Abstract
Introduction
Bone metastases confined to the posterior elements of the spine are rarely treated, as there exist no established radical surgical treatment options for this area. Herein, we present a case report of and technical note on a patient who underwent radical resection for a metastatic tumor in the thoracic spinous process.
Case presentation
A 34-year-old male presented with a nasopharyngeal carcinoma with a solitary metastatic focus in the spinous process of the 10th thoracic vertebra. Imaging revealed that the tumor was confined to the spinous process and the surrounding soft tissues. No tumor was noted in the pedicles, vertebral body, and cortical bone on the ventral side of the lamina, as well as within the spinal canal. As treatment for this solitary metastatic lesion, we decided to perform radical resection with sufficient margins that would include the involved spinous process and all surrounding soft tissues exhibiting evidence of tumor infiltration. The posterior elements of the 9th–11th vertebrae, multifidus muscles, and skin were widely resected en bloc using a T-saw. The posterior elements of the spinal column were resected at the level of pedicles without full visualization of the involved dural sac. The tumor-infiltrated soft tissues surrounding the T10 vertebral spinous process were excised without full visualization of the tumor. Adjuvant therapy was not administered postoperatively. During the second year of follow-up, no signs of recurrence or metastasis were noted.
Discussion
Our proposed technique allows wide resection of a solitary focus of metastasis in the posterior elements of the spine.
Similar content being viewed by others
Introduction
As metastatic spinal tumors favor well-vascularized cancellous bones, they often grow or settle in the central posterior part of the vertebral body [1,2,3,4]. Consequently, bone metastases confined to the posterior elements of the vertebral body are rarely treated. Only one case of a patient with rectal cancer metastasizing to a lumbar spinous process had been documented [1]. Demircay et al. [1]. performed excisional biopsy in this patient, however, they did not fully disclose their method, the extent of resection, or the patient’s postoperative course. No reproducible, curative surgical treatment for solitary metastasis in the posterior elements of the spine has been reported in the literature.
We report a rare case of a nasopharyngeal carcinoma (NPC) with a solitary metastatic focus in the spinous process of a thoracic vertebra. We resected this lesion en bloc to achieve local radical cure. This technical note describes a method for resecting tumors confined to the posterior elements of the spine. Our procedure may be performed even if the tumor being resected cannot be directly visualized. We also discuss the role of imaging in delineating the extent of resection.
Case presentation
A 34-year-old male presenting with a right neck mass and hearing loss was diagnosed with NPC (T2, N2, stage III) by an otolaryngologist. He received chemotherapy and proton-beam therapy, which resulted in complete remission after 1 year. At 2 years after the initial diagnosis, routine 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) revealed high uptake in the 10th thoracic (T10) spinous process. The patient was subsequently diagnosed with a metastatic tumor in the spinous process of the T10 vertebra and referred to our department.
Upon initial examination, the patient complained of tenderness in the area over the spinous process of the T10 vertebra. He also presented with bilateral upper- and lower- limb numbness and a taste disorder due to peripheral neuropathy from radiation and chemotherapy, but had no other neurologic findings.
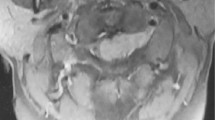
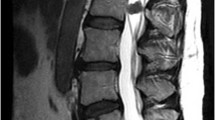
Magnetic resonance imaging (MRI) revealed a local signal change in the spinous process of the T10 vertebra, which presented as a low signal intensity on T1-weighted images and a high signal intensity on T2-weighted and gadolinium-enhanced T1-weighted images (Fig. 1a, c). Some signal changes were also observed in the soft tissues around the involved spinous process (Fig. 1a, c). No other intensity changes were noted in the pedicles or the main vertebral body. Computed tomography (CT) images revealed an osteolytic lesion in the spinous process of the T10 vertebra and tumor infiltration of the surrounding soft tissues (Fig. 1b, d). The pedicles and the vertebral body showed no signs of involvement (Fig. 1b, d). FDG-PET/CT revealed abnormal FDG uptake in the spinous process of the T10 vertebra (Fig. 1e). The standardized uptake value max was noted at 9.5 (Fig. 1e). There was no FDG accumulation in the other organs. We performed a biopsy of the lesion under local anesthesia. Histological examination revealed irregular nests of atypical cells with a high nucleocytoplasmic ratio (Fig. 2). These cells were positive for P40 and Epstein–Barr virus-encoded small RNA (Fig. 2). The pathological diagnosis was metastasis of NPC to the spinous process of the T10 vertebra.
