 8.8 g/dL and ferritin level 8 μg/L, she had tiredness and shortness of breath and received 800 mg iron intravenously.
A multidisciplinary team consisting of a pulmonologist from the HIMV unit, an obstetrician specialized in fetomaternal medicine, and an anesthetist specialized in obstetric anesthesia, planned her delivery. At the last antenatal visit at 32 + 1 weeks, the plan was discussed with the patient, her husband, and the HIMV unit’s nurse in charge. The patient reported that breathing was gradually becoming more strenuous. She is of short stature, 154 cm, and weighed 38 kg prior to pregnancy. Because of her size, deformed spine, and frailty, the growing uterus was compressing her diaphragm and diminishing her residual lung capacity more than usually. Fetal weight estimate was 1900 g. There were no signs of frequent contractions, but she reported that some contractions were detected during her maternity nurse visit.
An elective cesarean section (CS) at 34 + 0 weeks was chosen as the delivery mode. Even though tetraplegic patients have been reported to deliver vaginally [4, 11, 12], CS was preferred for several reasons. First, induction of labor at 34 weeks in a primipara may be challenging and the risk of CS is in any case increased in SCI patients [2]. Second, uterine contractions while painless could still provoke autonomic dysreflexia [11, 13]. In general, neither urgent nor emergency procedures would have been possible in standard time as merely her transfer to the operating room would have required extra caution. Hence, all procedures were aimed to be performed as electively and smoothly as possible. Her reported bladder tension-related headaches were signs of autonomic dysreflexia [11, 14] and indicated that proper hemodynamic control would require adequate anesthesia for the surgery.
The timing of the delivery was chosen at 34 + 0 weeks based on her subjective feeling of emerging difficulty in breathing and lying on her back, and after weighing the risks of prematurity. Betamethasone 12 mg intramuscularly to mature the newborn’s lungs were administered on days 4 and 5 prior the CS. A room suitable for her needs was pre-organized. All plans were meticulously documented in her patient file in case of her sudden arrival earlier, which fortunately did not happen as she came to the hospital a day before the CS as planned.
As she could speak with her tracheostomy, the anesthesia plan was to keep her on her own tracheostomy and ventilator and perform the operation under spinal anesthesia, which would be continued by epidural infusion should hemodynamics require it post-operatively. A combined spinal-epidural anesthesia was planned. The adequacy of the block was to be assessed by a lack of hemodynamic and spinal reflexes to pain. The secondary plan was to use general anesthesia with propofol-remifentanil and rocuronium in the event that muscle relaxation was needed.
Throughout the pregnancy her systolic blood pressure had been ~90 mmHg and there was uncertainty regarding hemodynamic responses to anesthesia and delivery. Invasive blood pressure monitoring was planned for the CS with the aim of maintaining the systolic blood pressure at 90–110 mmHg by phenylephrine or noradrenaline. Dalteparin was paused 24 h before the operation.
The CS was performed under combined spinal-epidural anesthesia placed in left supine position as the sitting position was impossible. Placement of the iv-cannula, invasive blood pressure monitoring, and the spinal anesthesia caused marked involuntary movements in the upper and lower extremities. Spinal bupivacaine at 8 mg, fentanyl at 15 μg, and morphine at 100 μg were used for the anesthesia.
A low horizontal incision was applied, and the operation went normally, although there was blood loss of 1200 mL, for which 1 g tranexamic acid was given, but no blood products were needed. A healthy boy weighing 2020 g was born and received Apgar scores 7–3–7 at 1–5–10 min, respectively. Subarachnoidal injection of bupivacaine and opioids caused a near-instant drop in blood pressure (Fig. 1), which responded well to phenylephrine.
Invasive blood pressure (red arrows) and heart rate (blue dots) during the cesarean delivery (A) and during catheterization 18 h postoperatively after the spinal anesthesia has worn off (B). The thicker, vertical lines indicate 5-min intervals and thin lines one-minute intervals. The horizontal green bar indicates the rate of administration of phenylephrine (0.1 mg/mL) infusion.
