Abstract
Introduction
Supernumerary phantom limb (SPL) is an uncommon phantom sensation where the patient experiences the illusory presence of one or more limbs in addition to their existing limbs. SPL after a spinal cord injury (SCI) is rare with few documented cases. There is minimal treatment guidance available, with some reports of visual–tactile feedback therapy used to manage SPL.
Case presentation
A 43-year-old male sustained a C4 ASIA Impairment Scale grade C SCI, developing the sensation of two SPL arms originating from his shoulders 6 days after injury. He developed a self-directed method of visual–tactile feedback as a means to improve the SPL sensations, consisting of shrugging his shoulders repeatedly for 1 min while observing the movement of his actual arms. After completion of this routine, the SPL moved to the same location as his arms, providing relief. Also, an elastic band was placed on a sensate region of his arm, providing additional visual–tactile feedback. The SPL improved and resolved by day 45.
Discussion
SPL after SCI is poorly characterized, usually occurring within 6–7 days of injury after a complete or incomplete cervical SCI. While the mechanism is unclear, the inability to integrate visual, tactile, and proprioceptive information after deafferentation may contribute to development. Similarities between SPL and phantom limb sensation after an amputation have resulted in the use of visual and visual–tactile feedback therapy for painful SPL management. This is the first case documenting successful use of visual–tactile feedback therapy to manage nonpainful SPL.
Similar content being viewed by others
Introduction
Phantom sensations are perceptions of sensory phenomenon in a deafferented region after an injury to the nervous system [1]. The classic example is phantom limb sensation after an amputation, where the individual continues to feel the presence of the absent limb [2,3,4]. However, phantom sensations have been reported below the level of injury in 60–100% of patients after a spinal cord injury (SCI) [1, 5]. Supernumerary phantom limb (SPL) is an uncommon phantom sensation where the patient experiences an illusory presence of one or more limbs in addition to their existing limbs [6]. SPL is typically encountered after a right sided cortical or subcortical stroke with the SPL originating from the limb with hemiparesis or hypoesthesia [7].
SPL after a traumatic SCI is extremely rare with few reported cases in the literature [6, 8,9,10,11,12]. While the underlying pathophysiology of SPL after an SCI is unclear, a mismatch between visual, tactile, and proprioceptive sensory processing is a proposed mechanism [6, 10]. In addition, there is minimal guidance for therapeutic and pharmacologic management of SPL with limited reports suggesting a role for visual or visual–tactile feedback therapy [8, 10, 11]. Presented here is a case of nonpainful SPL after a neurological incomplete traumatic SCI, with symptom improvement and eventual resolution after initiation of visual–tactile feedback therapy, in addition to a review of the literature.
Case presentation
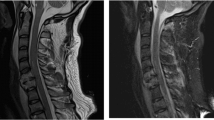
A 43-year-old male with a blood alcohol level of 320 mg/dl presented to the hospital after falling down a flight of stairs with subsequent upper and lower extremity weakness and decreased sensation. Computed tomography of the head showed no intracranial pathology and magnetic resonance imaging of his cervical spine revealed severe central canal stenosis at the C3–C4 level with spinal cord contusion at the C3–C5 level. He underwent C3–C6 posterior cervical discectomy and fusion 2 days after the injury. On day 5, a comprehensive neurological examination as per the International Standards for the Neurological Classification of SCI [13] revealed 0/5 bilateral upper extremity and 2/5 bilateral lower extremity strength in all key muscle groups. Sensation was decreased to light touch and pin prick evaluation below the right C4 dermatome and left C5 dermatome with sacral sparing and voluntary anal contraction, consistent with C4 American Spinal Injury Association (ASIA) Impairment Scale (AIS) C tetraplegia. On day 8, physiatry initiated pregabalin for treatment of lower extremity neuropathic pain. Pregabalin dose was increased over the subsequent week, which resulted in significant symptom improvement.
On day 18, the patient reluctantly disclosed that he felt as if he had “two extra arms.” This began on day 6 when he experienced chest discomfort due to feeling as if the two phantom arms, or SPL, were on his chest after awakening. Since onset, the SPL were present daily and intermittently occurred for hours prior to resolving. The SPL were equal in length to his arms, originated at the shoulders, and were oriented in one of two positions: crossed over his chest or extended over his abdomen with palms on his thighs (Fig. 1). The SPL were more pronounced when supine, improved with sitting, and more apparent in the morning, dissipating in the afternoon and evening.
