Abstract
Introduction
Postoperative infection is a potentially devastating complication of spine surgery and an appropriate strategy and timely decision-making are essential for successful treatment of deep surgical site infection (SSI) after spinal instrumentation surgeries. However, there is a lack of consensus on implant removal or retention. We report on a case of deep SSI after posterior lumbar interbody fusion (PLIF) surgery in which we achieved clinical cure by debridement and removal of the interbody fusion cage without removing the percutaneously inserted pedicle screws (PPS).
Case presentation
A case was a 53-year-old woman with deep SSI after PLIF surgery using the PPS system at the L4–5 level. Computed tomography (CT) showed no clear radiolucent line around the screws and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET)/CT demonstrated abnormal FDG uptake around the cages and no uptake around the pedicle screws. Intervertebral cages were removed and iliac bone grafts were inserted between the vertebral bodies, without removing the pedicle screws. The infection was cleared and bone fusion was achieved after the revision surgery.
Discussion
Targeting active infection using FDG-PET/CT is considered useful in narrowing the surgical margins and determining whether to preserve instrumentation in revision surgery after SSI. PLIF using the PPS system could be useful in preventing the easy spread of infection from the intervertebral space to the insertion point of PPS through the interstitial space.
Similar content being viewed by others
Introduction
Postoperative infection is a potentially devastating complication of spine surgery. An appropriate surgical strategy and timely decision-making are essential for the successful treatment of deep surgical site infection (SSI) after spinal instrumentation surgeries; however, a key problem is the lack of consensus concerning implant removal or retention [1]. Inappropriate retention of infected implants could aggravate infection due to biofilm formation and lead to sepsis, progressive bone destruction, neurological involvement, multiple surgeries, and poor outcome [2,3,4]. Alternatively, removal of instrumentation could lead to loss of fixation stability, progression of deformity, and long-term bed rest. Successful management of SSI with preservation of the implants, even including some parts of the spinal instruments, could allow for early ambulation and prevent activity of daily living decline after revision surgery. Therefore, the decision-making process concerning implant removal or retention is crucial for the treatment of deep SSI after spinal instrumentation surgery [2].
We report a case of deep SSI after posterior lumbar interbody fusion (PLIF) surgery in which we achieved clinical cure by debridement and removal of the interbody fusion cage without removing the percutaneously inserted pedicle screws (PPS). 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) localized SSI to the interbody space and facilitated decision-making on whether to retain the PPS.
Case report
A 53-year-old woman presented with a ten-year history of gradually progressive pain in the lower back and bilateral legs with gait disturbance. The patient was diagnosed with degenerative spondylolisthesis of the fourth lumbar vertebra.
The patient underwent PLIF with lumbar I/F cage (DePuy Synthes, Zuchwil, Switzerland) and bilateral pedicle screws (ES-2, Stryker, MI, USA) at the L4–5 levels. Bilateral decompression with a wide medical facetectomy (40–50%) and interbody fusion at L4–5 were performed via a midline incision, without exposing the outer aspect of the facet joints. Porous hydroxyapatite granules (Apaceram-AX, HOYA Technosurgical, Tokyo, Japan) were mixed with local bone to fill the interbody disc space and cages. Two additional small paramedian skin incisions were made, and the bilateral PPSs were fixed via the paramedian approach at the L4–5 level to achieve an oblique trajectory and separate the screws from the intervertebral space using soft tissue.
After surgery, there were no findings of fever or localized infection and the wound healed well. However, she had persistent postoperative low-back pain (visual analog scale (VAS): 5 (0 indicating no pain and 10 indicating the worst pain)). One month postoperatively, she developed a fever of 37.7 °C with intensification of low-back pain, and her C reactive protein (CRP) level increased to 2.9 mg/dl. She could not stand for over 5 min because of severe low-back pain (VAS: 8). She had no abnormal neurologic findings. Various microbiologic culture tests were performed, but a pathogenic bacterium could not be identified. Plain radiography and CT of the lumbar spine revealed bone destruction of the L4 lower and L5 upper vertebral end plates (Figs. 1 and 2). Magnetic resonance imaging (MRI) revealed high-signal intensity surrounding the intervertebral cages on T2-weighted imaging and low-signal intensity of the L4 and L5 vertebral body on T1-weighted imaging (Fig. 3). MRI findings were not accurate enough to demonstrate the localization of active infection because of the postoperative appearance and metal artifacts. FDG-PET/CT demonstrated abnormal FDG uptake around the cages and the spinal canal with a standardized uptake value (SUV) max of 15.3 (Fig. 4). By contrast, there was no uptake around the pedicle screws (Fig. 4). There was no clear radiolucent line around the pedicle screws (Fig. 4). We diagnosed deep SSI after PLIF. Despite the initiation of treatment with intravenous injection of 500 mg levofloxacin, bone destruction surrounding the cage progressed. We performed reoperation on the 40th postoperative day.
