Abstract
Study design
Systematic review.
Objectives
To systematically review the evidence on the use of local analgesics, specifically lidocaine or bupivacaine, to prevent autonomic dysreflexia (AD) during iatrogenic procedures or bowel and bladder care routines in individuals with spinal cord injury (SCI).
Methods
A keyword search of MEDLINE, CINAHL, CENTRAL, Cochrane Reviews, PsycInfo, Embase, and Web of Science databases identified all English-language studies evaluating the efficacy of local analgesics in reducing AD. Included studies were either randomized controlled trials (RCTs) or quasi-experimental studies. Participants were adults with chronic SCI who received local analgesics prior to AD-triggering procedures or routines. Additionally, studies were required to report blood pressure values as an outcome. The methodology of this review followed the PRISMA checklist and was registered with PROSPERO (CRD42021219506).
Results
Four RCTs and two quasi-experimental studies met inclusion criteria. Results were narratively synthesized as meta-analysis was not possible due to heterogeneity across studies included in the review. All six studies administered lidocaine. Lidocaine was found to have a beneficial effect on AD in three studies, no effect in two studies and a detrimental effect in one study.
Conclusions
Presently, RCTs and quasi-experimental studies on the use of lidocaine for reducing AD in individuals with SCI had small sample sizes and opposing findings. There is a strong need for definitive, well-monitored clinical trials with adequate sample sizes. Presently there is not enough compelling evidence to support or refute recommendations for the use of lidocaine from the AD management clinical practice guidelines.
Similar content being viewed by others
Introduction
Autonomic dysreflexia (AD) is a life-threatening hypertensive condition that commonly occurs in individuals with spinal cord injury (SCI), more frequently in those with injuries at the T6 spinal level and above [1]. Although defined as a rise in systolic blood pressure (BP) of ≥20 mmHg triggered by afferent stimuli originating below the level of injury, AD episodes are frequently associated with more pronounced elevation in arterial BP reaching up to 300 mmHg systolic [2, 3] and may result in devastating consequences [4, 5]. Typically, AD is accompanied by severe headache, anxiety, nasal congestion, blurred vision, and bradycardia, as well as flushing, piloerection, and sweating above the level of injury and dry and pale skin below the level of injury. AD is most commonly triggered by events in the lower urinary tract and in the colorectal area [6], and can be iatrogenic in nature, occurring during cystoscopy, urodynamic evaluation, penile vibration, electroejaculation, rectosigmoid distension and anal manipulation [6, 7]. Untreated episodes of AD have resulted in hemorrhagic stroke, retinal detachment, seizures, and death [8,9,10,11,12]. Therefore, timely recognition and management of AD in individuals with SCI is critical.
The latest version of clinical practice guidelines (published by Paralyzed Veterans of America; PVA) provides numerous pharmacological and non-pharmacological therapeutic modalities for prevention and management of AD [13]. These guidelines outline the steps for identifying and managing episodes of AD and provide an algorithm for treatment. Broadly, most management approaches for AD focus on either preventing or reducing noxious stimuli below the level of spinal cord lesion which trigger AD, accomplished by either removing the noxious stimuli or blocking afferent stimulation via the inactivation of nociceptors. For this reason, common topical analgesics such as lidocaine and bupivacaine are frequently used for bowel management at home and in the clinic. The PVA management guidelines for AD specifically recommend the use of lidocaine jelly for prevention of episodes of AD that could be triggered by urethral or anorectal irritation (e.g., instillation of 2% lidocaine jelly prior to urinary catheterization, rectal examination and stool removal, and the use of lidocaine solution during bladder irrigation) [6]. Local analgesics, such as lidocaine or bupivacaine, block afferent signals by blocking sodium channels [14] and is thus theorized to mitigate AD. However, despite topical analgesics being widely used and recommended clinically, the evidence for the impact of lidocaine and bupivacaine on episodes of AD triggered by bowel and bladder management remains inconclusive as several recent studies have reported contradictory findings [15, 16]. Therefore, this systematic review is aimed at assessing the current evidence on the use of topical analgesics to mitigate AD triggered by iatrogenic procedures and daily care routines in individuals with SCI.
