Abstract
Study design:
Prospective case series
Objectives:
To assess the effectiveness and safety of two vitamin D3 repletion protocols given to individuals with spinal cord injury (SCI).
Setting:
Publicly-funded intensive inpatient rehabilitation center, Montreal, Canada.
Methods:
Thirty adults with recent SCI complete or incomplete sensorimotor impairments were recruited upon admission from designated regional SCI trauma centers. Participants with serum 25OHD ≤ 30 nmol/L were given 10,000 IU of weekly and 1000 IU of daily vitamin D3 for 36.8 ± 11.9 days (higher dose: HD). Subjects with serum 25OHD > 30 nmol/L received 1000 IU of daily vitamin D3 for 38.2 ± 11.6 days (lower dose: LD). Outcomes were changes in 25OHD levels from baseline to the end of the study period and safety outcomes. Thresholds for vitamin D deficiency, insufficiency and sufficiency were: 25OHD levels ≤30 nmol/L, 30–74 nmol/L, and ≥75 nmol/L.
Results:
At baseline, 34 and 66% of participants had serum 25OHD < 30 and >30 nmol/L. Both protocols induced a rise in serum 25OHD with a greater increase in the HD vs. LD regimen (31.4 [95% CI: 16.7, 46.0] vs. 11.7 nmol/L [95% CI: 2.2, 21.2]). None of the participants given the HD remained vitamin D deficient, but only one achieved vitamin D sufficiency. Nearly all individuals on the LD regimen remained vitamin D insufficient with only two reaching vitamin D sufficiency. No adverse effects were observed over the course of the supplementation.
Conclusions:
Although 1000 IU of daily vitamin D3 alone or in combination with weekly 10,000 IU for an average of 37.6 days increased serum 25OHD, they were unsuccessful in improving the impaired vitamin D status during inpatient rehabilitation of individuals with a recent SCI.
Similar content being viewed by others
Introduction
Vitamin D has received much attention in recent years for its biological actions that go beyond its endocrine effects on the calcium and phosphorus homeostasis. The concomitant expression of the vitamin D-metabolizing enzyme, 1-α hydroxylase (CYP27B1), and the vitamin D receptor (VDR) in various extra-renal tissues has raised the possibility of a range of autocrine and paracrine actions targeting the skin, immune system, and musculoskeletal system, among other body systems [1]. The fact that the extra-renal CYP27B1 activity appears to be strongly influenced by the level of its substrate—namely circulating 25-hydroxyvitamin D (25OHD)—raises the importance of achieving and maintaining sufficient serum 25OHD levels to ensure activation of both the vitamin D endocrine and auto/paracrine pathways. The autocrine and paracrine actions of vitamin D may explain in part the association between vitamin D deficiency and multiple comorbidities including cardiovascular disease, inflammatory disorders, nephropathy, mood disorders, and chronic musculoskeletal pain [2]. Interestingly, people with spinal cord injury (SCI) experience similar health conditions post-injury [3], hence suggesting that attainment and maintenance of vitamin D sufficiency is a desirable clinical goal, as it may be linked to better health outcomes in these individuals.
Impaired vitamin D status has been largely reported in people with SCI in both the acute and chronic settings [4,5,6,7,8,9]. Contributing factors include insufficient sun exposure, limited oral intake, obesity, use of substances (e.g., some medications) or conditions (e.g., kidney/liver disease) affecting vitamin D metabolism [10]. Although people with SCI are at high risk of impaired vitamin D status, the Endocrine Society clinical practice guidelines on vitamin D have not included explicit population-specific recommendations [11]. Furthermore, only a few repletion protocols have been tested in adults with SCI with varying degrees of improvement [12,13,14,15]. However, most of these studies have been performed in adults with chronic SCI and information regarding vitamin D supplementation strategies shortly after an initial SCI is lacking. The high prevalence of vitamin D deficiency reported in adults who sustained a new SCI [8, 9] underscores the importance of finding safe and effective repletion regimens that could be initiated upon admission into inpatient rehabilitation so as to minimize the risk of secondary complications and optimize the benefits of rehabilitation interventions.
The goals of this study are to (1) characterize the vitamin D status, (2) document the effectiveness of two vitamin D3 protocols typically prescribed in our center in achieving vitamin D sufficiency (i.e., serum 25OHD levels ≥75 nmol/L), and (3) assess the safety of these protocols in adults with acute SCI admitted in a specialized inpatient SCI rehabilitation program.
