Abstract
Study design
Cross-sectional study.
Objectives
To compare muscle size, body composition, bone mineral density (BMD), and metabolic profiles in denervated versus innervated individuals with spinal cord injury (SCI).
Setting
Hunter Holmes McGuire Veterans Affairs (VA) Medical Center.
Methods
Body composition, bone mineral density (BMD), muscle size, and metabolic parameters were collected in 16 persons with chronic SCI (n = 8 denervated, n = 8 innervated) using dual-energy x-ray absorptiometry (DXA), magnetic resonance imaging (MRI), and fasting blood samples. BMR was measured by indirect calorimetry.
Results
Percent differences of the whole thigh muscle cross-sectional area (CSA; 38%), knee extensor CSA (49%), vasti CSA (49%), and rectus femoris CSA (61%) were smaller in the denervated group (p < 0.05). Leg lean mass was also lower (28%) in the denervated group (p < 0.05). Whole muscle intramuscular fat (IMF%; 15.5%), knee extensor IMF% (22%), and % fat mass (10.9%) were significantly greater in the denervated group (p < 0.05). Knee distal femur and proximal tibia BMD were lower in the denervated group, 18–22% and 17–23%; p < 0.05. Certain indices of metabolic profile were more favorable in the denervated group though were not significant.
Conclusions
SCI results in skeletal muscle atrophy and dramatic changes in body composition. Lower motor neuron (LMN) injury results in denervation of the lower extremity muscles which exacerbates atrophy. Denervated participants exhibited lower leg lean mass and muscle CSA, greater muscle IMF, and reduced knee BMD compared to innervated participants. Future research is needed to explore therapeutic treatments for the denervated muscles after SCI.
Similar content being viewed by others
Introduction
Skeletal muscle atrophy is a detrimental outcome following spinal cord injury (SCI). Previous work noted that muscle atrophy may start as early as 6 weeks post-SCI [1]. Furthermore, Castro et al. noted that muscle size is ~50% smaller in persons with SCI compared to age and weight matched able-bodied controls [2]. A previous study examining skeletal muscle atrophy following complete SCI showed 27–56% atrophy of type I, IIa, and IIax + IIx fibers from 6 to 24 weeks following injury [3]. Experimental animal models have identified potential bio-molecular regulators that may trigger myofiber apoptosis and subsequent muscle atrophy [4]. Muscle atrophy may lead to dysregulation in the balance between protein synthesis and protein breakdown (protein turnover). The process of muscle atrophy is associated with remarkable increases in intramuscular fat (IMF), referring to the sum of fat within and between muscle fibers and between muscle groups [5]. Ectopic adiposity is characterized by the storage of adipose tissue in non-subcutaneous sites, which is associated with metabolic and cardiovascular health consequences in persons with SCI [5,6,7]. Elder et al. noted that IMF and skeletal muscle mass are strong predictors of impaired glucose tolerance. Specifically, lipid surrounding skeletal muscle may account for up to 70% of glucose intolerance after SCI [6].
Most of the research in persons with SCI has focused on the impact of upper motor neuron (UMN) injury. UMN injury primarily results in the disruption of the corticospinal tract, which can lead to loss of voluntary motor control, paralysis, hypertonia, hyperreflexia (spasticity), and a positive Babinski sign in the lower limbs [8]. Furthermore, the final motor pathway remains undamaged as the peripheral skeletal muscle is still innervated by lower motor neurons (LMN) and their corresponding axons [8]. However, less attention has been paid to the effects of lower motor neuron (LMN) injury [6,7,8,9]. LMN injury can result from trauma to the spinal cord anterior horn cells, nerve roots, and peripheral nerves, resulting in denervation, i.e. an irreversible disconnection of skeletal muscle fibers from their damaged innervating motor neurons [9]. Persons with LMN injury may represent ~25% of the entire SCI population [10]. Additionally, LMN injury is often defined by a neurological insult below T10 level; however, previous work has suggested that determining whether an individual has a LMN or UMN lesion cannot be solely based by neurological level alone [8]. Prior works using muscle biopsies and imaging techniques have shown that denervated muscles are severely atrophic with fibrosis and infiltration of both intramyocellular fat and IMF [6, 9]. Moreover, medical image-based modeling has also been used to capture the morphological changes that occur following denervation [11]. However, we are unaware of any systematic research that has examined the effects of SCI-induced denervation on muscle size, body composition, bone mineral density (BMD), and metabolic profile in persons with LMN injury compared to age, time since injury (TSI), and body mass index (BMI)-matched individuals with UMN injury. It is well known that persons with SCI are at a greater risk of developing obesity, insulin resistance, and other cardiometabolic consequences as well as condylar fractures. The magnitude of these problems in SCI-induced denervation are rather unappreciated or yet to be determined. Furthermore, evaluating the magnitude of muscle atrophy, changes in body composition, and metabolic profile may provide researchers and clinicians with opportunities to effectively develop pharmaceutical, dietary, and rehabilitation countermeasures for this population. The purpose of this work was to compare muscle size, body composition, BMD, and metabolic profiles in SCI individuals with LMN compared to persons with UMN injury. We hypothesized that persons with LMN injury would exhibit reduced lean mass, increased fat mass, and reduced BMD.
