Abstract
Introduction
Schwannomas are the second most common type of intra-dural lesions involving the thoracic spine. They are frequently seen as solid and heterogeneous lesions. Totally cystic thoracic schwannomas represent a rare pathological schwannoma variant, with only four cases reported in the English literature to our knowledge.
Case presentation
We report an 80-year-old male who presented with upper back pain for 3 months. Magnetic resonance imaging (MRI) showed a cystic lesion at the level of T6–T7 with peripheral contrast enhancement. The lesion was removed in total surgically with complete resolution of the patient’s symptoms.
Discussion
The diagnosis of cystic schwannomas is often delayed due to the paucity of symptoms and the lack of meticulous investigation. The presence of rim enhancement on contrast-enhanced MRI may be the only clue for the diagnosis. It is important to consider cystic schwannomas in the differential diagnosis of cystic spinal lesions since the best surgical outcome is strongly related to earlier diagnosis and total resection of the lesion.
Similar content being viewed by others
Introduction
Schwannomas develop from Schwann cells of the nerve sheath. These tumours are benign and represent ~8% of primary intracranial and 29% of primary spinal tumours. Together with meningiomas, schwannomas are the most common type of intra-dural extra-medullary (IDEM) tumours in the thoracic spine. Degenerative changes such as haemorrhage, calcification, and fibrosis are commonly seen in schwannomas. Complete cystic changes are rarely seen alone. Cystic degeneration has been observed in various extra-spinal locations, such as the cranial nerves, tentorial hiatus, jugular foramen, pancreas, maxillary sinus, and pre-sacral region. While schwannomas occurring within the spine are not rare, predominantly cystic intra-spinal schwannomas within the IDEM compartment have been rarely reported, with a total of 38 cases reported in the English literature. To our knowledge, four cases of totally cystic schwannomas within the thoracic spine have been reported to date. Although they are generally slow-growing lesions, cystic schwannomas are prone to cause clinical deterioration secondary to a rapid expansion of the cysts in patients [1,2,3,4].
Schwannomas are not generally considered in the differential diagnosis of spinal–cystic lesions, as they are mostly solid and heterogeneous tumours. In such cases, the diagnosis of cystic nerve sheath tumours can be difficult. Contrast-enhanced magnetic resonance imaging (MRI), with its superior soft-tissue contrast and multi-planar imaging capability, is the ideal imaging modality to evaluate these tumours [5]. Herein, we report the fifth case of a thoracic IDEM cystic schwannoma in an 80-year-old male and describe the imaging findings and clinical details. Our main objective in presenting this particular case is to highlight the atypical presentation, radiological features, differential diagnosis, and management for this uncommon schwannoma variant.
Case presentation
An 80-year-old male presented to the emergency department (ED) with a history of mid-thoracic back pain for 3 months. The pain radiated bilaterally to the anterior chest wall along the T6 dermatome; there was no history of weakness in the lower limbs, loss of sensation, loss of sphincter control, antecedent trauma or constitutional symptoms. Apart from an up-going plantar reflex in the left lower limb, his examination revealed normal motor and sensory function. He was found to have fresh cautery marks over the chest wall on the left side and over the upper back near the midline, which he explained later as being caused by traditional treatment attempts. Due to the patient’s age, cardiac pathology was initially suspected at the time of his first visit to the ED; a cardiac workup including serial ECG and cardiac enzymes revealed no abnormalities, and the patient was reassured and discharged. He continued to have repeated visits to the ED for the same complaint, and he was investigated more thoroughly during his last visit.