Methods
Study design
A prospective case series reporting pre- and post-intervention measures of a two-arm interventional study.
Setting
A publicly-funded rehabilitation hospital, servicing the western part of the province of Quebec, Canada, where a 25-bed specialized SCI inpatient rehabilitation program is offered.
Participants
Between February 2015 and March 2016, all adults (≥18 years old) referred to the inpatient SCI rehabilitation program who had recently sustained a traumatic or non-traumatic SCI at or below the 5th cervical spine (neurological level) with ASIA Impairment Scale A-D were screened upon admission for study eligibility. Participants had to be deemed medically-stable, referred directly from a traumatology program at a partnered acute hospital and had no vitamin D supplementation initiated during the acute hospital stay. Exclusion criteria were: conditions or use of medications known to interfere with vitamin D or calcium absorption or metabolism, history of chronic disease (e.g., diabetes; cancer), impaired capacity for consent or current use of vitamin D supplements. Ethical approval was obtained from the Research Ethics Committee of the Center for Interdisciplinary Research in Rehabilitation of Greater Montreal. All participants provided informed consent.
Intervention
Participants were stratified into two subgroups based on initial serum 25OHD levels: deficiency (≤30 nmol/L) or insufficiency (>30 nmol/L) [16]. Individuals with serum 25OHD ≤ 30 nmol/L received 10 000IU vitamin D3/week in addition to a daily regimen of 1000 IU vitamin D3 (higher dose: HD). Individuals with serum 25OHD > 30 nmol/L were assigned to daily vitamin D3 (1000 IU) (lower dose: LD). These regimens were typically prescribed by physicians in our center. Given the absence of data on vitamin D repletion protocols in individuals with SCI during the acute and subacute (i.e., rehabilitation) phases, the selection of doses was based on local routine practices and primarily guided by safety considerations to prevent vitamin D toxicity. The selection of 10,000 IU/week was also made to minimize study burden for participants. A dose superior to 10,000 would have necessitated participants to take more than 1 pill at a time, which may have decreased their willingness to participate. In addition, all participants received 500 mg of calcium carbonate to meet their age- and sex-specific recommended dietary allowance (RDA) for calcium. Calcium supplementation is routinely given to individuals in our center to counteract the enhanced bone resorption and rapid bone loss occurring in the acute and subacute phases. Regimens were prepared by the pharmacist associated with the SCI program and supplements administered by the nursing staff and documented in the medication flow sheet. These interventions were carried out until rehabilitation center discharge. To be included in the analyses, participants had to take the supplements for at least 3 weeks. During inpatient stay, participants were followed by a multidisciplinary rehabilitation team and engaged in approximately one hour of physical therapy and one hour of occupational therapy on weekdays.
Outcomes
All outcomes were measured within 72 h both pre-intervention (i.e., baseline) and post-intervention (i.e., end).
Blood biochemistry
Serum total 25OHD levels were measured using an automated chemiluminescent immunoassay performed on a VITROS®ECIQ analyzer (Ortho Clinical Diagnosis, Mississauga, ON, Canada). The interassay coefficients of variation varied depending on the level of 25OHD detected and ranged from 3.4% when 25OHD levels were high (<175 nmol/L) to 14% when levels were low (<25 nmol/L). Vitamin D status was categorized according to serum 25OHD as follows: vitamin D deficiency (<30 nmol/L), insufficiency (30–74 nmol/L), and sufficiency (≥75 nmol/L) [11]. Safety laboratory measures included serum phosphorus and total and ionized calcium measured at baseline and at the end of the study. Clinical adverse health effects (e.g., nephrolithiasis, hypercalcemia) were monitored throughout the study.
Statistical analysis
Normality of data distribution was checked using the Shapiro-Wilk test. Continuous variables with a normal distribution are presented as means ± SD while data with a skewed distribution are expressed as medians (interquartile ranges; (IQR)). Categorical variables are presented as frequencies and percentages. Between-group and within-group differences were assessed by means of parametric (i.e., Student’s t-tests and paired t-tests) and non-parametric (i.e., Mann–Whitney U, χ2 and Fisher’s exact test) methods. All calculations were made with SPSS, version 24 (IBM Corp., Armonk, NY) with significance set at p < 0.05.
