Abstract
Study design
An international multi-centred, double-blinded, randomised sham-controlled trial (eWALK).
Objective
To determine the effect of 12 weeks of transcutaneous spinal stimulation (TSS) combined with locomotor training on walking ability in people with spinal cord injury (SCI).
Setting
Dedicated SCI research centres in Australia, Spain, USA and Scotland.
Methods
Fifty community-dwelling individuals with chronic SCI will be recruited. Participants will be eligible if they have bilateral motor levels between T1 and T11, a reproducible lower limb muscle contraction in at least one muscle group, and a Walking Index for SCI II (WISCI II) between 1 and 6. Eligible participants will be randomised to one of two groups, either the active stimulation group or the sham stimulation group. Participants allocated to the stimulation group will receive TSS combined with locomotor training for three 30-min sessions a week for 12 weeks. The locomotor sessions will include walking on a treadmill and overground. Participants allocated to the sham stimulation group will receive the same locomotor training combined with sham stimulation. The primary outcome will be walking ability with stimulation using the WISCI II. Secondary outcomes will record sensation, strength, spasticity, bowel function and quality of life.
Trial registration
ANZCTR.org.au identifier ACTRN12620001241921
Similar content being viewed by others
Introduction
Regaining the ability to walk is a priority for individuals with spinal cord injury (SCI) [1,2,3] and has been the focus of many studies and clinical trials [4]. While it is currently not possible to restore voluntary control of muscles paralysed after SCI, recent developments have been made [5]. A series of case studies indicate that epidural spinal stimulation in individuals with SCI can elicit step-like, rhythmic movement of the legs [6,7,8,9,10]. Similar effects have also been observed when transcutaneous spinal stimulation (TSS) is applied in able-bodied individuals [11, 12], with evidence that TSS activates the same neural structures as epidural stimulation [13]. Moreover, there is preliminary evidence that TSS may be able to restore voluntary movement and the ability to stand and walk in some individuals with SCI [14]. These improvements occur almost immediately in some individuals while others experience progressive improvements after combined spinal stimulation and intensive physiotherapy. Moreover, there is preliminary evidence that TSS can reduce spasticity [15], which may also contribute to improved walking ability.
Following a complete SCI, the human lumbosacral neural circuitry can generate rhythmic motor output in response to cutaneous and proprioceptive afferent signals elicited during assisted standing and walking [14, 16,17,18,19,20]. Similarly, TSS can elicit rhythmic muscle activity [10, 21,22,23]. Importantly, these therapeutic modalities—assisted standing and walking and TSS—interact with one another [24] and their combined application can elicit greater rhythmic muscle activity than either intervention alone [14, 22]. Moreover, repeated exposure to this combined therapy can augment, and potentially restore, connections with supraspinal centres and postural reflex pathways [25,26,27,28,29]. As a population on the rise [30,31,32], individuals with incomplete SCI are the most likely to regain the ability to walk [33], and TSS could help with recovery by increasing the excitability of lumbar spinal locomotor circuits that have partially lost their descending motor drive [34,35,36,37]. Preliminary evidence indicates that TSS can improve gait kinematics and locomotor muscle activity in these individuals.
Despite positive preliminary results, a lack of evidence from randomised controlled trials has prevented translation into standard clinical practice [38]. Therefore, the primary aim of this study is to determine the effectiveness of 12 weeks of TSS combined with locomotor training on walking ability in people with chronic SCI. For this purpose, we will compare a group randomised to receive TSS plus locomotor training to a group randomised to receive sham TSS plus locomotor training. This study will also investigate the effect of this training on sensory [39, 40], motor [41, 42] and bowel function [40, 43, see also 44], spasticity and quality of life. We hypothesise that TSS with locomotor training will improve walking ability in people with chronic SCI who have some residual lower limb motor function. We also hypothesise TSS with locomotor training will improve spasticity, sensation, lower limb muscle strength, bowel function and quality of life. Furthermore, we hypothesise that improvements will only be apparent in the presence of TSS.