Methods
Participants
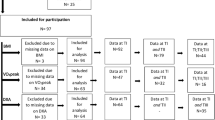
Sixteen individuals with chronic (>1-year post injury) SCI were recruited to participate in one of two clinical trials (NCT02660073 and NCT03345576). Study procedures were in accordance with the ethical standards of the 1964 Declaration of Helsinki. A neurological examination was performed per the International Standards for Neurological Classification of SCI (ISNCSCI) to determine the American Spinal Injury Association (ASIA) Impairment Scale (AIS) for each participant [12]. Physical characteristics of all participants are presented in Table 1. Participants were excluded for cardiovascular disease, uncontrolled type 2 diabetes mellitus (HbA1c > 7.5), uncontrolled hypertension (resting blood pressure >140/90 mmHg), insulin dependence, pressure sores stage 3 or greater, hematocrit above 50%, or severe urinary tract infection. Each participant signed an informed consent that was approved by the Hunter Holmes McGuire VA Medical Center IRB. Importantly, denervation was confirmed by a lack of response to electrical stimulation of the knee extensors. The data presented in this manuscript are cross-sectional baseline data that was analyzed prior to any study intervention.
Anthropometric parameters
Participants height and weight were measured using previously published methods [13, 14]. Measurements of waist, abdominal, thigh, and hip circumferences were all measured in a supine position [14, 15]. Measurements were reported to the nearest 0.1 cm. If there was a difference greater than 0.5 cm between readings the measurements were repeated until three measurements were within 0.5 cm range of each other, and the average of these measurements was used [13].
Dual-energy x-ray absorptiometry (DXA)
Total body DXA scans were performed using a GE Lunar iDXA (Lunar Inc., Madison, WI, USA) bone densitometer. DXA scans were used to quantify regional and total fat mass (FM), fat-free mass (FFM), %FM, lean mass (LM), as well as BMD. Participants were transferred onto the DXA and rested for 30 min in a flat supine position to account for potential fluid shift prior to the scan. All scans were performed and analyzed by a DXA trained researcher using enCORE GE Healthcare software version 16. A hard plastic triangular foot positioner and additional foam pad were used to strap the feet tightly and secure them prior to the scan. In addition, the leg prior to scanning was internally rotated to minimize the overlap between the tibia and fibula. Knee BMD of the distal femur and proximal tibia were analyzed using the GE iLunar orthopedic knee-specific scan module software [16, 17]. Longitudinal estimates of precision of total and regional body composition using DXA has previously been published in persons with SCI [18, 19].
Magnetic resonance imaging (MRI)
MRI was performed for each participant using a total body General Electric (GE) signa 1.5-T magnet [15]. Twelve to 15 trans-axial images (slice thickness of 0.8 cm and 1.6 cm; fast spin echo; repetition time, 850–1000 ms; echo time, 6.7 ms; imaging frequency, 63.8 MHz; echo number, 1; echo train length, 3; flip angle, 90°; field of view, 20 cm; matrix size, 256 × 256) were captured from the hip to knee joint (Fig. 1). A General Electric body array flex coil to measure thigh cross-sectional area (CSA) was used to improve the overall signal-to-noise ratio resulting in higher resolution images for analysis. Participants were instructed to lie still in a supine position inside the magnet and provided earplugs to protect from the magnet noise. The duration of the whole scan including preparation time per leg was 3.5 min. Both lower limbs were strapped together using a soft band to mitigate movement (involuntary muscle spasms) during the scan. Skeletal muscle CSAs of the knee extensor (KE), rectus femoris (RF), and vasti (KE minus RF) muscles were determined. Detailed analysis procedures and segmentation of MRI images into muscle and IMF pixels were previously described [1, 5, 6].