Methods
Methods of search, screening, and analysis were registered on PROSPERO (CRD42021219506). The review was done in accordance with the PRISMA checklist (Preferred Reporting Items for Systematic reviews and Meta-Analyses) [17]. A keyword search was conducted for English-language studies published after 1960 investigating the impact of local analgesics in reducing AD. Searches were conducted in the following databases: MEDLINE, CINAHL, CENTRAL, Cochrane Reviews, PsycInfo, Embase, Web of Science. Population keywords such as spinal cord injury, tetraplegia, paraplegia, and quadriplegia were paired individually with the intervention keywords lidocaine and bupivacaine and with the outcome keywords blood pressure and autonomic dysreflexia. Full details of the search strategy for the MEDLINE database are demonstrated in Fig. 1. Variations of this search were used, specific for each database. Studies were included if participants were adults (18 + years old) with chronic SCI ( >1 year) who received either bupivacaine or lidocaine through any method of administration before or during procedures or routines with potential to trigger AD. For inclusion, studies were required to report BP values. Study designs were limited to randomized control trials (RCTs) and quasi-experimental studies. Manual searches were conducted through references of included articles.
abstract and full-text review were performed by three independent reviewers, such that each study was rated by at least two reviewers. Conflicts were resolved by discussion leading to a mutual consensus. Twenty non-English studies were translated with Google translate and were excluded during abstract search because they did not meet inclusion criteria. Data extraction tables were designed by three authors in collaboration. Two reviewers independently collected data for each article. Information extracted from each paper included: (1) authors, country where study was conducted, study design and quality assessment results; (2) methodology (including participant population, procedure with potential to trigger AD, analgesic intervention, and outcome measures); and (3) primary outcomes and conclusions from each study (Table 1). See Fig. 1 for the exact search strategy used for the MEDLINE database. To assess quality and risk of bias in individual studies, we used version 2 of the Cochrane Risk of Bias Tool for RCTs [18] and the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for quasi-experimental studies [19]. To create a “Low, Moderate, or High” judgment of overall quality for each study, we used the principles outlined by the Cochrane Risk of Bias Tool [18]. The systematic review software Covidence was used for eligibility assessment, full-text review, and quality assessment.
Results
Study selection
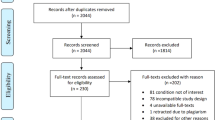
The database search produced 243 citations in total (Fig. 2). Once duplicates were removed, 187 citations were screened for eligibility. A review of titles and abstracts resulted in the exclusion of 120 studies as these did not meet the inclusion criteria. The full texts of 67 articles were reviewed in detail; in the end, 61 studies did not meet the inclusion criteria. No unpublished studies were included. No additional studies were identified via the manual search. In the end, 6 studies met the inclusion criteria and were ultimately included in the review [15, 16, 20,21,22,23].
Risk of bias
The results of the Cochrane Risk of Bias Tool for RCTs [18] and JBI Critical Appraisal Checklist for the quasi-experimental studies [19] are presented in Table 1. No studies were excluded on the basis of quality level. Of the four RCTs, one study was judged to have low risk of bias [21], another study had moderate risk of bias [20] and the other two studies had high risk of bias [15, 16]. With respect to the two quasi-experimental studies, one study was judged to have low risk of bias [23] and the other had high risk of bias [22].
Study characteristics
A meta-analysis was not appropriate for this systematic review due to limited number of relevant studies, with considerable heterogeneity across the design, procedures/routines, interventions and reported outcome measures. Therefore, the present review is focused on a qualitative narrative synthesis.