He denied pain associated with the SPL, but instead reported a bothersome sensation when they were in a different location than his actual arms. Since the time of initial onset, he had developed a method to alleviate this sensation which involved looking at his actual arms while shrugging his shoulders (i.e., activating the trapezius muscle), resulting in gross movement of his actual arms. After about 1 min, the SPL moved to the same location as his actual arms and provided relief. Of note, he denied any change in the SPL since starting pregabalin for neuropathic pain in his actual limbs. A detailed mental status exam was performed showing intact reasoning and insight. He was unable to see the SPL and denied hallucinations. Importantly, the patient confessed that he was initially reluctant to discuss the SPL for fear of disbelief by medical staff.
First, extensive reassurance and education about SPL were provided to the patient and medical staff. Next, the patient was given a structured shoulder shrugging method, being instructed to perform this every time he felt the SPL and at least three times daily. Finally, an elastic bandage was placed over his left upper arm, where sensation was intact in the C5 dermatome, to provide additional visual–tactile feedback. Within 5 days, he reported a decrease in SPL frequency and duration, now only occurring a few times per week for less than 1 h in duration, and no longer awakening with the SPL on his chest. He continued to utilize shoulder shrugging and elastic bandage techniques daily throughout his acute care admission. His bilateral lower extremity strength gradually improved to 3/5 in all key muscle groups, without change in sensation and upper extremity strength. By discharge to rehabilitation on day 45, the SPL had resolved without recurrence.
Discussion
Since its original description, SPL after an SCI has remained poorly understood with the minimal literature on epidemiology, diagnostic characteristics, and clinical management. Including this case, there are seven reports of SPL after a traumatic SCI (Table 1) [6, 8,9,10,11,12]. All reported cases occurred in males aged 22–71 years old and after sustaining a cervical SCI of varying severity from to C2–C6 levels. Three of the cases sustained either a complete (AIS A) classification and four cases had either an incomplete injury (AIS B or C) classification. When reported, SPL onset varied from 6 days to 2 years after injury, with four of the five cases experiencing onset at 6–7 days after injury [6, 8,9,10,11,12].
SPL after an SCI has multiple unique characteristics (Table 1). First, the SPL can consist of either entire limbs or only portions of limbs, such as the hands [6, 8,9,10,11,12]. Second, the SPL orientation varies, either laying parallel to the limbs, over the chest and abdomen, or angled from the body in different planes [6, 8, 10,11,12]. Third, the SPL can be continuously or intermittently present and more pronounced at specific times of day or with certain positions [6, 8, 10,11,12]. In addition, the SPL can be nonpainful or painful and can be accompanied by neuropathic pain in the actual limbs or episodes of chest discomfort when the SPL arms are crossed over the chest [6, 8, 10,11,12]. Finally, SPL can be associated with a telescoping phenomenon, or shortening of the SPL, as motor function, sensation, and proprioception improve [6, 8]. Clinicians can utilize these trends to aid with clinical diagnosis of SPL. It is plausible that SPL is an underrecognized occurrence after an SCI due to a combination of clinician unfamiliarity and patient reluctance to report this bizarre experience [6, 8, 11].
There are multiple proposed mechanisms for the development of SPL after SCI. One leading hypothesis is that SPL occurs due to disrupted communication between the brain and paralyzed limb, resulting in an altered central body schema perception [14, 15]. The perception of the body’s location in space is achieved through the central integration of afferent sensory information, including tactile sensation and proprioception, received from the environment. This allows the body schema, or the configuration of the body in space, to be established and updated with changes in body and limb orientation [16]. The loss of this afferent information, such as that proposed in SPL, may lead to a failure in generating a normal experience of self-location and subsequent construction of an illusory limb [8]. Another proposed mechanism is that SPL may result from cortical and subcortical reorganization and maladaptive plasticity after an SCI [8, 11, 17]. A third hypothesis is that a disturbance in the sensory-motor loop may lead to an efferent copy mismatch and SPL emergence [10, 18]. Nevertheless, to date, the pathophysiology behind SPL development remains poorly understood.
Currently, there is no standardized approach for the management of SPL after an SCI, with use of pharmacologic and therapy-based interventions in the literature (Table 1) [6, 8,9,10,11,12]. Four documented cases of SPL used medications as a part of management, with pregabalin used in all patients, either alone or with additional medication [6, 8, 11]. These selections took into consideration if the SPL was painful and if neuropathic pain was present in the actual limbs. None of the patients experienced direct improvement in SPL presence or intensity after initiation of medications. However, pregabalin was associated with improved neuropathic pain in the actual limbs and decreased neuropathic pain in the SPL in some cases (Table 1) [8, 11]. One possible explanation for this finding is that patients did not associate SPL improvement with the medication due to pregabalin’s delayed onset of action. In addition, three cases used pregabalin in combination with visual feedback therapy which may represent a synergistic effect of the two treatments [6, 11].
