Abstract
Introduction
Of the 23 cases of spinal intradural-extramedullary ependymomas which have been reported to date, 11 were diagnosed as anaplastic. Here we present a very rare case of a thoracic intradural-extramedullary (not intramedullary) anaplastic ependymoma in an adult along with a literature review.
Case presentation
A 29-year-old man presented with rapidly progressive gait disturbance, a sensory-deficit below the trunk and urination disorders that had begun a few months earlier. Magnetic resonance imaging of his thoracic spine revealed a dorsal-located intradural-extramedullary tumor at T4-5. The rapid deterioration of his symptoms within several months led him to refer to our department for surgery. Within one month the size of tumor increased to involve the T4-6 level, consequently worsening his gait disturbance. He underwent surgery and tumor mass was resected. However, there was leptomeningeal dissemination of the tumor cells on the surface of cord. A near-total resection was therefore achieved. Histopathology revealed the resected specimen had immunoreactivity for EMA/Vimentin/CD56/CD99/S-100/GFAP, with a Ki-67 index of ~35%. These factors led to the diagnosis of anaplastic ependymoma. Seven weeks postoperatively he received adjuvant radiotherapy to the whole brain and the whole spinal cord. He recovered as an independent ambulator without recurrence 1 year postoperatively.
Discussion
Because of their rarity, there are no clear treatment or adjuvant therapy guidelines for spinal anaplastic ependymoma. Adjuvant radiotherapy to the whole brain and spinal cord was necessarily indicated after near-total resection. Although the patient’s condition has not recurred 1 year after surgery, careful and serial follow-up is necessary for this individual.
Similar content being viewed by others
Introduction
Ependymoma is the most common intramedullary tumor of the central nervous system in adults, and arises from ependymal cells lining the ventricles and the central spinal canal [1]. According to the 2007 World Health Organization (WHO) classification of tumors of the central nervous system, ependymal tumors are classified into four types: subependymoma (grade I), myxopapillary ependymoma (grade I), ependymoma (grade II, cellular, papillary, clear cell, and tanycytic), and anaplastic ependymoma (grade III) [2]. The revised WHO classification in 2016 defined a new type of ependymoma; RELA fusion-positive type, which is defined by genetic abnormalities [3]. Ependymomas that arise from the intramedullary spinal cord are greatly different genetically from those that arise intracranially. Notably, most spinal ependymomas follow a relatively better course than intracranial tumors [1, 4]. Consequently, the golden standard of treatment for spinal ependymoma is surgical resection. However, cases of WHO grade III anaplastic ependymoma have worse outcomes than grade I or II masses [5], and the benefits of chemotherapy and radiation therapy for anaplastic ependymoma remain unclear because of its rarity [5]. To the best of our knowledge, only 23 cases of spinal intradural extramedullary ependymoma have been reported [6, 7], of which 11 were anaplastic [5,6,7,8,9,10,11,12,13,14,15]. We present a case of a thoracic intradural extramedullary (not intramedullary) anaplastic ependymoma in a patient with rapidly progressing myelopathy.
Case presentation
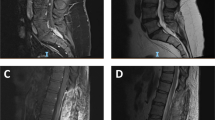
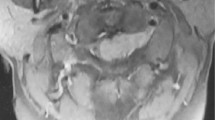
A 29-year-old healthy male was referred to our department with rapidly progressive gait disturbance and sensory deficits below the trunk over the past few months. He was diagnosed with a T4-5 level intradural extramedullary tumor on thoracic MRI at his previous hospital. He was unable to walk independently (modified McCormick scale [16] grade III), and needed support to maintain his standing position. He had numbness below T7 and hypoesthesia to pain and temperature below T4. Regarding the muscle strength of his lower extremities measured using the manual muscle testing (MMT) grading system, the scores of the right iliopsoas/quadriceps/biceps femoris/tibialis anterior were 4 and no other muscle weakness was noted. He had hyperactive bilateral patellar and Achilles tendon reflexes and had Babinski signs and ancle clonus bilaterally. He also exhibited a positive Romberg’s test, dysuria and hypotonia of the anal sphincter. His thoracic MRI revealed an intradural extramedullary tumor located at the T4-5 level that was hypointense on T1-weighted images, iso- to hyperintense on T2-weighted images, and demonstrated heterogeneous contrast enhancement with gadolinium (Fig. 1a–d). Over a period of one month, there was an obvious increase in the tumor’s size (Fig. 1e), and his gait disturbance worsened (wheelchair needed: modified McCormick scale grade V [16]). Given his rapidly progressive myelopathy manifest by gait apraxia and bladder/bowel dysfunction, the patient was referred to our hospital for surgery.