Results
Baseline characteristics of the participants
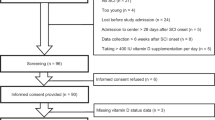
As illustrated in Fig. 1, 30 adults with SCI accepted to participate. Among those, one was excluded due to a missed assessment of serum 25OHD at study entry. Table 1 displays demographics, anthropometrics, laboratory results and clinical characteristics for all participants and for the two subgroups. Baseline serum 25OHD was low with 94% of individuals exhibiting either vitamin D deficiency or insufficiency even though nearly two-third of serum 25OHD levels were assessed in summer and fall. Among the 29 participants included into the analysis, 34.5% and 65.5% had serum 25OHD indicative of vitamin D deficiency and insufficiency, respectively.
Participants with 25OHD levels ≤30 and >30 nmol/L also differed in terms of seasonal variations in the occurrence of the SCI and blood sampling. Most SCI were sustained during spring and summer seasons in participants with serum 25OHD > 30 nmol/L while SCI occurred more frequently in the winter and spring seasons in vitamin D-deficient individuals. Since assessment of 25OHD levels was performed shortly after admission to inpatient rehabilitation, seasons of blood testing paralleled those of spinal cord lesions.
Six participants were excluded from the study during the follow-up period and most of them were allocated to the insufficiency subgroup (5/6 = 83.3%). Reasons for exclusion included compliance issues, missing serum 25OHD values at the end of intervention, and new diagnosis of malabsorption. Among participants who completed the study, duration of supplementation was similar in both groups (HD: 36.8 ± 11.9 vs. LD: 38.2 ± 11.6 days). Hence, analyses of the intervention effects were completed on 9 and 14 participants from the deficiency and insufficiency subgroups, respectively.
Effects of repletion regimens on vitamin D status
Fig. 2a illustrates individual serum 25OHD levels by treatment at baseline and at the end of the intervention. Within-groups, there was a mean increase of 31.4 (95% CI: 16.7, 46.0; p = 0.001) and 11.7 nmol/L (95% CI: 2.2, 21.2; p = 0.02) in serum 25OHD for the deficiency (i.e., HD) and insufficiency (i.e., LD) subgroups. The rise in serum 25OHD did not differ significantly according to the season of supplementation (p = 0.729), but was slightly greater in individuals who started supplementation in winter and spring (27.2 (95% CI: 7.0, 47.5) and 18.5 nmol/L (95% CI: −23.3, 60.3)) than summer and fall (17.7 (95% CI: 1.8, 33.7, and 14.2 nmol/L (95% CI: −11.7, 40.1)). All individuals in the HD regimen experienced an increase in serum 25OHD whereas two participants assigned to the LD regimen had a decrease in their serum 25OHD. In both cases, pre-intervention and post-intervention 25OHD levels were assessed in the summer and/or fall. None of the participants who received the HD regimen remained in the vitamin D deficiency range (Fig. 2b). Although all subjects in this subgroup improved their vitamin D status by the end of the study, only one achieved vitamin D sufficiency (25OHD > 75 nmol/L). Nearly all individuals receiving the LD regimen remained vitamin D insufficient and only two transitioned from an insufficient to a sufficient status.
a Serum 25-hydroxyvitamin D levels at baseline and at the end of the follow-up period. Individual 25OHD levels are represented by treatment group with the mean indicated by the straight gray line. The dotted line indicates the cut-point proposed for vitamin D sufficiency (75 nmol/L). Within-group comparisons were made by paired-t test. b Distribution of vitamin D status at baseline and at the end of the follow-up period in each treatment group. Black bar indicates vitamin D deficiency (serum 25OHD < 30 nmol/L); dashed bars indicate vitamin D insufficiency (serum 25OHD 30–74 nmol/L) and white bars indicate vitamin D sufficiency (serum 25OHD ≥ 75 nmol/L). Within-group comparisons were made by χ2 test
Adverse effects
The highest recorded individual 25OHD concentrations were 89.4 and 90.7 nmol/L in the HD and LD subgroups, which is below the upper limit (i.e., 250 nmol/L) associated with vitamin D toxicity [17]. There were minor fluctuations in serum calcium and phosphorus during the repletion period but none of clinical significance (Table 2). No episodes of hypercalcemia or nephrolithiasis and no serious health adverse effects occurred over the course of the supplementation.