Methods
Trial design and study setting
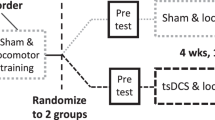
An international multi-centred, double-blinded, randomised sham-controlled trial will be conducted in four SCI research centres located in Australia, USA, Scotland and Spain. Fifty participants with chronic SCI will be randomised to one of two groups, namely the stimulation group or the sham group. Participants allocated to the stimulation group will receive TSS while they complete 30-min of locomotor training, three times per week for 12 weeks (Fig. 1A). Participants allocated to the sham group will receive the same locomotor training, but with sham TSS.
Participants
Recruitment and consent procedure
Participants will be recruited via existing research databases, health professionals and advertisements on websites of SCI organisations. Potential participants will be given a copy of the participant information sheet to read. They will be informed that their participation in the trial is voluntary and will not affect their current or future relationships with the study centres. Participants will be encouraged to ask questions and to discuss the trial with family and friends before providing consent. Once essential trial information has been provided, participants will be asked to give informed consent to participate in the trial by signing the Consent Form in the presence of a witness. These forms will be dated and retained by the investigator at the site where consent is gained, and a copy will be provided to the participant. The present plan is for the Sydney site, the sponsor, to recruit approximately 20 participants and each of the other sites to recruit approximately 10 participants each.
Inclusion and exclusion criteria
Table 1 presents the inclusion and exclusion criteria.
Allocation and randomisation of participants
A secure blocked random-allocation schedule will be computer-generated prior to the start of the trial by an independent person not directly involved in the trial. The random-allocation schedule will consist of 50 three-digit codes: 25 assigned to the stimulation group and 25 assigned to the sham group. The three-digit codes will be used to program the stimulator control unit. The random-allocation schedule will be uploaded to REDCap [45] to allow each study site to randomise participants. To maintain blinded allocation, the REDCap random-allocation schedule will display the three-digit code, not the participant’s allocated group. Once a participant is deemed eligible for the trial, a baseline assessment will be completed, and a study investigator will randomise the participant within REDCap. The participant will be considered enroled in the trial at this time.
Intervention
The physiotherapists and assistants delivering the locomotor training will be blinded to treatment allocation. Participants will be instructed to not discuss their perceived group allocation with the physiotherapists and assistants. The success of blinding will be recorded at week 12. When enroled in the trial, participants will be asked to stop any formal physiotherapy programs aimed at improving walking.
Locomotor training
Both groups will receive three 30-min locomotor training sessions per week for 12 weeks while receiving their allocated stimulation. Previous studies examining transcutaneous stimulation and locomotor training protocols vary considerably in frequency and duration [46], particularly stimulation studies in which there are currently no RCTs [14, 41]. Many of the studies examining the effects of traditional locomotor training programs, typically train participants between 8 to 12 weeks, 3–5 times a week, with improvements in walking ability seen in people with incomplete SCI [47,48,49]. Potential participants were contacted to gauge whether it would be feasible for them to train three times a week for 12 weeks, this was deemed feasible by all the people contacted. Therefore, three 30-min locomotor training sessions per week for 12 weeks was chosen as an adequate training dose, taking into account current established training protocols and the time commitment from participants. While overground and treadmill locomotor training have equivalent therapeutic benefits in people with incomplete SCI [24,25,26], body-weight support treadmill training is more ergonomic for therapists and allows for greater dosage (i.e., steps) when training individuals with impaired mobility (i.e., WISCI II scores from 1 to 6). Thus, participants will undergo five treadmill and one overground training session(s) per fortnight, for a total of 30 treadmill and six overground training sessions over 12 weeks. Training will be delivered by an experienced neurological physiotherapist and up to two trained assistants as required. Training will be provided to all therapists at each site on how to perform the locomotor training to ensure consistency across sites.