a, c Gadolinium-enhanced T1-weighted MRI shows a local signal change in the spinous process of the T10 vertebra. The region within the dotted line indicates the extent of the tumor, as estimated by MRI. b, d CT images reveal an osteolytic lesion in the spinous process of the T10 vertebra (white arrowheads), but no lesion in the pedicles and vertebral body. e FDG-PET/CT shows abnormal FDG uptake in the spinous process of the T10 vertebra, with a standardized uptake value max of 9.5. MRI, magnetic resonance imaging; CT, computed tomography; FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
a Hematoxylin and eosin staining shows irregular nests of atypical cells with a high nucleocytoplasmic ratio (scale bar, 100 μm). b The tumor cell nuclei are positive for Epstein–Barr virus-encoded small RNA in situ hybridization (scale bar, 100 μm). The histopathologic diagnosis is metastasis of nasopharyngeal cancer to the spinous process of the T10 vertebra.
Surgical plan
As treatment for this solitary metastatic lesion, we decided to perform radical resection with sufficient margins that would include the involved spinous process and all surrounding soft tissues exhibiting evidence of tumor infiltration. CT imaging confirmed that the cortical bone on the ventral side of the T10 vertebral arch was preserved and that there was no tumor invasion in the pedicles, dural sac, or spinal canal (Fig. 1). In planning the surgery, we recognized that we needed to resect the posterior elements of the spinal column at the level of pedicles without full visualization of the involved dural sac and to excise the dorsal elements, specifically the tumor-infiltrated soft tissues surrounding the T10 vertebral spinous process, without full visualization of the tumor. As such, we elected to perform a posterior elemental resection of the spine from the 9th to 11th (T9–T11) vertebrae, as well as all surrounding soft tissues. We discuss the steps in detail below.
Operative technique
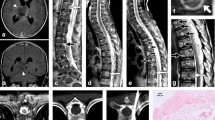
One day prior to the operation, embolization of the 9th and 11th intercostal arteries was performed. During this procedure, we also ensured that the right 10th intercostal artery, the Adamkiewicz artery, was preserved. The surgery itself proceeded as follows: a spindle-shaped skin incision with a 2-cm margin around the biopsy wound was made at the level of the 8th–12th (T8–T12) vertebrae. To avoid incising the tumor, the 9th–11th ribs were approached intermuscularly. We exposed the transverse processes of the T9–T11 vertebrae (Fig. 2a) and excised the inferior articular process and caudal lamina of the T8 vertebra. A cephalad T-saw was inserted into the spinal canal on the dura at the level of the T9 vertebra and was subsequently passed under the superior facet of the T9 vertebra and the transverse processes of the T9–T11 vertebrae, creating a reversed U-shape (Fig. 3a). The T-saw guides were positioned on the caudal side of the lamina and the transverse processes of the T11 and T12 vertebrae, respectively. The T-saw guides allow us to cut the pedicles at their connections to the vertebral bodies. All pedicles of the T9–T11 vertebrae were cut using the T-saw. This exposed the posterior elements of the T9–T11 vertebrae, multifidus muscles, and skin. These were then resected en bloc (Fig. 3b). Posterolateral fusion with fibular bone grafts of the 7th–12th vertebrae was performed (Fig. 3c). The surgical wound was closed without muscle-flap coverage. The operative time was 6 h and 15 min, and the volume of blood loss was 345 ml. No adjuvant therapy such as radiation or chemotherapy was administered following surgery. Pathological examination revealed that the resected tumor had negative margins, which meant that the 2-cm margin was curative (Fig. 3d). During the second year of follow-up, the patient showed no signs of recurrence, metastasis, or implant failure (Fig. 4).
a A spindle-shaped skin incision with a 2-cm margin around the biopsy wound (arrow heads) is made at the 8th–12th (T8–T12) vertebral level. The transverse processes of the T9–T11 vertebrae are exposed using an intermuscular approach. This approach also avoids incision of the tumor. After the inferior articular process and caudal lamina of the T8 vertebra are excised, a T-saw (arrows) is inserted under the lamina of the T9–T11 vertebrae. b After the pedicles on both sides of the T9–T11 vertebrae are cut using the T-saw, the posterior elements of the T9–T11 vertebrae, as well as the involved multifidus muscles and skin, are resected en bloc. c Posterolateral fusion with fibular bone grafts of the 7th–12th vertebrae is performed. d Pathological examination reveals that the resected tumor has negative margins, indicating that the 2-cm margin is curative. The surgical specimen also includes the biopsy route (red arrow).