a–d Preoperative MRI images 1 month prior to the surgery. Sagittal images of T1 (a), T2 (b), and T1 post-gadolinium (c) contrast administration and an axial image of T1 post-gadolinium (d) administration are presented. The intradural extramedullary tumor on the T4-5 level on the dorsal side of spinal canal was iso-intense on T1-weighted images, mixed iso/hyper intense on T2-weighted images and demonstrated strong and diffuse enhancement with gadolinium. (e) T2 sagittal images 1 month after the primary MRI demonstrating increased tumor size.
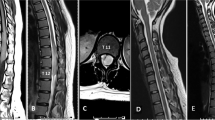
After a T4-6 laminectomy we confirmed the rostral and caudal edges of the tumor with intraoperative ultrasonography through the dura mater. As the tumor size increased since his preoperative MRI 1 week before surgery, we advanced our decompression to T3 before starting microscopic procedures. The dura and arachnoid maters were incised microscopically and the tumor was resected as much as possible. However, the tumor exhibited diffuse subpial dissemination on the dorsal surface of the cord with severe adhesions to the arachnoid mater. A near-total resection (NTR) of the coarse component of tumor was achieved (Fig. 2). Intraoperative pathology using frozen sections demonstrated a spindle cell tumor. Motor evoked potentials of the bilateral lower extremities were preserved during the surgery. We sutured the dura and arachnoid maters with an artificial membrane to prevent adhesive arachnoiditis and completed the surgery.
A dark red-colored bulk tumor (a, b) was located in the dorsal subarachnoid space. After the resection of as much of the dorsal tumor as possible, subpial dissemination (c) was detected on microscopic images. This could not be totally resected grossly. After applying an artificial membrane to prevent adhesive arachnoiditis, we had sutured the dura and arachnoid mater. Intraoperative ultrasound images showed that the central part of the tumor caused significant cord compression (e), and that the caudal end of the tumor had a smooth surface that was compressing the cord (f).
Histopathology was significant for perivascular pseudo-rosette formation with perivascular arrangement of the tumor cells with analogous to oval-shaped nuclei on hematoxylin-eosin staining. Higher magnification images showed a high cell density and mitotic appearance. Immunohistochemistry showed that the resected tumor had dot-like EMA immunoreactivity and was Vimentin/CD56/CD99/S-100/GFAP positive, with a Ki-67 index of ~35%. These findings led to the final diagnosis of anaplastic ependymoma (Fig. 3).
Hematoxylin and eosin staining showed perivascular pseudo-rosettes with a perivascular arrangement of tumor cells with round to oval-shaped nuclei (a) and some necrotic changes (b). On higher magnification images, proliferating tumor cells associated with multiple mitotic images and a large nuclear-cytoplasmic ratio were observed (c, d). Immunohistochemistry revealed that the tumor cells were all positive for GFAP (e), S-100 (f), Vimentin (g), CD56 (h), CD99 (i). Dot-like immunoreactivity to EMA was demonstrated (j). The ratio of Ki-67 positive cells was ~35% (k). Image magnifications; (a, b) x100, (c) x200, (d–k) x400.
The patient began physical therapy that included standing and walking training four days postoperatively. His preoperative instability and lower extremity muscle strength gradually recovered. He advanced to walking with double canes 4 weeks after surgery. Based on the intraoperative findings of subpial diffuse dissemination on the dorsal surface of the cord, adjuvant radiotherapy was started 7 weeks after surgery. Stereotactic external-beam radiotherapy was performed on the patient’s whole brain and spinal cord, with a total dose of 36 Gy on his brain and 14.4 Gy on his spinal cord. One year postoperatively the patient was an independent ambulator (modified McCormick scale grade II) without evidence of tumor recurrence or dissemination to his brain or spinal cord on MRI (Fig. 4).