Participants will be allowed standing, and if needed, seated rests during the training sessions. Stimulation will be stopped during seated rests, and this time will not count towards the 30 minutes training target. In contrast, stimulation will continue during standing rests, and this time will count towards the 30 minutes training target. For each session, a maximum of 60 minutes will be allotted to complete the 30 minutes of training. While it is possible a participant may not complete 30 minutes of training in a session if they require regular or prolonged seated rests, unpublished pilot work conducted in the Sydney centre found that participants rarely require seated breaks. Standing breaks, when taken, typically lasted no longer than 1–2 minutes. Thus, we expect most participants to complete the 30 minutes of training each session, with > 85% of each session spent walking.
Manual assistance of the lower limbs based on established locomotor protocols will be provided as required to improve walking patterns [50]. The amount of body-weight support required will be assessed on the first training day. Excessive knee flexion during the stance phase (i.e., > 40°) or toe dragging during the swing phase will indicate body-weight support needs to be increased [51]. Body-weight support will be reviewed regularly with the aim of walking with the greatest amount of weight-bearing that does not cause excessive knee flexion or toe drag. There will be no limit to the amount of body-weight support that can be provided, as long as the participant’s heels contact the treadmill. The speed of the treadmill will be monitored and adjusted by the physiotherapist throughout the 12-week training period, with the aim of walking at the fastest comfortable speed that allows for a correct walking pattern. For overground training sessions, participants will be encouraged to walk at a comfortable pace. To ensure safety and optimise the participant’s walking pattern during overground training sessions, orthoses, gait aides, parallel bars and safety harnesses will be used as required.
The details of each training session will be recorded by the therapist in a training diary. Changes in pain and spasticity will be recorded on a weekly basis. Changes in medication use will also be recorded.
Transcutaneous spinal stimulation
Transcutaneous spinal stimulation will be applied with the anode (5 × 10 cm) placed over the lower abdomen and the cathode (5 × 10 cm) placed over the lower back, both centred on the midline of the body [14, 22, 52, 53]. For the anode, the long edge of the electrode will be oriented horizontally. For the cathode, the long edge of the electrode will be oriented vertically. The preferred cathode placement will be with the top edge of the electrode in-line with the L1–L2 vertebral interspace. However, this location may be adjusted rostrally—to a maximum of the T11–T12 vertebral interspace—to accommodate for implanted metal hardware or the rare case that posterior root-muscle (PRM) reflexes cannot be elicited at the preferred electrode site. Prior to the first day of locomotor training plus stimulation (or sham), all participants will be briefed on what to expect with regards to the stimulation. Specifically, participants will be informed that each person experiences the stimulation differently based on their level of sensation and tolerance. Participants will also be informed that the sensations associated with the stimulation may change over time, including over the course of a single training session.
Studies, case studies and case series investigating the use of TSS to improve walking ability in people with SCI have used a variety of stimulation parameters [54]. The choice of parameters for the current trial was based on a study conducted in Sydney on 10 able-bodied participants and 10 participants with SCI [55] and a survey of the literature. The basic stimulation waveform will be 1 ms in duration, filled with a biphasic 10 KHz carrier frequency. This type of waveform has been used in several previous studies [12, 14, 39, 56,57,58]. While some studies have reported similar effects without the high carrier frequency [22, 41, 53], there is evidence that, with the high-frequency component present, motor responses can be elicited with less discomfort [59, 60], although a recent paper does not support this claim [61]. Moreover, there is recent evidence that, for the upper limb, including a carrier frequency may have suppressive effects on cortical excitability in people with SCI, which is associated with greater functional performance [62]. However, the mechanisms underlying improvements remain unclear for conventional or carrier frequency stimulation delivered either epidurally or transcutaneously.
Posterior root-muscle reflexes are evoked muscle responses elicited by electrically stimulating the posterior nerve roots of the spinal cord. To assess these, biphasic rectangular single pulses of 1 ms with a 10 kHz carrier frequency will be delivered using a Digitimer Biphasic Constant Current multi-modal stimulator (DS8R, Digitimer Ltd, UK), driven by a custom stimulator control unit. The minimal stimulation intensity required to induce PRM reflexes in the bilateral vastus medialis muscles (> 50 μV peak-to-peak amplitude above the background muscle activity in 5 of 10 consecutive trials in relaxed muscles) will be used to set TSS intensity during locomotor training [62]. Reflexes will be assessed in standing with approximately 90% body-weight support to enable some afferent feedback from the feet, yet no EMG activity from the vastus medialis muscles. The location of the EMG electrodes will be standardised across sites. This threshold will be assessed prior to randomisation and then re-assessed every 2 weeks, during the training period.