Statement of ethics
The authors certify that all applicable institutional and governmental regulations concerning the ethical use of the patient were followed during the course of this research. Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Discussion
Surgical treatment of solitary spinal metastasis is associated with a favorable long-term prognosis when wide or marginal excision is performed [5]. However, curative resection of solitary metastases involving the posterior elements of the spine has not been reported. Demircay et al. [1] documented a case of a 45-year-old man with rectal cancer who presented with solitary bone metastasis in the spinous process of the 4th lumbar vertebra. The patient underwent excisional biopsy; however, it was unclear whether recurrence occurred after 2 months postoperatively. This is the first report on a surgical treatment for solitary metastasis in the posterior elements of the spine that resulted in local radical cure.
To arrive at the best outcomes, resection must be performed with sufficient margins and must not disturb the tumor tissue. We achieved sufficient margins by planning the incision with the plastic-surgery department. We also utilized an inter-muscular approach to access the 9th–11th ribs. This exposed the corresponding transverse processes of the T9–T11 vertebrae without requiring manipulation of soft tissues surrounding the tumor. To detach the posterior elements of the spine, a total laminectomy, which was part of the total en bloc spondylectomy (TES), was performed using the T-saw [3, 5]. The TES technique consists of two steps that permit the complete surgical excision of a vertebra: (1) en bloc resection of the posterior elements (total laminectomy), and (2) en bloc resection of the anterior portion to salvage the spinal cord [3, 5]. The T-saw was passed into the spinal canal at the level of the T9–T11 vertebrae. The pedicles of the T9–T11 vertebrae were then cut bilaterally. The T-saw allows horizontal cutting of the pedicles even without full visualization of the tumor or the dural sac within which the tumor is located. To cut the pedicles near the vertebral body and avoid interference by the ribs, the guide pulleys of the T-saw were inserted caudally in a reversed U-shape. After the pedicles were removed, the posterior elements of the T9–T11 vertebrae, as well as the involved multifidus muscles and skin, were easily resected en bloc. This technique, which allows for resection of the tumor with sufficient margin without damaging the spinal cord, can also be applied to cases where the tumor has locally spread from the posterior elements of the spine into the spinal canal.
NPC is associated with early bone metastasis [6], which occurs with an estimated incidence of 54–80% [7, 8]. Bony metastases from NPC most frequently affect the bones of the spine. While locoregional control of primary NPC by radiotherapy and chemotherapy is excellent, with an overall survival rate of 80–90.5% at 5 years [6, 7, 9,10,11], the prognosis of NPC with spinal metastasis is significantly poorer [6, 8, 12]. As such, it is extremely important to detect spinal metastasis from NPC at an early stage and aim for radical treatment. NPC is highly radiosensitive and chemosensitive. In patients with bone metastatic NPC, platinum-based chemotherapy is the first line of treatment [6, 8, 12]. Comparatively, patients with spinal metastases from NPC do not respond as well to radiation or chemotherapy [7]. Surgical interventions had to be adopted to maximize neurologic function, avoid neurologic deterioration, and prolong life expectancy [7]. In our patient, spinal metastasis occurred, despite radical cure of the primary NPC by chemotherapy and proton-beam therapy. We decided that the patient was a good candidate for radical resection because he was young and otherwise healthy. Despite not receiving adjuvant radiation and chemotherapy, the patient remained disease-free during the second year of follow-up.
The estimated sensitivity of FDG-PET to detect bone metastases ranges from 62% to 100% [13]. FDG-PET/CT can detect bone metastases from NPC in the early stages [10]. However, benign FDG activity has also been reported in degenerative arthritis, inflammatory diseases, and nontumorous focal uptake around the spinous processes [14, 15]. Nishimatsu et al. [14]. analyzed the results of serial FDG-PET/CT scans in 27 patients. Among the study participants, 42 spinous-process regions were initially PET-positive. However, repeat scans showed no focal uptake in 35 of these 42 regions, which corresponded to 83% of the initial findings [14]. At 1 year prior to consultation, our patient underwent FDG-PET/CT, which revealed a mild FDG uptake that was considered to be nontumorous. However, a follow-up FDG-PET/CT scan showed increased FDG uptake. Subsequently, MRI revealed spinous-process metastasis. As such, while solitary spinous-process metastases are rare and can present as a false-negative result on FDG-PET/CT, repeat FDG-PET/CT scan and MRI are integral in ruling out the actual disease.