Discussion
Ependymoma is the most common spinal intramedullary tumor, and occurs primarily in adults [17]. Ependymomas are tumors that arise from the ependymal cells that line the ventricles of the brain, the choroid plexus, the central canal of the spinal cord, and the peripheral membrane. Ependymomas are divided into grades I-III depending on the degree of malignancy as defined by the WHO classification. The frequency of each grade was 6.6% grade I, 83.9% grade II and 9.5% grade III [5]. The current case—grade III anaplastic ependymoma—is thought to be the most aggressive type of ependymoma [18]. Based on previous reports, spinal anaplastic ependymomas have a female preponderance and are most commonly found in the thoracic spine [5, 19], and typically present with limb-muscle weakness, dysuria, and gait disturbances due to myelopathy [6].
The histopathologic features of ependymomas generally include a clear demarcation between the surrounding tissue and the ependymal rosette, perivascular pseudo-rosettes, and immunohistochemical positivity to GFAP, EMA, and CD56. Ki-67 index also considered an important prognostic marker that correlates with the grade of ependymoma [20]. The Ki-67 index of a grade III ependymoma is significantly higher (~10–15%) than that for grade I and II disease (~1–4%) [21]. The current case had a relatively increased Ki-67 index of 35%, indicating a high degree of malignancy and a worse prognosis without postoperative adjuvant therapy.
The golden standard for the treatment for spinal ependymomas is surgical total resection [22]. Grade I and II ependymomas exhibit slower tumor growth and a more indolent clinical course, and tend to compress rather than invade the adjacent spinal cord parenchyma [23]. Hence lower grades of ependymoma have a relatively clear margin between tumor and normal spinal cord tissue, and have a better prognosis after gross total resection [22,23,24]. However, the degree of spinal cord involvement differs by each WHO classification, and gross total resection may therefore not always be possible for all cases, especially for grade III tumors. In grade III ependymomas the normal spinal cord parenchyma is likely to be invaded by tumor cells. As a result, only a partial tumor resection is possible, and the prognosis is poor. A high recurrence rate of grade III ependymomas has been reported even with postoperative additional radiation therapy. Kobayashi et al. reported 5-year recurrence-free survival rates of 90%, 91%, and 20% for grades I, II and III ependymomas, respectively [25]. A retrospective study by Li et al. showed that 82.9% of individuals with grade II or III spinal ependymoma were amenable to radical surgery. However, when divided into three groups based on the tumor length (less than 5 cm, 5–10 cm, and greater than 10 cm), patients with larger tumors had worse postoperative functional outcomes than those with small masses, with especially poor outcomes when the tumor was >10 cm [19]. Even worse, patients with larger tumors also were more likely to develop postoperative neuropathic pain and proprioceptive deficits [19]. The current case was diagnosed relatively early and the size of that patient’s tumor was 4.3 cm rostro-caudally. As a result, a near-total resection was achieved and the patient remains neurologically stable 1 year after surgery and adjuvant radiotherapy. The early diagnosis and surgery were deemed important contributors to better neurologic outcomes.