Discussion
Given the lack of standardized vitamin D therapies for individuals with acute SCI, we undertook this study to assess the effectiveness of two vitamin D3 repletion regimens, currently administered over the course of the inpatient stay at our rehabilitation center, in improving suboptimal serum 25OHD levels. After approximately 1 month of dosing regimens providing either 1000 IU of daily vitamin D3 or 1000 IU of daily vitamin D3 combined with weekly doses of 10,000 IU vitamin D3, very few participants achieved vitamin D sufficiency despite a significant rise in serum 25OHD levels.
The majority of participants (n = 27/29 = 93%) had serum 25OHD below 75 nmol/L at study entry, a rate comparable to what has been previously reported in adults with acute SCI living in northern USA [8], but above that found in adults with SCI from southern US locations [9]. Since circulating 25OHD has a half-life of 3 weeks, the measured levels may partly reflect the pre-SCI vitamin D status as time from injury ranged from 15 to 92 days. However, according to the most recent Canadian Health Measures Survey, the proportion of Canadians with serum 25OHD below 30 nmol/L is 10% [18], which is lower than the rate found in our study (i.e., 35%), suggesting that SCI-related factors, such as sunlight deprivation due to hospitalization and decreased vitamin D oral intake due to anorexia and swallowing disorders, may contribute to the deterioration of the vitamin D status.
The combined intake of daily 1000 IU and weekly 10,000 IU of vitamin D3 over an average of 37 days by vitamin D-deficient adults with SCI resulted in a mean serum 25OHD increase of 31.4 nmol/L whereas daily 1000 IU given alone raised 25OHD levels by 11.7 nmol/L. Although the HD regimen corrected vitamin D deficiency in all participants, most subjects remained vitamin D insufficient. The LD regimen was clearly insufficient to improve vitamin D status as only two individuals had their 25OHD levels increased from insufficient to optimal. Direct comparison to the current literature is difficult, as efficacy of vitamin D therapies have been solely reported in adults with a chronic SCI in the community. The only vitamin D study performed in the acute setting was done in adults with a traumatic SCI who received, within 8 h of injury, 5 μg/kg of oral vitamin D3 for up to 5 days [19]. Since the main objective of this last study was to assess the effects of early administration of vitamin D3 and progesterone on functional outcomes and recovery, the authors neither measured baseline 25OHD levels nor evaluated the impact of the supplementation on serum 25OHD. Studies done in individuals with chronic SCI have tested various dosing regimens and durations. Bauman et al. first reported a mean serum 25OHD increase of 29.4 nmol/L following 800 IU of daily vitamin D3 for 12 months and concluded that this replacement protocol was insufficient for clinical use[12]. A few years later, the same group assessed the efficacy and safety of a higher dose (i.e., 2000 IU) given over 3 months to vitamin D-deficient adults with SCI and reported a significant rise in serum 25OHD of 85 nmol/L and the correction of suboptimal vitamin D status in nearly all participants [13]. These observations are not consistent with our results whereby a HD regimen, corresponding to an equivalent daily dose of 2429 IU, induced a lesser increase in serum 25OHD and failed to normalize vitamin D status in the majority of vitamin D-deficient participants. Possible reasons explaining these discrepancies may relate to injury stage. Soon after injury, individuals are known to experience acute inflammation as evidenced by the C-reactive protein (CRP) levels above the reference range (i.e., > 10 mg/L) in 45% of the present sample (n = 13/29). It has been reported that serum 25OHD decreased acutely and markedly in adults who underwent primary knee arthroplasty and that this decline lasted for up to 3 months [20, 21]. Interestingly, at study entry, 60% of vitamin D-deficient individuals (n = 6/10) had high CRP levels compared to 37% of non-deficient subjects (n = 7/19). Although it is impossible to ascertain the temporality of this relationship, the presence of inflammation in the acute phase potentially explains the low 25OHD levels observed in our participants and may thus represent a factor affecting responsiveness to supplementation. Nevertheless, in light of the potential decline of serum 25OHD during rehabilitation as a result of compromised sun exposure and reduced vitamin D intake, our study underscores that vitamin D repletion protocols prevent further deterioration of vitamin D status during the subacute phase. Consistent with other studies [6, 22, 23], level of motor impairment did not affect serum 25OHD in our study. Baseline and post-intervention 25OHD levels did not differ between AIS A + B and C + D individuals. However, these findings should be interpreted cautiously given the small number of AIS A + B individuals in our sample (1: HD and 3: LD).