Design and participant characteristics
Table 1 summarizes the design and participant characteristics of the 6 studies included in the review. Four studies were RCTs [15, 16, 20, 21] and two studies lacked randomization and thus were of quasi-experimental design [22, 23]. Three studies were conducted in the USA [20, 21, 23], two in Canada [16, 22] and one in Japan [15]. The publication years of the studies spanned greater than 20 years from 1997 to 2020 and included 280 procedures and 165 patients (some procedures were performed more than once in the same patient). Gender and level/severity of SCI were reported for all studies. Most participants were male, consisting of 89.7% of all participants. All participants had either high thoracic or cervical SCI.
Procedures and interventions
For consistency and clarity, “procedure” refers to any potential AD triggering event that participants underwent. Four studies were related to bowel care routines or procedures [15, 16, 20, 21], one study was related to urological care (e.g., catheterization) [23] and one was related to functional electrical stimulation [22]. Although bupivacaine was included as a search term during the database search, none of the included studies used bupivacaine; only lidocaine or a lidocaine/prilocaine combination were used as the intervention in all studies [15, 16, 20,21,22,23]. As such, from this point forward, “intervention” refers to administration of lidocaine. The administration methods included anal block injection [20], rectal insertion of lubricant [15, 16, 21], intravesical instillation of lubricant [23] and topical cream [22].
Outcomes
The primary outcome of most studies was the impact of lidocaine on AD during procedures, reported as SBP changes and/or AD incidence by number of participants (Table 1). All studies either reported peak SBP or change in SBP from baseline. AD symptoms such as headache, sweating or flushing were also reported when present. Additional outcomes reported by some studies included time-to-completion of procedure, heart rate (HR) and cardiac rhythm. No study reported on adverse events or complications due to prolonged AD such as retinal hemorrhage or death.
Effects of lidocaine on AD severity
Overall, three studies reported lower SBP values or AD incidence with use of lidocaine compared to control/placebo [15, 20, 23] and two studies reported no difference in SBP values or AD incidence [21, 22]. One study reported that the use of lidocaine may worsen AD because the absolute maximum SBP was higher in the lidocaine condition compared to the placebo condition [16]. Regarding the secondary outcome, HR changes, two studies reported no differences in HR changes with use of a lidocaine compared to placebo [15, 22]. Only one study investigated cardiac arrhythmias but the relationship between lidocaine interventions and cardiac arrhythmias was unclear [16].
Bowel sub-group synthesis
Intricacies of lidocaine’s effect on AD are revealed by examining the four studies related to bowel care in more depth. Two studies compared the use of lidocaine versus placebo lubricant during bowel care routines and had conflicting findings [15, 16]. Furusawa et al. [15] demonstrated lidocaine lubricant was effective in reducing the severity of AD although AD was not completely prevented. In this study, SBP during the bowel routine in the lidocaine condition was still elevated compared to baseline values; however, maximal SBP was significantly lower than in the placebo condition, and SBP returned to baseline values at an earlier point during the routine in the lidocaine condition compared placebo. Additionally, four patients reported AD symptoms in the lidocaine condition compared to ten patients in the placebo condition. There were no HR changes in either condition [15]. On the other hand, Lucci et al. [16] concluded that lidocaine lubricant was not only ineffective at reducing AD but may even worsen AD. Maximal SBP was significantly higher and time to complete the bowel care routine (i.e. time spent at risk of triggering AD) was significantly longer in the lidocaine condition compared to placebo. However, there was no significant difference between conditions for mean SBP during bowel care nor when maximal SBP was compared to baseline, which could be attributed to the lack of stable BP during baseline due to distended bowel [16, 24, 25].
Two other studies compared lidocaine to placebo during a variety of anorectal procedures and found differing results [20, 21]. Cosman, Vu & Plowman found that topical lidocaine did not significantly reduce the SBP elevation from baseline compared to placebo, regardless of the type of anorectal procedures [21]. The same group later found an anal block with lidocaine injections was effective in reducing the maximal SBP increase from baseline compared to placebo during procedures involving flexible sigmoidoscopy [20]. However, lidocaine anal block was ineffective at blocking AD during procedures involving anoscopy, which is theorized to have a stronger sphincter stretch stimuli.