F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) images of the lumbar spine before revision surgery. FDG-PET/CT demonstrated abnormal FDG uptake around the intervertebral cage and the spinal canal with a standardized uptake value max of 15.3. There was no uptake around the pedicle screws. Axial computed tomography of lumbar spine demonstrated that there was no clear radiolucent line around the pedicle screws
First, the intervertebral cages were removed using the midline incision, without opening up the wound where the pedicle screws were percutaneously inserted. The intervertebral cages were easily removed owing to the destruction of the vertebral end plates surrounding the cage. There was no segmental instability at L4–5 after removing the cages and the pedicle screws were not found to be loose. The pedicle screws were retained because there was no screw loosening and no active infection around the pedicle screws based on FDG-PET/CT findings. After performing curettage and irrigation thoroughly of the intervertebral disc space and the vertebral bodies at L4–5, tricortical iliac bone grafts were inserted between the vertebral bodies (Fig. 5). One gram of vancomycin (VCM) powder was applied to the subfascial layer before closing the wound.
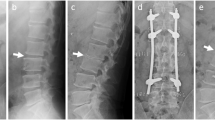
Plain radiographs and computed tomography (CT) images of the lumbar spine after revision surgery. Plain radiographs in the anteroposterior (a) and lateral view (b) and sagittal CT (c) of the lumbar spine 1 week after revision surgery. The intervertebral cages were removed and tricortical iliac bone grafts were inserted between the vertebral bodies at the L4–5 level. Percutaneous pedicle screws were retained. Sagittal CT showed that bone fusion was achieved 6 months after revision surgery (d)
VCM was initiated at a dose of 3000 mg daily (1500 mg per os every 12 h) based on a blood concentration simulation after the revision surgery. However, VCM was switched to linezolid at 3 days after surgery due to acute renal failure. Linezolid was initiated at a dose of 1200 mg daily (600 mg per os every 12 h). CRP levels returned to normal 15 days after the revision surgery. At 4 weeks following surgery, the antibiotic was changed to oral sulfamethoxazole and trimethoprim combination. The antibiotic treatment was continued for 4 months after revision surgery. Bone fusion was achieved at 6 months after revision surgery (Fig. 5).
Discussion
The ideal goal in the treatment of postoperative deep infection after spinal fusion surgery is to clear the infection without lowering the patients’ ability to perform activities of daily life. It is very important to determine whether the spinal implants should be removed in patients with deep SSI after instrumented spinal surgery. Kanayama et al. reported that the MRI findings of vertebral osteomyelitis and/or intervertebral abscess are key to the decision-making process of implant removal for deep wound infection after instrumentation surgery [2]. They suggest that the interbody cage must be removed when MRI reveals high signal intensity change in the cage-inserted intervertebral disc space on T2-weighted imaging, indicating intervertebral abscess surrounding the inserted cage [2]. Furthermore, the pedicle screws should be removed when low signal intensity extending throughout the entire vertebral body on T1-weighted imaging, which indicates vertebral osteomyelitis, is found in the vertebra with the screw inserted [2]. However, MRI artifacts caused by the metallic instrumentation make it difficult to evaluate whether the infection has spread to the pedicle screws.