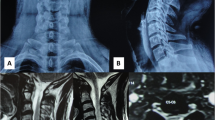
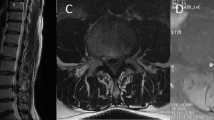
Lateral thoracic spine X-ray was requested to investigate the back pain; it showed no destructive lesion or fracture. Magnetic resonance imaging of the thoracic spine revealed a well-defined cystic lesion dorsal to the spinal cord at the level of T6–T7. The lesion showed peripheral contrast enhancement with focal nodular wall thickening inferiorly, causing a significant mass effect and anterior displacement of the spinal cord. No associated abnormal signal intensity was observed within the cord. The tumour measured 2.1 × 1.3 × 1.3 cm overall in craniocaudal, anteroposterior, and transverse dimensions. A diagnosis of cystic meningioma or schwannoma was suspected (Fig. 1).
A Lateral thoracic X-ray showing no bone abnormalities. Sagittal B T1- and C T2-weighted MRI showing a round lesion with lower and higher signal intensities, respectively. Post-contrast D axial and E sagittal T1-weighted images showing the well-defined cystic mass with thick circumferential wall enhancement. F Axial T2 images showing the location of the intra-dural extra-medullary lesion with severe compression of the thoracic segment of the cord.
A posterior midline approach utilising T5–T7 laminectomy was used to expose the tumour. Under microscopic visualisation, the dura was opened in the midline. The tumour was well-demarcated, encapsulated, yellowish, and soft in consistency. It was found to be slightly adherent to the arachnoid, marginally displacing the neighbouring nerve roots. It was not attached to the spinal cord. The left dorsal rootlets of the T6 spinal nerve were attached to both tumour ends and had to be sacrificed. Total resection was achieved with no clinical sequelae.
Upon histopathological evaluation, gross examination showed a single piece of the tan-brown, circumscribed, cystic mass measuring 1.5 × 1 × 1 cm. The cut section showed a cystic cavity. Microscopic examination showed spindle-cell lesions with cellular fibrillary areas (Antoni A) and paucicellular areas (Antoni B) surrounded by clusters of nuclei (Verocay bodies). Thick hyalinized blood vessels were present with extensive cystic degeneration. Neither nuclear atypia nor mitosis was noted. The lesion was encapsulated by fibrous tissue (Fig. 2). Immuno-histo-chemical staining showed diffuse positivity for S100 (Fig. 3A) and NFP (Fig. 3B) stains and negativity for the EMA stain, confirming the diagnosis of World Health Organization (WHO) grade I schwannoma.
After an unremarkable postoperative period, the patient experienced complete relief of his preoperative symptoms and remained asymptomatic after 2 months of follow-up.
Discussion
Epidemiology
Schwannomas are slow-growing benign tumours that were first reported in 1910. They commonly arise from the dorsal sensory roots of the spinal nerves [1, 3, 5]. Males and females are affected equally, with a higher tendency for occurrence between the fourth and sixth decades of life [1, 2, 6].
Spinal schwannomas represent approximately one-third of all IDEM tumours, with the lumbar region being the most common location of occurrence [7]. The percentage of totally intra-dural nerve sheath tumours increases from 8% at high cervical levels to 80% at the thoracolumbar junction [3]. In a retrospective review, Conti et al. [8] concluded that the thoracolumbar junction was the second most common location for IDEM schwannomas after the lumbar region. More than 50% of schwannomas are IDEM tumours, 25% are totally extradural, and 15% are both intra-dural and extradural; only a small number of cases were found to be intramedullary [6]. Cystic schwannomas are more commonly seen in the lumbar region and less frequently seen in the thoracic and cervical regions [9].
Clinical presentation
Cystic schwannomas are usually asymptomatic, and such a diagnosis is challenging in the absence of symptoms [2]. Few symptoms are present until the tumour reaches a large size. Tumours arising from the cauda-equina and the cervical region can become larger than those in the thoracic region because of the spacious canal at these levels [9]. However, they are collectively prone to cause clinical deterioration secondary to the rapid expansion of cysts [6, 10, 11]. The characteristic paucity of symptoms seen with schwannomas is related to their longitudinal and indolent proliferation, which in turn delays the diagnosis until proper imaging studies are performed [3, 4]. Symptoms are commonly caused by the mass effect on the spinal cord, nerve roots, or both. The presentation of such lesions highly varies depending on the tumour location, and related symptoms include local pain, radiculopathy, paraesthesia, motor weakness, and sphincter control disturbances [6, 9]. Moreover, non-specific chest or abdominal pain may be the only manifestation of the tumour [3]. In a series of six patients with spinal–cystic schwannomas, those harbouring thoracic lesions presented with myelopathy in the form of spastic paraparesis, while patients with thoracolumbar junction and lumbar lesions presented with backaches associated with symptoms of radiculopathy [10]. In our case, there was a remarkable paucity of symptoms in relation to the tumour size and the degree of compression on the spinal cord.