Previous authors have suggested that the pathophysiology behind SPL after SCI is similar to phantom limb sensation and pain after amputation [6, 8, 10, 11]. One established treatment for phantom limb sensation and pain is visual feedback therapy [19]. Patients undergoing visual feedback therapy see the absent limb, either through mirrors or virtual video systems, and attempt to synchronize the phantom limb movements with the observed movements [4, 19]. The role of the visual system in SPL was detailed in a patient with a C3 AIS C tetraplegia who developed SPL arms primarily when supine [6]. In this case, the rubber hand illusion paradigm was administered while the patient was sitting and SPL were absent. Within minutes of initiating the vision-touch protocol, the previously reported SPL appeared [20]. This supports the idea that SPL may arise from a breakdown in the ability to integrate visual, tactile, and proprioceptive information [6, 10, 15].
Previous authors have proposed that the visual system’s role in proprioceptive mismatch can assist in SPL treatment by reconciling the altered perception. Multiple novel visual feedback techniques have been used for the management of SPL sensations and pain (Table 1) [8, 10, 11]. One case report utilized visual–tactile stimulation therapy for the management of two painful SPL legs by instructing the patient to identify the paralyzed lower extremities and tap his legs with a stick three times daily for 10–15-min sessions. The patient began to experience gradual improvement in SPL sensations and pain, starting on day 8 of treatment [8]. In another example, a patient with two painful SPL arms was placed on a tilt table in front of a mirror, body covered from the neck down, while a video projection of moving extremities was shown. The patient was then instructed to imagine moving his hands and feet as seen in the projected video. The authors reported a decrease in SPL pain after completion of the treatment [10]. In a third example, a patient experiencing two nonpainful SPL hands over the abdomen was instructed to look at his actual hands while either thinking about or attempting to move them. Of note, this patient also received repetitive transcranial magnetic stimulation as a separate intervention [11].
In the currently presented case, two visual–tactile feedback therapies were utilized for SPL management. The first was the patient’s method of performing shoulder shrugs for 1 min, while watching his arms move, which likely allowed for the integration of the visual, tactile and proprioceptive systems. This reconciled the discrepancy between the sensory systems by moving his SPL to the same location as his arms. Second, wrapping the elastic bandage around his arm at the distal-most site with intact sensation provided him with additional visual–tactile feedback both at rest and when performing shoulder shrugs. The patient’s altered sensation below the C5 dermatomal level may have permitted some degree of visual, tactile, and proprioceptive information processing, allowing for the visual–tactile feedback therapy to reconcile the miscommunication between sensory systems. Finally, patient and medical provider education and reassurance about SPL is essential given that the interventions detailed above are patient and staff driven. The patient presented in this case experienced symptom improvement within 5 days of starting the above treatment measures and complete resolution by day 45 after injury. This is the first case documenting successful use of visual–tactile feedback therapy in the management of nonpainful SPL.
The outcomes for patients with SPL after SCI vary in the reported cases. Of the six cases where outcomes were reported, three had resolution of the SPL, while three others documented improvement of SPL intensity or pain (Table 1) [6, 8, 10,11,12]. For the patients who experienced SPL resolution, all had sustained an incomplete SCI and the timing varied between 45 days and 8 months after initial injury. In addition, all patients with resolution had received pregabalin and two received some form of visual feedback therapy [6, 11]. In this presented case, pregabalin alone was not effective for SPL management and only after addition of visual–tactile feedback therapy was there symptom improvement.
In summary, SPL is a rarely reported phenomenon after complete and incomplete SCI with symptom onset usually within 1 week of injury. There are many characteristics the clinician can use to assist in SPL diagnosis (Table 1). The proposed similarity between phantom limb sensation after an amputation and SPL after SCI has resulted in the use of multiple types of visual feedback therapies with varying success. Though the role of pregabalin in the treatment of SPL remains unclear at this time, it may help treat painful SPL, have long term benefit for SPL recovery, and may have a synergistic effect with visual feedback therapy. The case presented here serves to bolster the current literature on SPL after an SCI by helping to further characterize this rare condition and by detailing two novel methods of visual–tactile feedback therapy. Finally, this is the first case describing the use of visual–tactile feedback treatment for the successful management of nonpainful SPL, emphasizing the importance of both patient and staff education as key parts of management. Also, it is unclear if SPL improves as neurologic recovery occurs and if an incomplete injury confers a better prognosis for SPL resolution. Additional studies are needed to clarify the underlying neural mechanisms, identify risk factors, and to develop standardized management strategies for SPL after SCI.