Basal metabolic rate (BMR)
After an overnight fast (10–12 h), participants were kept in a dark room with a thermoneutral environment for ~20–30 min to reach a resting state [20, 21]. A canopy was placed over the participant’s head while lying in a supine position and BMR was measured as previously described [20].
Metabolic biomarkers
Once BMR testing was complete, blood samples were collected. Total testosterone was measured using liquid chromatography with isotope dilution mass spectrometry detection, after supported liquid extraction (ESOTERIX INC.). Lipid profile, including low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), total cholesterol, and high-density lipoprotein cholesterol (HDL-C) was determined by collecting 10 ml of blood from an indwelling venous catheter using standard analysis procedure. After the fasting blood samples, an intravenous line was added to enable the infusion of glucose and glucose tolerance was measured as previously described [16]. Twenty minutes after the glucose injection, insulin sensitivity was determined by injecting a bolus of insulin (0.02 U/kg, regular short acting insulin, Humulin; Lilly).
Statistical analysis
All data were tested for normality using Shapiro–Wilk tests (p < 0.05), and if required data were log-transformed prior to any statistical analysis. Independent t-tests were used to examine significant differences in physical characteristics, body composition, BMD, anthropometrics, and metabolic parameters between innervated and denervated groups. Physical characteristics and results are expressed as mean ± standard deviations (SD). Statistical analyses were performed using SPSS (SPSS statistics version 28.0.0.0, IBM Corp, Armonk, USA). Statistical significance was set at an alpha of p < 0.05.
Results
Physical characteristics of the participants
Participants’ physical characteristics for each group are described in Table 1. Seven participants in the denervated group were AIS A, while one participant was AIS C. In the innervated group three participants were AIS A, two AIS B, and three AIS C. Participants were matched for age, weight, BMI, TSI, and injury classification (i.e., paraplegia; Table 1).
Skeletal muscle cross-sectional area (CSA)
In the denervated group, the right and left whole thigh muscle CSAs were 38% smaller than the innervated group (p = 0.002; Table 2 and Fig. 2). The average size of both right and left KE muscle (m.) CSA was 49% smaller in the denervated group (right: p = 0.0001; left: p = 0.0003). The denervated group had a smaller vasti m.CSA (49%, right: p = 0.001; 48%, left: p = 0.0002). Rectus femoris m.CSA was smaller in the denervated group compared to the innervated group (61%, right: p = 0.001; 58%, left: p = 0.003) (Table 2).
Comparisons of whole thigh muscle and knee extensor CSA between innervated (black bar) and denervated (gray bar) participants. A Right whole thigh muscle CSA, B left whole thigh muscle CSA, C right knee extensor CSA, D left knee extensor CSA. Right and left whole thigh muscle CSA was significantly lower in denervated participants (A, B respectively). Right and left knee extensor CSA was significantly lower in denervated participants (C, D). *Indicates significant difference between groups (p < 0.05).
Intramuscular fat (IMF)
Relative %IMF for the whole thigh m. CSA was significantly greater in the denervated group (13%, right: p = 0.014; 18%, left: p = 0.001) (Fig. 1). Right and left KE IMF% was significantly greater in the denervated group compared to the innervated group (24%, right: p = 0.036; 20%, left: p = 0.037), respectively. There were no significant differences between groups for whole thigh IMF CSA (21%, right: p = 0.266; 35%, left: p = 0.127). Average right and left KE IMF CSA were also not significantly different between groups (14%, right: p = 0.377; 16.5%, left: p = 0.363) (Table 2).