Imaging characteristics
Schwannomas are usually entirely solid or heterogeneously solid tumours. Predominantly, cystic spinal schwannomas are uncommon lesions and may pose a preoperative diagnostic dilemma [10]. Schwannomas causing significant destruction of vertebrae are rarely seen; plain X-ray and computed tomography (CT) may reveal widening of the neural foramen, erosion of the pedicle, an increased interpedicular distance, and scalloping of the adjacent vertebral body [3]. Magnetic resonance imaging, however, has been considered the best tool to investigate IDEM spinal schwannomas [12]. These tumours have iso- to hypo-intense signals on T1-weighted (T1W) images and variable degrees of hyper-intensity on T2-weighted (T2W) images compared to the spinal cord [3, 5, 6, 12]. Compared to the adjacent CSF, these tumours are usually hyper-intense on T1W and slightly hypo-intense on T2W images because of their solid cellular elements [5]. The hyper-intense signals observed on T2W images commonly represent cyst formation, while the hypo-intense signals may be related to haemorrhage, high cellularity, and collagen composition [6, 7, 12]. The presence of a fluid–fluid interface and hypo-intense sediments on T2W sequences also support repeated haemorrhagic episodes in some cases [13]. In our case, it was notable that the lesion was not easily identifiable as an IDEM lesion on MR imaging, mimicking an arachnoid cyst, with no significant bone remodelling noted on the thoracic spine X-rays.
Differential diagnosis
Despite their rarity, IDEM cystic schwannomas should be considered in the differential diagnosis of spinal–cystic lesions [14]. Generally, the enhancement patterns for schwannomas vary, from strong and homogenous at times to only a thin rim of enhancement at other times [5,6,7]. In the case of cystic schwannomas, rim enhancement of the lesion should suggest such a diagnosis [3, 9, 11, 15].
The differential diagnosis of cystic lesions in the thoracic IDEM compartment includes cystic meningiomas, neurenteric cysts, hydatid cysts, and arachnoid cysts, which can be differentiated from cystic schwannomas by their unique imaging features [6, 7, 10, 11]. Although scantly reported, cystic IDEM meningiomas represent the main differential diagnosis to cystic schwannomas in the thoracic spine. These lesions are more commonly seen in middle-aged females. They typically compress and displace the spinal nerve root and, less commonly, circumferentially surround it. A thin rim of enhancement is noted after contrast administration that is contiguous with the dura, commonly known as the dural tail sign, which might be the only finding differentiating it from a cystic schwannoma [11]. Spinal neurenteric cysts represent a rare type of foregut duplication cyst, accounting for 1% of all spinal cord lesions. They are usually associated with other vertebral or CNS abnormalities. They are commonly seen as non-enhancing and multi-lobulated cysts that extend through a long segment along the anterior IDEM compartment. Hydatid cysts have multiple well-defined cystic lesions with a multi-loculated appearance [6]. Arachnoid cysts are iso-intense to cerebrospinal fluid (CSF) and follow CSF signal intensity on all pulse sequences. They are usually located posterior to the thecal sac and primarily occur in adolescents and young adults [6, 11]. A thin rim of enhancement of the cyst’s wall is suggestive of a cystic schwannoma or meningioma rather than an arachnoid or a hydatid cyst, which will exhibit rim enhancement only if complicated by rupture or infection [10].