Prolonged immobilization occurring in the acute phase induces a marked elevation in bone resorption, which results in hypercalcemia and PTH suppression [24]. The fear of triggering or aggravating hypercalcemia and hypercalciuria may impede the widespread implementation of vitamin D supplementation during the acute phase. Our findings indicate that administration of either of the two regimens for at least 1 month did not cause any adverse effects. Although we cannot exclude that deleterious effects may occur over time, studies done in adults with chronic SCI failed to report any toxicity with larger doses and over longer periods than those tested in this study [13, 14].
Adults with a new SCI may experience rapid bone loss shortly after injury that increases risk of osteoporosis and fractures [25, 26]. Intervening early in the course of the rehabilitation with appropriate vitamin D repletion protocols, alone or in combination with anti-osteoporosis agents and/or mechanical therapy, may have a positive effect on the rate of bone loss. In the only study that has looked at factors associated with the development of osteoporosis within 12 months of SCI, the authors failed to find an association between baseline 25OHD levels and the occurrence of osteoporosis [26]. They explained this finding by the fact that participants with low serum 25OHD received vitamin D supplements during the follow-up period, which may have obscured the association.
This study is the first to report the effectiveness of two vitamin D3 replacement strategies during inpatient rehabilitation for SCI; however, limitations should be acknowledged. First, the sample size is small and is explained, in most part, by the comprehensive list of exclusion criteria developed to circumscribe the effects of the vitamin D supplementation. In fact, out of the 156 adults with SCI admitted to the rehabilitation center and screened for eligibility, 119 did not qualify based on the inclusion and exclusion criteria whereas only 7 refused to participate. In addition, two-thirds of our participants were AIS D, a situation reflecting the shift in the admission patterns towards more incomplete injuries. The reasons underlying such shift are unclear but certainly explain the limited inpatient rehabilitation length of stay of our participants. Such selection bias limits the applicability of the findings to the entire population of individuals with a recent SCI admitted to rehabilitation and may lead to an underestimation of the prevalence rates of vitamin D deficiency and insufficiency reported in this study. A pragmatic approach with the inclusion of all adults with a SCI admitted to rehabilitation, regardless of their comorbidities, would have portrayed more accurately the vitamin D status of individuals with a recent SCI. Second, the main objective of this study was to assess the effect of vitamin D3 repletion protocols in the acute phase of SCI; therefore, no attempt was made to correlate these changes to clinical or functional outcomes. Indeed, durations of supplementation were too short to capture any association with clinical improvements. Third, although our participants did not experience any clinical and laboratory adverse effects, we did not have data on urinary calcium excretion—a recognized marker of vitamin D intoxication. Finally, there was no control group made of non-supplemented individuals in the present study. Given the high rate of vitamin D insufficiency in this population, it was deemed unethical to not provide supplements to these individuals.
In conclusion, administration of 1000 IU daily vitamin D3 alone or in combination with 10,000 IU weekly vitamin D3 during intensive inpatient rehabilitation significantly increased serum 25OHD but was not successful in correcting the vitamin D deficiency and insufficiency found in individuals with a recent SCI admitted to rehabilitation. These findings will inform clinicians working in inpatient rehabilitation settings about the effectiveness of these two repletion protocols and provide a basis for the design of future studies aiming to test the efficacy and safety of higher-dose regimens in achieving vitamin D sufficiency at this particular stage post-injury.
References
Morris HA, Anderson PH. Autocrine and paracrine actions of vitamin D. Clin Biochem Rev. 2010;31:129–38.
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408.
Jensen MP, Truitt AR, Schomer KG, Yorkston KM, Baylor C, Molton IR. Frequency and age effects of secondary health conditions in individuals with spinal cord injury: a scoping review. Spinal Cord. 2013;51:882–92.
Bauman WA, Zhong YG, Schwartz E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism. 1995;44:1612–6.
Gaspar AP, Brandao CM, Lazaretti-Castro M. Bone mass and hormone analysis in patients with spinal cord injury: evidence for a gonadal axis disruption. J Clin Endocrinol Metab. 2014;99:4649–55.
Hummel K, Craven BC, Giangregorio L. Serum 25(OH)D, PTH and correlates of suboptimal 25(OH)D levels in persons with chronic spinal cord injury. Spinal Cord. 2012;50:812–6.