Lean and fat mass
Leg lean mass (LLM; kg) was significantly lower (28%) in the denervated group compared to the innervated group (p = 0.017). Leg % fat mass (%FM) was significantly greater (10.9%) in the denervated group (p = 0.004). There was also a trend of greater total %FM (23%) in the denervated group (p = 0.059). Furthermore, total body LM (kg) (18.6%, p = 0.104), leg fat (kg) (13.5%, p = 0.283), total body FM (kg) (17%, p = 0.228), trunk LM (kg) (2.6%, p = 0.392), and trunk FM (kg) (23.8%, p = 0.183) were not significantly different between groups (Table 2).
Bone mineral density (BMD)
Left and right distal femur BMD (g/cm2) were significantly lower in the denervated group compared to the innervated group, 18%; p = 0.040 and 22%; p = 0.007, respectively (Table 3). Right knee proximal tibia BMD (g/cm2) was significantly lower (23%; p = 0.010) in the denervated group. While the left knee proximal tibia BMD (g/cm2) was not significantly different between groups, it trended toward significance and the denervated group had a 17% lower average BMD (p = 0.062, Table 3 and Fig. 3). Whole right and left femur BMD (g/cm2) were not significantly different between groups (11.5%, right: p = 0.177; 0.4%, left: p = 0.490). In addition, leg BMD (g/cm2) and total BMD (g/cm2) were not significantly different between groups, (11.1%; p = 0.096 and 5.1%; p = 0.117), respectively (Table 3).
Comparisons of knee distal femur and proximal tibia BMD between innervated (black bar) and denervated (gray bar) participants. A Right knee distal femur BMD, B left knee distal femur BMD, C right knee proximal tibia BMD D left knee proximal tibia BMD. Right and left distal femur BMD was significantly lower in denervated participants (A, B respectively). Right and left proximal tibia BMD was lower in denervated participants (C, D respectively). *Indicates significant difference between groups (p < 0.05). #Indicates trending toward significance.
Metabolic biomarkers
HDL-C (mg/dl) was 24% greater in the denervated group and trended toward significance (p = 0.051; Table 4). LDL-C (mg/dl) (0.2%; p = 0.497), Non-HDL-C (mg/dl) (4.6%, p = 0.376), total cholesterol (mg/dl) (2.4%; p = 0.414), and TG (mg/dl) (26%; p = 0.096) were not significantly different between groups. Total testosterone (ng/dl) and BMR (kcal/day) were also not significantly different between groups, 11%; p = 0.339 and 4.6%; p = 0.301, respectively. While there was no significant difference in fasting glucose (4.9%; p = 0.291) or fasting insulin levels between groups, fasting insulin (µU/ml) was two-fold lower in the denervated group compared to the innervated and trended toward significance (46%; p = 0.093; Table 4).
Discussion
The major findings indicated that whole thigh m. CSA was 38% smaller in denervated individuals with LMN injury compared to the matched innervated individuals. Additionally, KE m. CSA for both thighs was 49% smaller in the denervated group. Furthermore, whole thigh IMF and KE IMF% was significantly greater in the denervated group. The denervated group on average had a lower leg lean mass (kg) compared to matched innervated participants. Left and right knee distal femur BMD (g/cm2) was lower in the denervated group, 18% and 22%, respectively. Additionally, both right and left knee proximal tibia BMD (g/cm2) were lower in the denervated group, 23% and 17%, respectively. Fasting insulin (µU/ml) was 46% lower in the denervated group compared to the innervated group. However, HDL-C was noticeably higher in the denervated compared to the innervated group.
Injury to the conus and cauda equina regions result in permanent LMN denervation, which is associated with severe muscle atrophy and flaccid paralysis in the lower limbs [8]. An immediate loss of function following nerve injury leads to fibrotic changes in tissue architecture due to increasing dominance of adipocytes [22]. Denervation is followed by several stages, which ultimately proceed to terminal atrophy of the paralyzed muscles below the neurological level of injury [22].
Loss of lean tissue and increased IMF is accelerated due to extreme disuse following SCI and reduced physical activity [1, 23]. Previous studies have shown that spasticity is significantly correlated with skeletal muscle mass and may attenuate thigh muscle atrophy [24, 25]. Individuals with spastic knee extensors were found to have 22% greater KE m. CSA and a lower degree of IMF infiltration compared to non-spastic persons with SCI [25]. Indeed, innervated individuals with higher levels of spasticity may experience preserved skeletal muscle mass in comparison to individuals with denervated muscle.