Pathological examination
Histopathological examination forms the basis for differentiating cystic schwannomas from other cystic lesions within the spine [3, 13]. Schwannomas consist of spindle-shaped cells with a fine eosinophilic cytoplasm. The cell arrangement is compact in Antoni A type and loose in Antony B type tumours [2, 9]. Ancient schwannoma, an extremely benign histological schwannoma variant that was first described by Ackerman and Taylor in 1951, is principally formed by Antoni B tissue and depicts tumour degeneration in the form of cyst formation, calcification, haemorrhage, fibrosis and cytological atypia [1,2,3]. Although ancient schwannomas are not uncommon, cystic degeneration occurring alone without the other features of ancient schwannomas is rarely observed [1, 7].
Aetiology of cyst formation
Various theories have been hypothesised to clarify the aetiology of the cystic transformation that occurs in schwannomas. Sakamoto suggested that central ischaemic necrosis secondary to tumour growth eventually resulted in the formation of a cystic cavity with central necrosis [16]. Another theory was proposed by Enzinger, stating that the degeneration of Antoni B areas resulted in cyst formation, which coalesced over time and formed a single cystic cavity [17].
Treatment
The treatment of choice for schwannomas is complete surgical excision, which is safe and feasible given the advances in surgical techniques and equipment [7, 9, 14, 18]. Despite the fact that schwannomas originate from nerve tissue, only half of these lesions are directly related to a nerve. Adhesion of the tumour capsule to the surrounding structures can complicate excision; prompt identification of the arachnoid plane without opening the cyst with sharp dissection may be useful in such cases. Total resection without deficits is often possible on the condition that there is no nerve root entrapment [3]. However, if the tumour adheres to many nerve roots, incomplete removal is judicious for preventing the risk of postoperative neurologic impairment [13].
Complete resection is the standard of care when aiming for a cure with the lowest probability of recurrence [3, 9, 12, 18]. The best surgical outcome is strongly related to earlier diagnosis, meticulous investigation, and total resection [3, 12]. Nevertheless, it is unknown whether irradiation is effective for benign schwannomas, but it has been well established that recurrence after incomplete removal is rare irrespective of irradiation [13].
Prognosis and outcome
The prognosis for cystic schwannomas is similar to that of solid schwannomas, which is usually excellent [3, 13, 19]. Cystic schwannomas rarely undergo malignant transformation [1]. Malignant cystic schwannomas represent a rare subset of cystic schwannomas reported in the mediastinum by Suzuki et al. [20], who proposed the likelihood of malignant transformation in cystic schwannomas. Within the spine, Vilanova et al. [21] also reported a case of malignant schwannoma of the lumbar region with haemorrhage and fluid–fluid levels. Hence, long-term follow-up of such lesions is recommended.
The behaviour of cystic schwannomas involving the thoracic spine needs further research. According to the information we currently have about this variant, the treatment of choice should involve complete resection of the tumour when feasible, hence preventing recurrence. However, those not amenable for complete excision need to be closely followed for a long period of time to evaluate the tumour growth rate and to intervene as indicated.
Conclusion
Cystic schwannomas are rare and difficult to diagnose. Such lesions should be suspected in patients in their fourth to sixth decades of life, in patients who present with mild but rapidly evolving symptoms, and in patients who have well-defined cystic lesions on MRI studies. Surgical excision is the mainstay of treatment, and complete excision should be attempted when feasible.
References
Borges G, Bonilha L, Proa JrM, Fernandes YB, Ramina R, Zanardi V, et al. Imaging features and treatment of an intradural lumbar cystic schwannomaI. Arq Neuropsiquiatr. 2005;63:681–4.
Santhosh K, Kesavadas C, Thomas B, Gupta AK, Kapilamoorthy TR, Radhakrishnan V. Fluid-fluid levels in cystic lumbosacral schwannomas: a report of three cases. Singap Med J. 2009;50:16–21.
Jaiswal A, Shetty AP, Rajasekaran S. Giant cystic intradural schwannoma in the lumbosacral region: a case report. J Orthop Surg. 2008;16:102–6.