References
Siddall PJ, McClelland J. Non-painful sensory phenomena after spinal cord injury. J Neurol Neurosurg Psychiatry. 1999;66:617–22.
Anderson ML. What phantom limbs are. Conscious Cognit. 2018;64:216–26.
Luo Y, Anderson TA. Phantom limb pain: a review. Int Anesthesiol Clin. 2016;54:121–39.
Ramachandran VS, Hirstein W. The perception of phantom limbs. The DO Hebb lecture. Brain: J Neurol. 1998;121:1603–30.
Bors E. Phantom limbs of patients with spinal cord injury. AMA Arch Neurol Psychiatry 1951;66:610–31.
Curt A, Yengue CN, Hilti L, Brugger P. Supernumerary phantom limbs in spinal cord injury. Spinal Cord. 2011;49:588–95.
Bourlon C, Urbanski M, Quentin R, Duret C, Bardinet E, Bartolomeo P, et al. Cortico–thalamic disconnection in a patient with supernumerary phantom limb. Exp Brain Res. 2017;235:3163–74.
Choi JY, Kim HI, Lee KC, Han Z-A. Atypical supernumerary phantom limb and phantom limb pain in a patient with spinal cord injury: case report. Ann Rehabilit Med. 2013;37:901.
Davis R. Pain and suffereing following spinal cord injury. Clin Orthop Relat Res. 1975;112:76–80.
Katayama O, Iki H, Sawa S, Osumi M, Morioka S. The effect of virtual visual feedback on supernumerary phantom limb pain in a patient with high cervical cord injury: a single-case design study. Neurocase.2015;21:786–92.
Lu Y-S, Tong P, Guo T-C, Ding X-H, Zhang S, Zhang X-J. Effects of combined rTMS and visual feedback on the rehabilitation of supernumerary phantom limbs in a patient with spinal cord injury: a case report. World J Clin Cases. 2019;7:3120.
Ohry A, Gur S, Zeilig G. ‘Duplicate limbs’ sensation in acute traumatic quadriplegia. Spinal Cord. 1989;27:257–60.
International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI). American Spinal Injury Association (ASIA) and International Spinal Cord Society (ISCOS). 2019. https://asia-spinalinjury.org/international-standards-neurological-classification-sci-isncsci-worksheet/.
Brugger P. Supernumerary phantoms: a comment on Grossi et al.'s (2002) spare thoughts on spare limbs. Percept Motor Skills. 2003;97:3–10.
Khateb A, Simon SR, Dieguez S, Lazeyras F, Momjian‐Mayor I, Blanke O, et al. Seeing the phantom: a functional magnetic resonance imaging study of a supernumerary phantom limb. Ann Neurol. 2009;65:698–705.
Giummarra MJ, Gibson SJ, Georgiou-Karistianis N, Bradshaw JL. Mechanisms underlying embodiment, disembodiment and loss of embodiment. Neurosci Biobehav Rev. 2008;32:143–60.
Tidoni E, Tieri G, Aglioti S. Re-establishing the disrupted sensorimotor loop in deafferented and deefferented people: the case of spinal cord injuries. Neuropsychologia. 2015;79:301–9.
McCabe CS, Blake DR. Evidence for a mismatch between the brain’s movement control system and sensory system as an explanation for some pain-related disorders. Curr Pain Headache Rep. 2007;11:104–8.
Herrador Colmenero L, Perez Marmol JM, Martí-García C, Querol Zaldivar MdlÁ, Tapia Haro RM, Castro Sánchez AM, et al. Effectiveness of mirror therapy, motor imagery, and virtual feedback on phantom limb pain following amputation: a systematic review. Prosthet Orthot Int. 2018;42:288–98.
Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391:756.
Acknowledgements
We would like to thank Dr. Kathryne Bartolo, MD, for creating the artwork in Fig. 1 and reviewing the final paper.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the writing of this paper. Within this paper there is discussion of off-label use of medication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Written informed consent was obtained from the patient for use in this case report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diaz-Segarra, N., McKay, O., Kirshblum, S. et al. Management of nonpainful supernumerary phantom limbs after incomplete spinal cord injury with visual–tactile feedback therapy: a case report. Spinal Cord Ser Cases 6, 62 (2020). https://doi.org/10.1038/s41394-020-0312-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-020-0312-7