Bone health
Deteriorating bone health following SCI manifests as disproportionate bone resorption resulting in osteoporosis, thus dramatically increasing the risk of fractures. Disuse of the lower extremities following SCI substantially affects the muscle tissue and ultimately bone quality. It has been reported that after SCI, leg muscle mass is correlated with the magnitude of bone loss [26]. BMD reportedly decreases by almost 50% and 60% at the distal epiphyses of the femur and the proximal epiphyses of the tibia, respectively, 3–4 years after SCI [27]. The lack of muscle activity due to LMN denervation has a major effect on bone resorption and can lead to accelerated osteoporosis [28]. Additionally, sciatic neurectomy (a model of denervation) performed in murine models, showed a considerable level of trabecular bone loss in growing female rats due to increased bone resorption and decreased bone formation [29]. As previously mentioned, the lack of innervation to the lower leg muscles results in atrophy and a consequent accumulation of IMF. The accumulation of IMF likely affects muscle stiffness, which may reduce the muscle force applied on the bone and further reduce bone health [7]. However, the majority of the literature on bone loss in SCI focuses primarily on UMN lesions and less attention has been paid to bone health following denervation in persons with SCI. Previous work by Garland et al. suggested that a BMD value of 0.6 g/cm2 at the distal femur and proximal tibia represents the bone fracture threshold in SCI [30]. The rate of bone loss is dependent on the severity of injury, TSI, BMI, and age [30].
We attempted to control for age, TSI, and BMI by closely matching groups. Cirnigliaro et al. reported distal femur and proximal BMD values for different epochs of TSI in 105 individuals with SCI, and noted a significant decline in BMD after the first decade of injury in comparison to able-bodied controls and <1-year TSI [31]. More specifically, in 36 individuals with TSI (1–5 years) average BMD at the distal femur and proximal tibia (0.886, 1.084 g/cm2; respectively) was found to be higher compared to BMD values into the second decade after SCI (0.714, 0.781 g/cm2; respectively) [31]. In the innervated group, three individuals had TSI values ranging from 1.5 to 4 years and three individuals had TSI values ranging from 9 to 20 years, which may explain the higher distal femur and proximal tibia BMD values reported. Our findings show that denervated participants had a significantly lower BMD at the distal femur and proximal tibia for both legs compared to innervated participants. Therefore, it is likely that denervated individuals are at a higher risk of reaching this BMD threshold (0.6 g/cm2) compared to innervated individuals. Due to this increased risk following LMN injury, larger studies are warranted to address these concerns more adequately in this population.
Gargiulo et al. demonstrated restoration of muscle structures, tendons, and improved bone strength using functional electrical stimulation [11]. Additionally, Chandrasekaran et al. has shown that the denervated musculature may be activated using a form of non-invasive electrical stimulation called long pulse width stimulation [10]. Moreover, due to the concomitant detrimental changes in body composition seen in LMN injury along with the increased risk for hypogonadism in chronic SCI, testosterone replacement therapy may be particularly effective in this population. Previous work has shown that testosterone replacement therapy has beneficial effects on LM, FM, and bone health [32].
Metabolic profile
Previous studies have emphasized that persons with SCI are at a high risk of developing insulin resistance and impaired glucose tolerance due to decreased levels of physical activity and dramatic changes in body composition [33, 34]. Infiltration of fat within the muscle and visceral sites is known to worsen the metabolic profile of persons with SCI [35]. Aksnes et al. reported the dissociation between whole body insulin-mediated glucose uptake and insulin action in skeletal muscle is potentially due to decreased skeletal muscle mass in individuals with tetraplegia [34]. Indeed, denervated muscle may contribute to impaired glucose tolerance and increased insulin resistance [36]. The current results showed that fasting insulin levels were higher in the innervated group compared to the denervated group. While this difference was not significant, there was a 46% difference between groups. Glucose uptake has been found to be higher in paralyzed spastic legs in persons with SCI compared to able-bodied controls [30]. Moreover, previous work has shown that contracting skeletal muscle releases myokines that may signal to the pancreas and impact insulin release, which may explain the higher fasting insulin levels in our innervated sample [37]. It is probable that the lack of muscle contraction after denervation (non-spastic) impacts cross-talk between skeletal muscle and pancreatic β-cells, which may explain the differences in fasting insulin between groups [37]. Additionally, mitochondrial function is positively associated with LM, carbohydrate and lipid profile, and negatively associated with adipose tissue [38]. Previous work has demonstrated that reduced skeletal muscle mass following SCI leads to mitochondrial dysfunction, which may impair insulin metabolism [17]. Indeed, Sergi et al. showed that a decrease in mitochondrial oxidative capacity plays a role in the development of insulin resistance [39].