Kasliwal MK, Kale SS, Sharma BS, Suri V. Totally cystic intradural extramedullary schwannoma. Turkish Neurosurg 2008;18:404–6.
Parmar H, Patkar D, Gadani S, Shah J. Cystic lumbar nerve sheath tumours: MRI features in five patients. Australas Radiol. 2001;45:123–7.
Karatas A, IS M, Yildirim U, Akyuz F, Gezen F. Thoracic intradural cyst schwannoma: a case report. Turkish Neurosurg. 2007;17:193–6.
Vikram M, Pande A, Vasudevan MC, Ravi R. Cervical solitary long segment cystic Schwannoma. Br J Neurosurg. 2010;24:208–10.
Conti P, Pansini G, Mouchaty H, Capuano C, Conti R. Spinal neurinomas: retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol. 2004;61:35–44.
Kumar S, Gupta R, Handa A, Sinha R. Totally cystic intradural schwannoma in thoracic region. Asian J Neurosurg. 2017;12:131–3.
Savardekar A, Singla N, Mohindra S, Ahuja CK, Gupta SK. Cystic spinal schwannomas: a short series of six cases. Can we predict them preoperatively? Surg Neurol Int. 2014;5:S349–53.
Netra R, Hui MS, Gang MZ, Ming Z. Spinal cystic schwannoma: an MRI evaluation. J Coll Physicians Surg Pak. 2014;24:145–7.
Hsieh C, Tsai W, Liu M. Intradural lumbar cystic schwannoma. Neuroscience (Riyadh). 2011;16:366–8.
Himmiche M, Benzagmout M, Alami B, Benabdellah IS, Chakour K, Chaoui ME. Giant cystic schwannoma of the cauda equina. Ann Afr Med. 2019;18:180–3.
Akhaddar A, Ajja A, Albouzidi A, Elmostarchi B, Boucetta M. A cystic schwannoma of the cauda equina mimicking hemangioblastoma. Neurochirurgie. 2008;54:101–3.
Attiah MA, Syre PP, Pierce J, Belyaeva E, Welch WC. Giant cystic sacral schwannoma mimicking tarlov cyst: a case report. Eur Spine J 2016;25:84–88.
Sakamoto M, Harayama K, Furufu T. A case of cystic spinal schwannoma presented unusual clinical findings. Orthopedics 1985;3:1185–9.
Enzinger FM. Schwannomas. In: Harshberger, SE, editor. Soft tissue tumors. St Louis:Mosby; 1983. p. 585–97.
Albert AF, Kirkman MA, Plessis D, Sacho R, Cowie R, Tzerakis NG. Giant solitary cystic schwannoma of the cervical spine: a case report. Clin Neurol Neurosurg. 2012;114:396–8.
Desheng WU, Zhaoyu BA, Huang Y, Zhao W, Shen B, Kan H. Totally cystic schwannoma of the lumbar spine. Orthopedics 2013;5:679–82. (36)
Suzuki H, Yamaguchi Y, Kimura H, Shiba M, Baba M, Hiroshima K. Malignant mediastinal schwannoma associated with von Recklinghausen’s disease – a resected case. Nippon Kyobu Geke Gakkai Zaoshi. 1996;44:864–8.
Vilanova JC, Dolz JL, Maestro de Leon JL. MR imaging of a malignant schwannoma and an osteoblastoma with fluid-fluid levels. Report of two new cases. Eur Radio. 1998;8:1359–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samman, A.M., Bardeesi, A.M. & Alzahrani, M.T. Thoracic cystic schwannoma: case report and review of literature. Spinal Cord Ser Cases 7, 7 (2021). https://doi.org/10.1038/s41394-020-00376-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-020-00376-0
This article is cited by
-
Atypical presentation of an ancient retroperitoneal schwannoma mimicking a renal hydatid cyst: a case report and literature review
African Journal of Urology (2024)