Regarding the intraspinal location of ependymomas, to the best of our knowledge only 23 cases of intradural extramedullary ependymoma have been reported [6, 7], of which 11 were anaplastic [5,6,7,8,9,10,11,12,13,14,15] (Table 1). Since ependymomas are tumors that arise from ependymal cells that line the ventricles of the spinal cord, the origin of intradural extramedullary ependymomas provokes a controversy. Although the exact origin of intradural extramedullary ependymomas (except for grade I myxopapillary ependymoma arising at the lumbar spine) is unclear, a previous report speculated that the existence of ectopic ependymal tissues might lead to an atypical ependymoma [26]. Ependymomas (especially myxopapillary type) occuring outside the central nervous system were presumed to be originated from coccygeal medullary vestige remnants or ependymal rest [27]. The extramedullary ependymoma might be originated from ectopic ependymal tissues such as ependymal rest. Of the 11 total cases of an intradural extramedullary anaplastic ependymoma, 5 were followed for more than 1 year [5,6,7, 10, 12]. Robles et al. reported that despite a gross total resection (GTR), the tumor recurred at 1 after surgery without additional postoperative radiation therapy [10]. In the cases described by Pomeraniec et al. and Chakravorty et al., even though they resected the tumor partially (subtotal resection (STR)), after adjuvant postoperative radiotherapy to the whole brain and spinal cord no recurrence was observed over more than 1 year of follow-up [6, 7]. While Schuurmans et al. reported the recurrence of STR even after radiation therapy to the whole brain and spinal cord 2 years postoperatively [12], postoperative adjuvant radiation therapy was considered mandatory for anaplastic ependymomas. Furthermore, our intraoperative findings clearly showed leptomeningeal dissemination of tumor onto the surface of the cord. These taken together we conducted adjuvant whole brain and spinal cord irradiation in the present case to prevent recurrence.
In conclusion, a rare case of a thoracic intradural extramedullary (not intramedullary) anaplastic ependymoma that underwent near-total resection followed by adjuvant radiation therapy to the whole brain and spinal cord is reported. One year postoperatively the patient has had a favorable clinical course and preserved ambulatory ability. Although there is no solid evidence of the effectiveness of adjuvant radiotherapy against spinal anaplastic ependymoma, we believe that the additional radiotherapy to the whole brain and spinal cord after the near-total resection of tumor permitted better neurologic recovery and no obvious tumor recurrence 1-year after surgery. Careful and serial follow-up is necessary for this individual.
References
Guarnieri G, Tecame M, Izzo R, Zeccolini F, Genovese L, Muto M. Multisegmental diffuse intradural extramedullary ependymoma. An extremely rare case. Neuroradiol J. 2014;27:179–85.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–20.
Lee CH, Chung CK, Kim CH. Genetic differences on intracranial versus spinal cord ependymal tumors: a meta-analysis of genetic researches. Eur Spine J. 2016;25:3942–51.
Liu X, Sun B, Xu Q, Che X, Hu J, Gu S, et al. Outcomes in treatment for primary spinal anaplastic ependymomas: a retrospective series of 20 patients. J Neurosurg Spine. 2013;19:3–11.
Pomeraniec IJ, Dallapiazza RF, Sumner HM, Lopes MB, Shaffrey CI, Smith JS. Anaplastic extramedullary cervical ependymoma with leptomeningeal metastasis. J Clin Neurosci. 2015;22:1871–6.
Chakravorty A, Frydenberg E, Shein TT, Ly J, Earls P, Steel T. Multifocal intradural extramedullary anaplastic ependymoma of the spine. J Spine Surg. 2017;3:727–31.
Oliver B, de Castro A, Sarmiento MA, Arguello C, Blazquez MG. [Dorsal extramedullary ependymoma (author’s transl)]. Arch Neurobiol. 1981;44:215–24.
Katoh S, Ikata T, Inoue A, Takahashi M. Intradural extramedullary ependymoma. A case report. Spine (Philos Pa 1976). 1995;20:2036–8.
Robles SG, Saldana C, Boto GR, Martinez A, Zamarron AP, Jorquera M, et al. Intradural extramedullary spinal ependymoma: a benign pathology? Spine (Philos Pa 1976). 2005;30:E251–4.
Cerase A, Venturi C, Oliveri G, De Falco D, Miracco C. Intradural extramedullary spinal anaplastic ependymoma- case illustration. J Neurosurg Spine. 2006;5:476.
Schuurmans M, Vanneste JA, Verstegen MJ, van Furth WR. Spinal extramedullary anaplastic ependymoma with spinal and intracranial metastases. J Neurooncol. 2006;79:57–9.
Guppy KH, Hou L, Moes GS, Sahrakar K. Spinal intradural, extramedullary anaplastic ependymoma with an extradural component: case report and review of the literature. Surg Neurol Int. 2011;2:119.