Persons with SCI have increased levels of LDL-C, TG, total cholesterol, and decreased HDL-C [35, 40]. However, it is unknown if cholesterol levels differ significantly between individuals that are denervated or innervated. In the current study, there were no significant differences between groups; however, there was a trend for HDL-C to be higher in the denervated group (p = 0.051). While this finding is intriguing, it should be noted that this may be due to a myriad of factors, including dietary habits, medication usage, genetic predisposition, level of impairment, and degree of physical activity [41]. Specifically, more severe AIS grades (i.e., A and B) have been associated with lower total cholesterol and HDL-C, independent of the neurological level of injury [41]. Importantly, while we matched the groups for sex, age, BMI, and TSI, there were differences in the AIS grades between groups. This discrepancy along with the small sample size may explain the lack of significant differences between groups in the current study.
Limitations
This is a correlative analysis based on baseline cross-sectional data and does not precisely identify the complex causal relationships between body composition, metabolic health, and bone health in individuals with LMN injury. This study used a relatively small sample size due to constraints such as access to this specific population and the cost of MRI and DXA. This study also used a sample of relatively healthy individuals (i.e., no cardiovascular disease, type 2 diabetes, pressure ulcers, or common medical and psychiatric comorbidities), limiting the generalizability of findings beyond individuals with similar levels of function. Additionally, the heterogeneity of impairment in our innervated sample (incomplete versus complete) is important to note as some individuals may have spared motor control, thus having lower magnitudes of muscle atrophy. It is important to note the addition of two participants designated to the LMN group, had an NLI of T9 and T7, respectively, as recruitment for this sub-population of SCI was rather difficult. Moreover, one cannot determine the type of lesion (UMN versus LMN) on the basis of neurological injury, as a detailed neurological examination, including sacral reflexes is required [8]. As mentioned previously, denervation was confirmed by a lack of response to electrical stimulation of the knee extensors. Importantly, considering these limitations, the current findings identify important differences between individuals with LMN and UMN injury. This is highly important due to the dearth of information regarding the effects of LMN injury on body composition and bone health. Lastly, multicenter trials examining changes in the variables over time are necessary to further elucidate the differential effects of innervation versus denervation in persons with SCI. Future large multicenter trials may be required to further explore differences in metabolic profiles between innervated and denervated individuals with SCI.
Conclusion
Denervated individuals with LMN injury exhibited smaller muscle size and lower LM, BMD, and higher levels of FM and IMF compared to matched innervated individuals with UMN injury. Specifically, leg lean mass was significantly lower in the denervated group, and total LM was lower in the denervated group. Leg region % fat, total % FM, whole muscle IMF %, and KE IMF % were significantly higher in the denervated group. Lastly, left and right distal femur BMD and right proximal tibia BMD were significantly lower in the denervated group, while left proximal tibia BMD trended toward significantly lower in the denervated group. Interestingly, fasting insulin trended toward significance and was lower in the denervated group, but further exploration is needed to determine the mechanisms underlying this result. While we found no significant differences in metabolic profiles between groups, this may have been due to the relatively small sample size. Future trials with larger sample sizes should explore potential therapeutics for denervated individuals with LMN injury, including testosterone replacement therapy and long pulse width electrical stimulation.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request and after receiving approval from our research office for data sharing. The data were uploaded as Supplementary Material for review process only. After acceptance of the paper, the data will be available upon email communications with the corresponding author.
References
Gorgey A, Dudley G. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45:304–9.
Castro MJ, Apple DF Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol. 1999;80:373–8.
Castro MJ, Apple DF Jr, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999;86:350–8.