FDG-PET/CT has been reported to have clinical utility in suspected infection and inflammation [5, 6]. Nakahara et al. [6] reported that metabolic assessment using FDG-PET/CT allows for the accurate diagnosis of active infection of the spine. FDG-PET/CT was reported to be useful for the quantitative evaluation of the severity of spinal infection foci, using the SUV value. In this case, it was impossible to determine whether the infection had spread to the pedicle screws using conventional imaging modalities such as plain radiography, CT, and MRI, which are not accurate enough to demonstrate active regions in spinal infection. Moreover, MRI tends to show more compartments with abnormal signal and metal artifacts, which makes it difficult to accurately diagnose vertebral osteomyelitis. In this case, FDG-PET/CT demonstrated that the SUV value surrounding the intervertebral cage was 15.3, whereas there was no uptake observed around the pedicle screws. An optimal SUVmax threshold for active spinal infection was reported to be 4.2 [6]. The PET-CT findings of this case showed that there was active infection only around the intervertebral cages, and the active infection did not spread to the pedicle screws. Targeting active infection using FDG-PET/CT is considered to be useful in order to narrow the surgical margins and determine whether to preserve instrumentation in revision surgery after SSI [6].
PPS techniques in spine surgery are increasing in popularity due to potential advantages, including reduced intraoperative blood loss, minimal soft tissue trauma, and decreased postoperative pain [7, 8]. In the present case, the infection did not spread to the percutaneous pedicle screws on the basis of FDG-PET/CT assessment, and was cleared without removal of the pedicle screws. Regarding control of deep SSI, the PPS technique could also have other advantages due to the screw insertion trajectory and insertion point. The PPS technique could provide a more oblique trajectory using a lateral starting point through the longissimus muscle as compared to conventional open techniques for pedicle screw placement. Therefore, the percutaneously inserted screws were separated from the intervertebral cages by soft tissue, including the paravertebral muscles, which could prevent the spread of infection from the intervertebral space to the insertion point of the PPS via the interstitial space.
In conclusion, we report a case of deep SSI following PLIF using the PPS technique. The focus of active infection was identified using FDG-PET/CT, and the infection was cleared without removal of the pedicle screws. Targeting the active infection using FDG-PET/CT is considered useful in narrowing the surgical margins and determining whether to preserve instrumentation in revision surgery after SSI. PLIF using the PPS system could be valuable for the prevention of infection spreading from the intervertebral space to the insertion point of PPS via the interstitial space.
References
Tsubouchi N, Fujibayashi S, Otsuki B, Izeki M, Kimura H, Ota M et al. Risk factors for implant removal after spinal surgical site infection. Eur Spine J 2017 [Epub ahead of print].
Kanayama M, Hashimoto T, Shigenobu K, Oha F, Iwata A, Tanaka M. MRI-based decision-making of implant removal in deep wound infection after instrumented lumbar fusion. Clin Spine Surg. 2017;30:E99–E103.
Pappou IP, Papadopoulos EC, Sama AA, Girardi FP, Cammisa FP. Postoperative infections in interbody fusion for degenerative spinal disease. Clin Orthop Relat Res. 2006;444:120–8.
Thalgott JS, Cotler HB, Sasso RC, LaRocca H, Gardner V. Postoperative infections in spinal implants. Classification and analysis—a multicenter study. Spine. 1991;16:981–4.
Vaidyanathan S, Patel CN, Scarsbrook AF, Chowdhury FU. FDG-PET/CT in infection and inflammation—current and emerging clinical applications. Clin Radiol. 2015;70:787–800.
Nakahara M, Ito M, Hattori N, Magota K, Takahata M, Nagahama K, et al. 18F-FDG-PET/CT better localizes active spinal infection than MRI for successful minimally invasive surgery. Acta Radiol. 2015;56:829–36.
Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine. 2016;24:416–27.
Mobbs RJ, Sivabalan P, Li J. Technique, challenges, and indications for percutaneous pedicle screw fixation. J Clin Neurosci. 2011;18:741–9.
Author contributions
Conception and design of the study: K.Y. and M.T. Data acquisition: K.H., K.Y., and T.K. Drafting of the article: K.H. and K.Y. Critical revision: M.T. Supervision: N.I. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Haraya, K., Yamada, K., Kokabu, T. et al. 18F-FDG-PET/CT localizes intervertebral disc space infection following posterior lumbar interbody fusion surgery leading to successful retention of percutaneously inserted pedicle screws: a case report. Spinal Cord Ser Cases 4, 81 (2018). https://doi.org/10.1038/s41394-018-0119-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-018-0119-y