As planned, postoperatively she was taken to the high dependency unit for the first 24 h for hemodynamic and respiratory control. She was ventilated with her own ventilator with normal pregnancy settings throughout the surgery and postoperative period. She experienced no postoperative pain and there was no need for vasoactive medications even during uterine massaging. Thereafter she stayed in the postpartal ward and was discharged on the 6th postoperative day. The newborn recovered expectedly and was discharged from the neonatal ward 3 weeks later.
Ventilator settings were gradually returned to those before pregnancy. At her follow-up visit 6 weeks after delivery all findings were normal, although breastfeeding had not succeeded. Her spasticity symptoms had returned and she had restarted her medications. Any subsequent pregnancies were also considered risk pregnancies and therefore could not be encouraged, but as this is a personal decision, they could not be definitively contradicted either.
Discussion
We report a successful pregnancy and cesarean delivery of a SCI patient totally dependent on continuous mechanical ventilation. No previous reports of pregnancies in patients with severe SCI dependent on HIMV were found. Most cases in the previous series involved either a markedly lower level of injury or partial injury with preserved motor/sensory functions [1, 2, 4,5,6,7,8,9,10].
The strengths of this report are the authors’ thorough analysis and consideration of this novel subject, careful planning of the delivery, the satisfactory recovery of both the mother and the child, and the unique experience gathered that can be shared with others. Its limitation is the size including only one patient, and the lack of similar previous reports to compare with.
Respiratory problems related to SCI may vary in severity [15]. Home invasive mechanical ventilation is an extremely rare treatment, with a prevalence of 2.0 in 100,000 in Finland in 2019 [16]; altogether there were 97 HIMV patients, 28 of whom (20%) had SCI [16]. Despite the severity of the condition of HIMV-treated SCI patients their survival is long, although only a few studies exist. A 25-year retrospective study from Britain revealed a mean survival of 10.5 years among SCI patients with HIMV, with the survival being longer for younger patients [17]. In a Finnish study, mean HIMV duration in SCI patients was 9.4 years and the treatment duration for deceased patients was 7.4 years [16]. As SCI patients are often young and otherwise healthy, they live longer and go through normal events of life, such as studying, working, and starting a family [1, 3, 9, 10].
The timing of the delivery was based on maternal well-being, which was considered to be at increasing risk with advancing pregnancy due to increasing respiratory need and reducing lung capacity. Her inability to sense contractions also raised concerns over her going into labor unknowingly [11]. It was therefore decided that her delivery should take place in a controlled fashion and at 34 weeks’ gestation. The newborn weighed 2020 g, which was only 100 g more than the last ultrasound estimate 13 days earlier, and thus markedly less than the expected normal weekly weight gain of 200–250 g. A misestimate with ultrasound is possible, but the growth had been regular and the measurements technically satisfactory. Therefore, placental malfunction in the last weeks of pregnancy is a possible explanation for growth retardation and could reflect a gradually decreasing maternal oxygen supply. This is in line with previous reports on SCI parturients’ children being more often small for gestational age [2, 18]. Hence, the timing of the delivery seemed appropriate as the newborn also recovered well. Late-preterm infants have higher morbidity compared to term infants, but generally manage without significant problems related to prematurity [19].
While our patient’s hemodynamics could be easily controlled peripartally, careful hemodynamic control is required for SCI patients since uncontrolled hypertension can cause severe morbidity or mortality in parturients [13] and the susceptibility to hypertensive responses may persist for several days [20]. Our patient showed the most marked hemodynamic effects after the slow oxytocine injection and upon urinary catheterization once the spinal anesthesia had worn off.
Developments in antenatal follow-up and care enable more women with disabilities to achieve motherhood. Pregnancies among SCI patients are increasing [1, 10], but uncertainty is experienced by both the caregivers and patients [8, 9]. A multidisciplinary team is necessary in the pre-conceptual counseling, antenatal follow-up, and delivery [1, 2, 8, 18].
Our patient had a successful pregnancy and delivery. She has read the case report and given consent to publish it. We do not expect a large series of similar cases but believe that our experience may be of use to other colleagues.
Data availability
Additional data that do not identify the patient in question are available from the corresponding author upon reasonable request.
References
Iezzoni L, Chen Y, Jackson McLain AB. Current pregnancy among women with spinal cord injury: findings from the U.S. National Spinal Cord Injury Database. Spinal Cord2015;53:821–6.