Siu PM, Alway SE. Response and adaptation of skeletal muscle to denervation stress: the role of apoptosis in muscle loss. Front Biosci Landmark Ed. 2009;14:432.
Ogawa M, Lester R, Akima H, Gorgey AS. Quantification of intermuscular and intramuscular adipose tissue using magnetic resonance imaging after neurodegenerative disorders. Neural Regen Res. 2017;12:2100.
Elder C, Apple D, Bickel C, Meyer R, Dudley G. Intramuscular fat and glucose tolerance after spinal cord injury—a cross-sectional study. Spinal Cord. 2004;42:711–6.
Ghatas MP, Khan MR, Gorgey AS. Skeletal muscle stiffness as measured by magnetic resonance elastography after chronic spinal cord injury: a cross-sectional pilot study. Neural Regen Res. 2021;16:2486.
Doherty JG, Burns AS, O’Ferrall DM, Ditunno M Jr, John F. Prevalence of upper motor neuron vs lower motor neuron lesions in complete lower thoracic and lumbar spinal cord injuries. J Spinal Cord Med. 2002;25:289–92.
Carraro U, Boncompagni S, Gobbo V, Rossini K, Zampieri S, Mosole S, et al. Persistent muscle fiber regeneration in long term denervation. Past, present, future. Eur J Transl Myol. 2015;25:4832.
Chandrasekaran S, Davis J, Bersch I, Goldberg G, Gorgey AS. Electrical stimulation and denervated muscles after spinal cord injury. Neural Regen Res. 2020;15:1397.
Gargiulo P, Jens Reynisson P, Helgason B, Kern H, Mayr W, Ingvarsson P, et al. Muscle, tendons, and bone: structural changes during denervation and FES treatment. Neurol Res. 2011;33:750–8.
Rupp R, Biering-Sørensen F, Burns SP, Graves DE, Guest J, Jones L, et al. International standards for neurological classification of spinal cord injury: revised 2019. Top Spinal Cord Inj Rehabil. 2021;27:1–22.
Gorgey AS, Martin H, Metz A, Khalil RE, Dolbow DR, Gater DR. Longitudinal changes in body composition and metabolic profile between exercise clinical trials in men with chronic spinal cord injury. J Spinal Cord Med. 2016;39:699–712.
Sumrell RM, Nightingale TE, McCauley LS, Gorgey AS. Anthropometric cutoffs and associations with visceral adiposity and metabolic biomarkers after spinal cord injury. PLoS ONE. 2018;13:e0203049.
Wade RC, Gorgey AS. Anthropometric prediction of skeletal muscle cross-sectional area in persons with spinal cord injury. J Appl Physiol. 2017;122:1255–61.
Ghatas MP, Sutor TW, Gorgey AS. Prediction of distal femur and proximal tibia bone mineral density from total body dual energy X-ray absorptiometry scans in persons with spinal cord injury. J Clin Densitom. 2022;25:252–60.
Morse LR, Biering-Soerensen F, Carbone LD, Cervinka T, Cirnigliaro CM, Johnston TE, et al. Bone mineral density testing in spinal cord injury: 2019 ISCD official position. J Clin Densitom. 2019;22:554–66.
Ifon DE, Ghatas MP, Davis JC, Khalil RE, Adler RA, Gorgey AS. Long-term effect of intrathecal baclofen treatment on bone health and body composition after spinal cord injury: a case matched report. World J Orthop. 2020;11:453.
Gorgey AS, Cirnigliaro CM, Bauman WA, Adler RA. Estimates of the precision of regional and whole body composition by dual-energy x-ray absorptiometry in persons with chronic spinal cord injury. Spinal Cord. 2018;56:987–95.
Nightingale TE, Gorgey AS. Predicting basal metabolic rate in men with motor complete spinal cord injury. Med Sci Sports Exerc. 2018;50:1305–12.
Gorgey AS, Khalil RE, Gill R, Gater DR, Lavis TD, Cardozo CP, et al. Low-dose testosterone and evoked resistance exercise after spinal cord injury on cardio-metabolic risk factors: an open-label randomized clinical trial. J Neurotrauma. 2019;36:2631–45.
Carlson BM. The biology of long-term denervated skeletal muscle. Eur J Transl Myol. 2014;24:5–11.