One limitation of this technique is that extensive resection of the posterior spinal elements, including the muscle and skin, may result in a defect, if the defect exceeds 6–7 cm in width, additional myocutaneous procedures may be necessary. Another limitation is the potential spinal cord injury that may occur during the blind insertion of the T-saw into the spinal canal, therefore, care must be exercised in cases of severe spinal canal stenosis.
In conclusion, we report a radical en bloc resection of a solitary metastatic spinal tumor confined to the spinous process of the thoracic vertebrae. We resected the posterior elements of the spine and the involved multifidus muscles using a T-saw, which ensured that our technique was safe to perform even without full visualization of the tumor. Recent advances in the treatment of malignant tumors have increased the life expectancy of patients with carcinoma, and there could be more opportunities to treat solitary spinal metastases such as this case in the future. In this case of thoracic spine metastasis, the tumor has not involved the spinal canal or spinal cord, however, metastatic tumors of the thoracic spine have a high risk of spinal cord injury. This technique can also be applied to cases where the tumor has locally spread from the posterior elements of the spine into the spinal canal. We consider our procedure to be an effective treatment for solitary spinous-process bone metastases that may induce spinal cord injury.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Demircay E, Civelek E, Demiralay E. Solitary spinous process metastasis: a case report. Eklem Hastalik Cerrahisi. 2013;24:58–61.
Heary RF, Bono CM. Metastatic spinal tumors. Neurosurg Focus. 2001;11:e1.
Tomita K, Kawahara N, Murakami H, Demura S. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci. 2006;11:3–12.
Sciubba DM, Petteys RJ, Dekutoski MB, Fisher CG, Fehlings MG, Ondra SL, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine. 2010;13:94–108.
Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T, et al. Surgical strategy for spinal metastases. Spine. 2001;26:298–306.
Kumar N, Tan JJ, Zaw AS, Lim JL, Wai KL, Malhotra R, et al. Evaluation of scoring systems and prognostic factors in patients with spinal metastases from nasopharyngeal carcinoma. Spine J. 2014;14:2946–53.
Yang J, Hu J, Wang D, Jia Q, Jiao J, Xiao J, et al. Surgical treatment outcomes of spinal metastases of nasopharyngeal carcinoma: the first report of 30 patients from a single center. Cancer Manag Res. 2020;12:6999–7008.
Shen L, Dong J, Li S, Wang Y, Dong A, Shu W, et al. M1 stage subdivision and treatment outcome of patients with bone-only metastasis of nasopharyngeal carcinoma. Oncologist. 2015;20:291–8.
Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104:286–93.
Liu FY, Chang JT, Wang HM, Liao CT, Kang CJ, Ng SH, et al. [18F]fluorodeoxyglucose positron emission tomography is more sensitive than skeletal scintigraphy for detecting bone metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2006;24:599–604.
Lin S, Pan J, Han L, Guo Q, Hu C, Zong J, et al. Update report of nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy and hypothesis of the optimal margin. Radiother Oncol. 2014;110:385–9.
Lu T, Guo Q, Cui X, Chen Z, Lin S, Xu L, et al. Prognostic evaluation of nasopharyngeal carcinoma with bone-only metastasis after therapy. Yonsei Med J. 2016;57:840–5.
Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–53.
Nishimatsu K, Nakamoto Y, Ishimori T, Togashi K. FDG uptake observed around the lumbar spinous process: relevance to Baastrup disease. Ann Nucl Med. 2015;29:766–71.
Rosen RS, Fayad L, Wahl RL. Increased 18F-FDG uptake in degenerative disease of the spine: characterization with 18F-FDG PET/CT. J Nucl Med. 2006;47:1274–80.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: AI and KY. Data acquisition: CI, AI, KY, and AS. Drafting of the article: CI, AI, and KY. Critical revision: MT. Supervision: NI. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ishii, C., Iwata, A., Yamada, K. et al. Radical resection for solitary thoracic spinous-process metastasis: a case report and technical note. Spinal Cord Ser Cases 8, 8 (2022). https://doi.org/10.1038/s41394-022-00479-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-022-00479-w