Discussion
The objective of this systematic review was to investigate the evidence on the clinical use of lidocaine for reducing AD in individuals with SCI. Given how commonly AD occurs in the SCI population [26] and the severity of complications that may result from AD [5], having effective strategies for managing AD is of utmost importance [6]. Patients with SCI undergo frequent medical procedures and engage in daily care routines, both of which can be triggers of AD [3, 7, 13]. Identifying effective strategies to prevent AD during medical procedures and routines would significantly reduce the risk of complications.
Of the six studies that were included in our review, lidocaine was found to be beneficial in three studies. However, two studies found no effect of lidocaine on AD triggered by procedures and one study found a detrimental effect. The administration method of the lidocaine and the AD triggering procedures varied widely across the included studies, preventing any patterns from emerging. Additionally, the overall risk of bias in four RCTs and two quasi-experimental studies is inconsistent, making it challenging to compare findings across studies. Finally, no meta-analysis or summary statistics were performed due to significant heterogeneity of studies. Overall, insufficient number of studies and wide diversity of the AD triggering procedures prevented any conclusive recommendation regarding the general use of lidocaine during AD triggering procedures.
Despite the majority of studies focusing on bowel care routines and anorectal procedures, no clear conclusions could be drawn due to opposing findings. With respect to reducing severity of AD during bowel care routines, the results of Furusawa et al. [15] support the use of lidocaine lubricant applied rectally prior to the routine; while Lucci et al. [16] found a detrimental effect of lidocaine lubricant on AD under similar clinical circumstances.
Two studies investigated the use of lidocaine to prevent or reduce the severity of AD during bowel procedures such as anoscopy and/or flexible sigmoidoscopy [20, 21]. These two studies are interesting to compare to one another because they were done by the same research group with one study using topical lidocaine [21] and the other using injectable lidocaine to create an anal block [20]. In the study by Cosman and Vu [20], it is unclear why a lidocaine anal block was effective at reducing AD during flexible sigmoidoscopy but not during anoscopy. We theorize that this could occur because anoscopy is theoretically a stronger stimulus for the anal sphincter than flexible sigmoidoscopy. Additionally, the dense innervation of the anal sphincter could make anoscopy a stronger stimulus when compared to stimulation that involves visceral organs such as the rectum or the colon. Higher concentrations or stronger analgesics may be necessary to counteract stronger stimuli. However, this relationship requires further investigation.
At the present time, clinical practice guidelines by the Consortium for Spinal Cord Medicine recommend the use of 2% lidocaine jelly prior to catheter change and bowel care in individuals with SCI [6]. However, these guidelines rely heavily on clinical consensus. More robust studies are needed to make an accurate decision about whether to support or revise these guidelines. Overall, from a clinical standpoint, our review found no clear conclusion to offer practitioners caring for patients with SCI regarding the use of lidocaine during AD triggering procedures.
Limitations
A limitation of note for the current systematic review includes the lack of consistent outcome measures limited comparisons between studies. As a meta-analysis was not performed, no request was made to authors to provide additional data. While obtaining consistent outcome measures across studies may have eased comparisons, given the small number of studies identified by this review, little additional insight would have been gained. Additionally, gray literature was not included in this review; therefore, the possibility exists that this literature could resolve some of the conflicts identified by this review. Finally, since the studies did not consistently report AD symptomology concurrent with the increase in SBP, it is challenging to differentiate between average increase in SBP and symptomatic AD based on the current literature.