Spungen AM, Wang J, Pierson RN Jr, Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol. 2000;88:1310–5.
Cha S, Yun JH, Myong Y, Shin HI. Spasticity and preservation of skeletal muscle mass in people with spinal cord injury. Spinal Cord. 2019;57:317–23.
Gorgey A, Dudley G. Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury. Spinal Cord. 2008;46:96–102.
Bauman WA, Spungen AM, Wang J, Pierson RN Jr, Schwartz E. Relationship of fat mass and serum estradiol with lower extremity bone in persons with chronic spinal cord injury. Am J Physiol-Endocrinol Metab. 2006;290:E1098–103.
Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, et al. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–80.
Jiang S, Jiang L, Dai L. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol. 2006;65:555–65.
Zeng Q, Jee W, Bigornia A, King J Jr, D’souza S, Li X, et al. Time responses of cancellous and cortical bones to sciatic neurectomy in growing female rats. Bone. 1996;19:13–21.
Garland DE, Adkins RH, Kushwaha V, Stewart C. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27:202–6.
Cirnigliaro CM, Myslinski MJ, Asselin P, Hobson JC, Specht A, La Fountaine MF, et al. Progressive sublesional bone loss extends into the second decade after spinal cord injury. J Clin Densitom. 2019;22:185–94.
Nightingale TE, Moore P, Harman J, Khalil R, Gill RS, Castillo T, et al. Body composition changes with testosterone replacement therapy following spinal cord injury and aging: a mini review. J Spinal Cord Med. 2018;41:624–36.
LaVela SL, Weaver FM, Goldstein B, Chen K, Miskevics S, Rajan S, et al. Diabetes mellitus in individuals with spinal cord injury or disorder. J Spinal Cord Med. 2006;29:387–95.
Aksnes A, Hjeltnes N, Wahlstrom E, Katz A, Zierath J, Wallberg-Henriksson H. Intact glucose transport in morphologically altered denervated skeletal muscle from quadriplegic patients. Am J Physiol-Endocrinol Metab. 1996;271:E593–600.
Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile–Part I. J Spinal Cord Med. 2014;37:693–702.
Raymond J, Harmer A, Temesi J, Van Kemenade C. Glucose tolerance and physical activity level in people with spinal cord injury. Spinal Cord. 2010;48:591–6.
Barlow JP, Solomon TP. Do skeletal muscle-secreted factors influence the function of pancreatic β-cells? Am J Physiol-Endocrinol Metab. 2018;314:E297–307.
O’Brien LC, Wade RC, Segal L, Chen Q, Savas J, Lesnefsky EJ, et al. Mitochondrial mass and activity as a function of body composition in individuals with spinal cord injury. Physiol Rep. 2017;5:e13080.
Sergi D, Naumovski N, Heilbronn LK, Abeywardena M, O’Callaghan N, Lionetti L, et al. Mitochondrial (Dys) function and insulin resistance: from pathophysiological molecular mechanisms to the impact of diet. Front Physiol. 2019;10:532.
Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86:142–52.
Laclaustra M, Van Den Berg ELM, Hurtado-Roca Y, Castellote JM. Serum lipid profile in subjects with traumatic spinal cord injury. PLoS ONE. 2015;10:e0115522.
Acknowledgements
The work was supported by the Department of Veteran Affairs (VA) Merit Program [Application # 1 I01 RX002649-01A; Protocol ID: B2649-R] by ASG. We would like to thank all the SCI participants who contributed their time and effort to complete the current work.
Author information
Authors and Affiliations
Contributions
AMA: formal analysis, investigation, writing original draft, reviewed scientific evidence, software, development of figures. JAG: assisted with original draft, formal analysis, critical feedback, data curation. REK and MRK aided in data curation, software, investigation, and resources. ASG: formal analysis, investigation, writing original draft, reviewed scientific evidence, critical feedback, supervision and approving of final draft as well as providing the funding source.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The authors certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alazzam, A.M., Goldsmith, J.A., Khalil, R.E. et al. Denervation impacts muscle quality and knee bone mineral density after spinal cord injury. Spinal Cord 61, 276–284 (2023). https://doi.org/10.1038/s41393-023-00885-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-023-00885-3