Kinsman MJ, Callahan JD, Hattab EM, Cohen-Gadol AA. Extramedullary spinal ependymoma: a diagnostic challenge and review of the literature. Clin Neurol Neurosurg. 2011;113:661–4.
Kim BS, Kim SW, Kwak KW, Choi JH. Extra and intramedullary anaplastic ependymoma in thoracic spinal cord. Korean J Spine. 2013;10:177–80.
Matsuyama Y, Sakai Y, Katayama Y, Imagama S, Ito Z, Wakao N, et al. Surgical results of intramedullary spinal cord tumor with spinal cord monitoring to guide extent of resection. J Neurosurg Spine. 2009;10:404–13.
Nishikawa R. Pediatric and adult gliomas: how different are they? Neuro Oncol. 2010;12:1203–4.
Ellison DW, Kocak M, Figarella-Branger D, Felice G, Catherine G, Pietsch T, et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7.
Li TY, Chu JS, Xu YL, Yang J, Wang J, Huang YH, et al. Surgical strategies and outcomes of spinal ependymomas of different lengths: analysis of 210 patients: clinical article. J Neurosurg Spine. 2014;21:249–59.
Manasa LP, Uppin MS, Sundaram C. Correlation of p53 and KI-67 expression with grade and subtype of ependymoma. Indian J Pathol Microbiol. 2012;55:308–13.
Zawrocki A, Izycka-Swieszewska E, Papierz W, Liberski PP, Zakrzewski K, Biernat W. Analysis of the prognostic significance of selected morphological and immunohistochemical markers in ependymomas, with literature review. Folia Neuropathol. 2011;49:94–102.
Tsuji O, Nagoshi N, Ishii R, Nori S, Suzuki S, Okada E et al. Poor prognostic factors for surgical treatment of spinal intramedullary ependymoma (World Health Organization Grade II). Asian Spine J. 2020. https://doi.org/10.31616/asj.2020.0064 [Epub ahead of print].
Karikari IO, Nimjee SM, Hodges TR, Cutrell E, Hughes BD, Powers CJ, et al. Impact of tumor histology on resectability and neurological outcome in primary intramedullary spinal cord tumors: a single-center experience with 102 patients. Neurosurgery. 2011;68:188–97. Discussion 197.
Volpp PB, Han K, Kagan AR, Tome M. Outcomes in treatment for intradural spinal cord ependymomas. Int J Radiat Oncol Biol Phys. 2007;69:1199–204.
Kobayashi K, Ando K, Kato F, Kanemura T, Sato K, Kamiya M, et al. Surgical outcomes of spinal cord and cauda equina ependymoma: Postoperative motor status and recurrence for each WHO grade in a multicenter study. J Orthop Sci. 2018;23:614–21.
Bonfield CM, Amin D, Hamilton RL, Gerszten PC. Extramedullary ependymoma near the conus medullaris with lumbar nerve root attachment: case report. Neurosurgery. 2011;68:E831–4.
Pulitzer DR, Martin PC, Collins PC, Ralph DR. Subcutaneous sacrococcygeal (“myxopapillary”) ependymal rests. Am J Surg Pathol. 1988;12:672–7.
Acknowledgements
We thank for Dr. Kota Kojima and Dr. Yuki Miwa for their critical advice. We also thank for Ms Makiko Miyazaki, Ms Yukari Yamanishi, and Ms Kaoru Yasumuro for their assistance.
Author information
Authors and Affiliations
Contributions
Writing – original draft: SA and OT. Writing – review and editing: NN, SN, SS, EO, MY, and KW. Data curation – SA, OT, MN, and MM. Pathological analyses – RI. OT was responsible for all working related to this submission as corresponding author. Also, all authors approved the final version manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ando, S., Tsuji, O., Nagoshi, N. et al. Grade III intradural extramedullary anaplastic ependymoma managed with near-complete resection and adjuvant radiotherapy: a case report. Spinal Cord Ser Cases 7, 1 (2021). https://doi.org/10.1038/s41394-020-00367-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-020-00367-1