Conclusion
Presently there is inconclusive evidence regarding whether lidocaine is effective in reducing iatrogenic AD during medical procedures or care routines for patients with SCI. Half of the published literature states that lidocaine is effective at reducing AD whereas the other half states that it is ineffective or detrimental. In sum, there is no compelling evidence to support or refute the use of lidocaine from the AD management clinical practice guidelines. Regardless, there is a strong need for definitive, well-monitored clinical trials with adequate sample sizes to strengthen the evidence regarding the role of lidocaine in AD management.
Data availability
Additional data may be provided by the corresponding author on reasonable request.
References
Teasell RW, Mehta S, Aubut JL, Foulon B, Wolfe DL, Hsieh JTC, et al. A systematic review of pharmacologic treatments of pain after spinal cord injury. Arch Phys Med Rehabil. 2010;91:816–31.
Sachdeva R, Nightingale TE, Krassioukov AV. The blood pressure pendulum following spinal cord injury: implications for vascular cognitive impairment. Int J Mol Sci. 2019;20:2464.
Faaborg PM, Christensen P, Krassioukov A, Laurberg S, Frandsen E, Krogh K. Autonomic dysreflexia during bowel evacuation procedures and bladder filling in subjects with spinal cord injury. Spinal Cord. 2014;52:494–8.
Vaidyanathan S, Soni BM, Mansour P, Oo T. Fatal collapse due to autonomic dysreflexia during manual self-evacuation of bowel in a tetraplegic patient living alone: lessons to learn. Int Med Case Rep. J. 2017;10:361–5.
Wan D, Krassioukov AV. Life-threatening outcomes associated with autonomic dysreflexia: A clinical review. J Spinal Cord Med. 2014;37:2–10.
Krassioukov A, Warburton DE, Teasell R, Eng JJ. Spinal cord injury rehabilitation evidence research team. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil. 2009;90:682–95.
Liu N, Zhou M, Biering-Sørensen F, Krassioukov AV. Iatrogenic urological triggers of autonomic dysreflexia: a systematic review. Spinal Cord. 2015;53:500–9.
Pine ZM, Miller SD, Alonso JA. Atrial fibrillation associated with autonomic dysreflexia. Am J Phys Med Rehabilitation. 1991;70:271.
Eltorai I, Kim R, Vulpe M, Kasravi H, Ho W. Fatal cerebral hemorrhage due to autonomic dysreflexia in a tetraplegic patient: case report and review. Paraplegia. 1992;30:355–60.
Dolinak D, Balraj E. Autonomic dysreflexia and sudden death in people with traumatic spinal cord injury. Am J forensic Med Pathol. 2007;28:95–98.
Valles M, Benito J, Portell E, Vidal J. Cerebral hemorrhage due to autonomic dysreflexia in a spinal cord injury patient. Spinal Cord. 2005;43:738–40.
Yarkony GM, Katz RT, Wu YC. Seizures secondary to autonomic dysreflexia. Arch Phys Med Rehabil. 1986;67:834–5.
Krassioukov A, Linsenmeyer TA, Beck LA, Elliott S, Gorman P, Kirshblum S, et al. Evaluation and management of autonomic dysreflexia and other autonomic dysfunctions: preventing the highs and lows: management of blood pressure, sweating, and temperature dysfunction. J Spinal Cord Med. 2021;44:631–83.
Barkin RL. The pharmacology of topical analgesics. Postgrad Med. 2013;125(4 Suppl 1):7–18.
Furusawa K, Sugiyama H, Tokuhiro A, Takahashi M, Nakamura T, Tajima F, et al. Topical anesthesia blunts the pressor response induced by bowel manipulation in subjects with cervical spinal cord injury. Spinal Cord. 2009;47:144–8.
Lucci VM, McGrath MS, Inskip JA, Sarveswaran S, Willms R, Claydon VE, et al. Clinical recommendations for use of lidocaine lubricant during bowel care after spinal cord injury prolong care routines and worsen autonomic dysreflexia: results from a randomised clinical trial. Spinal Cord. 2020;58:430–40.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
Higgins J, Altman DG Chapter 8: Assessing risk of bias in included studies. In: Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.0.0: The Cochrane Collaboration.; 2008.
Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis: JBI; 2020.
Cosman BC, Vu TT. Lidocaine anal block limits autonomic dysreflexia during anorectal procedures in spinal cord injury: A randomized, double-blind, placebo-controlled trial. Dis Colon Rectum. 2005;48(AUG):1556–61.
Cosman BC, Vu TT, Plowman BK. Topical lidocaine does not limit autonomic dysreflexia during anorectal procedures in spinal cord injury: a prospective, double-blind study. Int J Colorectal Dis. 2002;17(MAR):104–8.
Matthews JM, Wheeler GD, Burnham RS, Malone LA, Steadward RD. The effects of surface anaesthesia on the autonomic dysreflexia response during functional electrical stimulation. Spinal Cord. 1997;35:647–51.
Solinsky R, Linsenmeyer TA. Intravesical lidocaine decreases autonomic dysreflexia when administered prior to catheter change. J Spinal Cord Med. 2019;42:557–61.
Gray K, Sheehan W, Wecht J, Linsenmeyer TA, Sachdeva R, Krassioukov AV, et al. Response to “Clinical recommendations for use of lidocaine lubricant during bowel care after spinal cord injury prolong care routines and worsen autonomic dysreflexia: results from a randomised clinical trial”. Spinal Cord. 2021 -09-30:1-2.
Lucci VM, McGrath MS, Inskip JA, Sarveswaran S, Willms R, Claydon VE, et al. Response to “Clinical recommendations for use of lidocaine lubricant during bowel care after spinal cord injury prolong care routines and worsen autonomic dysreflexia: results from a randomized clinical trial”—the authors reply. Spinal Cord. 2021;59:1311–2.
Hubli M, Gee CM, Krassioukov AV. Refined assessment of blood pressure instability after spinal cord injury. Am J Hypertension. 2015;28:173–81.
Acknowledgements
We thank Dr. Trina Fyfe, the Health Sciences Librarian for the Northern Medical Program at UBC, for the theoretical and conceptual assistance during the database search process. Dr. Krassioukov’s laboratory is supported by funds from the Canadian Institute for Health Research, Heart and Stroke Foundation, Canadian Foundation for Innovation, BC Knowledge Development Fund, PRAXIS Spinal Institute, Wings for Life Spinal Cord Research Foundation, International Spinal Research trust (ISRT), Craig H. Neilsen Foundation; and Seed grants from International Collaboration on Repair Discoveries (ICORD). Dr. Krassioukov holds Endowed Chair in Spinal Cord Rehabilitation Research, Department of Medicine, UBC. Dr. Sachdeva is supported by the Wings for Life Spinal Cord Research Foundation and has fellowships from Canadian Institutes of Health Research, Michael Smith Foundation for Health Research (MSFHR), and Faculty of Medicine, University of British Columbia (Bluma Tischler Fellowship). Dr. Krogh is supported by the Novonordisk Foundation.
Author information
Authors and Affiliations
Contributions
WS, KG, RS, and AK designed the review. WS and KG conducted the database search. WS, KG, and LM screened and selected eligible articles, extracted the data and analyzed the quality of the included studies. Any discordance was settled by consensus between WS, KG and LM. WS, KG, and LM interpreted the results and wrote the first draft of the report. RS and AK provided theoretical feedback throughout the review process. WS, KG, KK, RS, and AK provided feedback on the manuscript and made edits to the final version. All authors approved the submission of the manuscript and declare no conflicts of interest.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gray, K., Sheehan, W., McCracken, L. et al. Are local analgesics effective in reducing autonomic dysreflexia in individuals with spinal cord injury? A systematic review. Spinal Cord 61, 1–7 (2023). https://doi.org/10.1038/s41393-022-00840-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-022-00840-8