Javidan AN, Sabour H, Latifi S, Vafa M, Shidfar F, Khazaeipour Z, et al. Calcium and vitamin D plasma concentration and nutritional intake status in patients with chronic spinal cord injury: a referral center report. J Res Med Sci. 2014;19:881–4.
Nemunaitis GA, Mejia M, Nagy JA, Johnson T, Chae J, Roach MJ. A descriptive study on vitamin D levels in individuals with spinal cord injury in an acute inpatient rehabilitation setting. PMR. 2010;2:202–8. quiz 28.
Oleson CV, Patel PH, Wuermser LA. Influence of season, ethnicity, and chronicity on vitamin D deficiency in traumatic spinal cord injury. J Spinal Cord Med. 2010;33:202–13.
Lamarche J, Mailhot G. Vitamin D and spinal cord injury: should we care? Spinal Cord. 2016;54:1060–75.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
Bauman WA, Morrison NG, Spungen AM. Vitamin D replacement therapy in persons with spinal cord injury. J Spinal Cord Med. 2005;28:203–7.
Bauman WA, Emmons RR, Cirnigliaro CM, Kirshblum SC, Spungen AM. An effective oral vitamin D replacement therapy in persons with spinal cord injury. J Spinal Cord Med. 2011;34:455–60.
Flueck JL, Schlaepfer MW, Perret C. Effect of 12-Week Vitamin D supplementation on 25[OH]D status and performance in athletes with a spinal cord injury. Nutrients. 2016;8.
Amorim S, Teixeira VH, Corredeira R, Cunha M, Maia B, Margalho P, et al. Creatine or vitamin D supplementation in individuals with a spinal cord injury undergoing resistance training: A double-blinded, randomized pilot trial. J Spinal Cord Med. 2017;1–8.
Shah S, Chiang C, Sikaris K, Lu Z, Bui M, Zebaze R, et al. Serum 25-Hydroxyvitamin D insufficiency in search of a bone disease. J Clin Endocrinol Metab. 2017;102:2321–8.
Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–6S.
Canada S. Vitamin D blood levels of Canadians 2015. https://www.statcan.gc.ca/pub/82-624-x/2013001/article/11727-eng.htm-n14.
Aminmansour B, Asnaashari A, Rezvani M, Ghaffarpasand F, Amin Noorian SM, Saboori M, et al. Effects of progesterone and vitamin D on outcome of patients with acute traumatic spinal cord injury; a randomized, double-blind, placebo controlled study. J Spinal Cord Med. 2016;39:272–80.
Waldron JL, Ashby HL, Cornes MP, Bechervaise J, Razavi C, Thomas OL, et al. Vitamin D: a negative acute phase reactant. J Clin Pathol. 2013;66:620–2.
Reid D, Toole BJ, Knox S, Talwar D, Harten J, O’Reilly DS, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011;93:1006–11.
Barbonetti A, Sperandio A, Micillo A, D’Andrea S, Pacca F, Felzani G, et al. Independent association of vitamin D with physical function in people with chronic spinal cord injury. Arch Phys Med Rehabil. 2016;97:726–32.
Barbonetti A, Vassallo MR, Felzani G, Francavilla S, Francavilla F. Association between 25(OH)-vitamin D and testosterone levels: Evidence from men with chronic spinal cord injury. J Spinal Cord Med. 2016;39:246–52.
Maynard FM. Immobilization hypercalcemia following spinal cord injury. Arch Phys Med Rehabil. 1986;67:41–4.
Bauman WA, Cardozo CP. Osteoporosis in individuals with spinal cord injury. PMR. 2015;7:188–201. quiz
Gifre L, Vidal J, Carrasco JL, Muxi A, Portell E, Monegal A, et al. Risk factors for the development of osteoporosis after spinal cord injury. A 12-month follow-up study. Osteoporos Int. 2015;26:2273–80.
Funding
: JL was supported by a master’s scholarship and GM and DHG by research scholars from FRQ-S (Fonds de Recherche du Québec en Santé) at the time of the study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mailhot, G., Lamarche, J. & Gagnon, D.H. Effectiveness of two vitamin D3 repletion protocols on the vitamin D status of adults with a recent spinal cord injury undergoing inpatient rehabilitation: a prospective case series. Spinal Cord Ser Cases 4, 96 (2018). https://doi.org/10.1038/s41394-018-0129-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-018-0129-9