Crane DA, Doody DR, Schiff MA, Mueller BA. Pregnancy outcomes in women with spinal cord injuries: a population-based study. PM R. 2019;11:795–806.
Courtois F, Alexander M, McLain AB. Women’s sexual health and reproductive function after SCI. Top Spinal Cord Inj Rehabil. 2017;23:20–30.
Skowronski E, Hartman K. Obstetric management following traumatic tetraplegy and literature review. Aust NZ J Obstet Gynaecol. 2008;48:485–91.
Cross LL, Meythaler JM, Tuel SM, Cross AL. Pregnancy following spinal cord injury. West J Med. 1991;154:607–11.
Ghidini A, Healey A, Andreani M, Simonson MR. Pregnancy and women with spinal cord injuries. Acta Obstet Gynecol Scand. 2008;87:1006–10.
Sterling L, Keunen J, Wigdor E, Semer M, Maxwell C. Pregnancy outcomes in women with spinal cord lesions. J Obstet Gynaecol Can. 2013;35:39–43.
Bertschy S, Pannek J, Meyer T. Delivering care under uncertainty: Swiss providers’ experiences in caring for women with spinal cord injury during pregnancy and childbirth – an expert interview study. BMC Pregnancy Childbirth. 2016;16:181.
Hocaloski S, Elliott S, Hodge K, McBride K, Hamilton L, McBride CB, et al. Perinatal care for women with spinal cord injuries: a collaborative workshop for consensus on care in Canada. Top Spinal Cord Inj Rehabil. 2017;23:386–96.
Bertschy S, Schmidt M, Fiebag K, Lange U, Kues S, Kurze I, et al. Guideline for the management of pre-, intra-, and postpartum care of women with a spinal cord injury. Spinal Cord. 2020;58:449–58.
Saunders D, Yeo J. Pregnancy and Quadriplegia–the problem if autonomic dysreflexia. Aust N Z J Obstet Gynaec. 1968;8:152–4.
Robertson K, Dawood R, Ashworth F. Vaginal delivery is safely achieved in pregnancies complicated by spinal cord injury: a retrospective 25-year observational study of pregnancy outcomes in a national spinal injuries centre. BMC Pregnancy Childbirth. 2020;20:56.
Abouleish E. Hypertension in a paraplegic parturient. Anesthesiology. 1980;53:348.
Soh SH, Lee G, Joo MC. Autonomic dysreflexia during pregnancy in a woman with spinal cord injury: a case report. J Int Med Res. 2019;47:3394–9.
Berlowitz DJ, Wadsworth B, Ross J. Respiratory problems and management in people with spinal cord injury. Breathe. 2016;12:328–40.
Kotanen P, Kreivi HR, Vainionpää A, Laaksovirta H, Brander P, Siirala W, et al. Home invasive mechanical ventilation in Finland in 2015–9. ERJ Open Res. 2020;6:00223–2020.
Watt JWH, Wiredu E, Silva P, Meehan S. Survival after short- or long-term ventilation after acute spinal cord injury: a single-centre 25-year retrospective study. Spinal Cord. 2011;49:404–10.
Obstetric management of patients with spinal cord injuries. ACOG committee Opinion No. 808. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2020;135:e230–6.
Bénin A, Blanc M, Chollat C, Jarreau PH, Goffinet F, Tsatsaris V, et al. The cause of birth is associated with neonatal prognosis in late preterm singletons. J Gynecol Obstet Hum Reprod. 2020;49:101920.
Brian J, Clark RB, Quirk JG. Autonomic hyperreflexia, cesarean section and anesthesia. J Reprod Med. 1988;33:645–9.
Author information
Authors and Affiliations
Contributions
All authors (RJ, H-RK and AV) have equally contributed in the preparing and writing of the case report. All authors have approved the final version of the case report.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jernman, R., Väänänen, A. & Kreivi, HR. Successful pregnancy and cesarean delivery in a tetraplegic, home-invasively-mechanically-ventilated patient – case report. Spinal Cord Ser Cases 8, 62 (2022). https://doi.org/10.1038/s41394-022-00528-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-022-00528-4
This article is cited by
-
Multiple drugs
Reactions Weekly (2022)