Transcutaneous spinal stimulation during locomotor training will consist of the same biphasic rectangular pulse delivered tonically at 20 Hz. TSS intensity will be 100% of the threshold intensity determined during the most recent PRM reflex testing session. Various TSS intensities have been used in studies involving people with SCI, with beneficial effects reported for intensities that are below the motor threshold, above the motor threshold and at the motor threshold [41, 62, 63]. We chose to not use a subthreshold target intensity as this is difficult to set reliably, especially across therapists, sessions and study sites [64]. Moreover, a study conducted in Sydney confirmed that the proposed TSS intensity elicits only very small lower limb muscle contractions, which do not interfere with the locomotor training or with walking by the participants.
Various stimulation frequencies have been used to induce locomotor-like muscle activity using TSS, ranging from 5–50 Hz [14, 22, 40, 65]. The amplitude of step-like movements increase as the stimulation frequency increases from 5 Hz to 40 Hz [12]. More recently, lower stimulation frequencies (< 15 Hz) are preferred to assist standing, while higher frequencies are used to assist walking [14, 66]. As discomfort due to the stimulation is a concern in people with incomplete SCI, and unpublished pilot experiments found 20 Hz stimulation to be more comfortable than 30 Hz stimulation, the present trial will use 20 Hz TSS (1 ms pulses filled with biphasic 10 kHz carrier frequency).
Sham transcutaneous spinal stimulation
The experimental set-up, procedures and training will be identical for participants randomised to the Sham group, except that they will receive sham TSS during the training sessions. Several studies have successfully employed sham electrical stimulation [67,68,69,70,71]. We have adapted these procedures to reduce further the possibility that participants will be able to determine (or guess) their group allocation. To reduce the risk of unblinding, details related to the novel sham TSS will only be reported with the results of the trial. Providing details of the sham stimulation prior to the trial being completed would make them accessible to blinded research staff and participants, which could lead to real or perceived unblinding and thus bias trial results. However, if others wish to replicate or adapt our sham stimulation prior to completion of the current trial, details will be shared upon reasonable request.
Outcomes
Primary and secondary outcomes were selected based on a review of previous studies investigating the benefits of locomotor training or TSS in people with SCI, as well as consultation with spinal physicians and the SCI community. Outcome measures will be assessed at baseline prior to randomisation and 12 weeks after randomisation. Participants will also be followed up four weeks after the intervention period (week 16, Fig. 1B). Assessments will be completed by an independent blinded assessor at each trial site; the assessor will be a physiotherapist with experience treating individuals with SCI and assessing the outcome measures. Participants will be instructed to not discuss their perceived group allocation with the blinded assessor. At weeks 12 and 16, some outcomes are assessed twice, once with the stimulation or sham and once without the stimulation or sham. The order of these assessments (i.e., with and without stimulation or sham) will be randomised across participants.
Adverse events directly related to the treatment will also be monitored throughout the trial.
Primary outcome
Walking ability with stimulation or sham at 12 weeks
Walking ability will be measured using the WISCI II. This is a functional capacity scale that rank orders the ability of a person with a SCI to walk 10 m from most to least impaired [72]. An a priori between-group difference of 2 points on the WISCI II has been determined as the minimally worthwhile treatment effect [73].
Secondary outcomes
Walking ability with stimulation or sham at 16 weeks
Measured using the WISCI II.
The following outcomes will be measured at 12 and 16 weeks.
Walking ability without stimulation or sham
Measured using the WISCI II.
Lower extremity motor function with and without stimulation or sham
Strength will be assessed with the lower extremity motor score from the ISNCSCI [74]. Scores will be summed, with a total possible score of 50.
Spasticity with and without stimulation or sham
Lower limb spasticity will be assessed with the Modified Ashworth Scale [75]. Three lower limb muscle groups will be tested: knee extensors, ankle plantar flexors and hip flexors. Scores will be summed, with a total possible score of 30.
Sensation without stimulation or sham
Sensation will be assessed with the sensory score of the ISNCSCI [74]. Scores will be summed, with a total possible score of 224.
Bowel function
Bowel function will be assessed with the Neurogenic Bowel Dysfunction score, a validated 10-item questionnaire commonly used to assess bowel symptoms in individuals with SCI [76]. This questionnaire produces a severity score out of 47.
Quality of life
Quality of life will be assessed using the EuroQol-5 Dimension—5 Level questionnaire (EQ-5D-5L) [77], a standardised preference-based measure of health status. The EQ-5D-5L comprises a short questionnaire and a visual analogue scale out of 100.
Participant characteristics
The participant characteristics that will be collected are age, gender, time since injury, height, weight, American Spinal Injury Association (ASIA) Impairment Scale (AIS), and neurological, motor and sensory levels according to ISNCSCI.
Statistical methods
Sample size calculation
A sample size of 50 (25 stimulation, 25 sham) gives a > 90% probability of detecting a between-group difference of 2 points on the primary outcome: WISCI II [73]. This assumes an alpha of 0.05, a SD of 2 points [48, 78] and a dropout rate of 15%.
Statistical analysis
We will use an intention-to-treat analysis to draw accurate and unbiased conclusions regarding the effectiveness of our intervention. That is, participants will be analysed according to the group to which they were allocated, regardless of compliance with the intervention. Statistical analysis will be conducted blind to treatment allocation.
All outcomes will be analysed with multi-level (i.e., mixed) models. Ordinal measures with few scale values (e.g., Modified Ashworth Scale) will be dichotomised and assessed using multi-level logistic regression. Continuous measures and ordinal measures with many scale values (e.g., WISCI II) will be assessed using multi-level linear regression. In both cases, the participant will be a random factor with a random intercept. We will verify the appropriateness of statistical procedures (diagnostic tests) a priori and, if required, identify appropriate alternative analyses (e.g., data transformations, robust analyses) and the order in which they should be applied.
We are primarily interested in whether improvements in outcomes differ between the stimulation group and the sham group following 12 weeks of locomotor training. Baseline values will be included as a covariate. All contrasts will be performed and results reported as mean effects and 95% confidence intervals.
Adverse events and serious adverse events
All adverse events will be recorded and reported to the Principal Investigator. The Principal Investigator will be responsible for reporting any serious adverse events to the Ethics Committee as soon as possible. All adverse events and serious adverse events will be followed until they have abated, or until a stable situation has been reached. Depending on the event, additional tests or medical procedures may be required, as well as a review by a general medical practitioner or SCI physician.
Data collection, management and confidentiality
All information collected for this trial will be de-identified and kept confidential and secure. All files containing participants’ personal details will remain at the trial site where they were collected. Moreover, case report forms will only contain participant ID codes and upon trial completion will be stored at the trial site where they were collected. Electronically transcribed data will be stored on the secure REDCap system managed by Neuroscience Research Australia. Access to data will only be granted to the Principal Investigators and other research staff directly involved in the study. Individual names of the participants will not be considered in data analysis and participants will not be identified in published data. Any data stored for future analysis will be de-identified.
Trial monitoring will be undertaken by the Principal Investigator, an independent Data Monitoring and Safety Committee (DMSC), and an independent trial monitor. Best practice conduct of the trial will be ensured through frequent monitoring by the responsible Investigators and the clinical trial monitor, with the purpose of facilitating the work and fulfilling the objectives of the trial.
Ethics and dissemination
All design features important for minimising bias will be adhered to and the trial has been registered with the Australian and New Zealand Clinical Trials register (ACTRN12620001241921). This study has been approved by the ethics committee in Sydney and is currently awaiting approval in the other sites. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers will be followed during the course of this research.
Results will be presented at national and international conferences or similar. Participant’s individual results will be available on request from the Principal Investigator at their site.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed. For the main trial, full de-identified data used to generate all results will be made available with the publication.
References
Brown-Triolo DL, Roach MJ, Nelson K, Triolo RJ. Consumer perspectives on mobility: implications for neuroprosthesis design. J Rehabil Res Dev. 2002;39:659–70.
Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–83.
Simpson LA, Eng JJ, Hsieh JT. Wolfe, the Spinal Cord Injury Rehabilitation Evidence Research Team DL. The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma. 2012;29:1548–55.
Mehrholz J, Harvey LA, Thomas S, Elsner B. Is body-weight-supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord. 2017;55:722–9.
Willyard C. How a revolutionary technique got people with spinal-cord injuries back on their feet. Nature. 2019;572:20–6.
Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl J Med. 2018;379:1244–50.
Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 2011;377:1938–47.
Alam M, Ling YT, Wong AY, Zhong H, Edgerton VR, Zheng YP. Reversing 21 years of chronic paralysis via non‐invasive spinal cord neuromodulation: a case study. Ann Clin Transl Neurol. 2020;7:829–38.
Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24:1677–82.
Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans a. Ann N. Y Acad Sci. 1998;860:360–76.
Gerasimenko Y, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, et al. Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J Neurophysiol. 2015;113:834–42.
Gorodnichev R, Pivovarova E, Puhov A, Moiseev S, Savochin A, Moshonkina T, et al. Transcutaneous electrical stimulation of the spinal cord: a noninvasive tool for the activation of stepping pattern generators in humans. Hum Physiol. 2012;38:158–67.
Hofstoetter US, Freundl B, Binder H, Minassian K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: elicitation of posterior root-muscle reflexes. PloS One. 2018;13:e0192013.
Sayenko DG, Rath M, Ferguson AR, Burdick JW, Havton LA, Edgerton VR, et al. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J Neurotrauma. 2019;36:1435–50.
Hofstoetter US, McKay WB, Tansey KE, Mayr W, Kern H, Minassian K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med. 2014;37:202–11.
Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811.
Dietz V, Colombo G, Jensen L, Baumgartner L. Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol. 1995;37:574–82.
Dietz V, Müller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–34.
Beres-Jones JA, Harkema SJ. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain. 2004;127:2232–46.
Dobkin B, Harkema S, Requejo P, Edgerton V. Modulation of locomotor-like EMG activity in subjects with complete and incomplete spinal cord injury. J Neurol Rehabil. 1995;9:183–90.
Minassian K, Jilge B, Rattay F, Pinter M, Binder H, Gerstenbrand F, et al. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord. 2004;42:401–16.
Minassian K, Hofstoetter US, Danner SM, Mayr W, Bruce JA, McKay WB, et al. Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord–injured individuals. Neurorehabil Neural Repair. 2016;30:233–43.
Minassian K, Persy I, Rattay F, Pinter MM, Kern H, Dimitrijevic MR. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum Mov Sci. 2007;26:275–95.
Dy CJ, Gerasimenko YP, Edgerton VR, Dyhre-Poulsen P, Courtine G, Harkema SJ. Phase-dependent modulation of percutaneously elicited multisegmental muscle responses after spinal cord injury. J Neurophysiol. 2010;103:2808–20.
Courtine G, Gerasimenko Y, Van Den Brand R, Yew A, Musienko P, Zhong H, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–42.
Musienko PE, Zelenin PV, Orlovsky GN, Deliagina TG. Facilitation of postural limb reflexes with epidural stimulation in spinal rabbits. J Neurophysiol. 2010;103:1080–92.
Field-Fote EC. Exciting recovery: augmenting practice with stimulation to optimize outcomes after spinal cord injury. Prog Brain Res. 2015;218:103–26.
Harkema SJ. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res Rev. 2008;57:255–64.
Colombo G, Wirz M, Dietz V. Effect of locomotor training related to clinical and electrophysiological examinations in spinal cord injured humans. Ann N. Y Acad Sci. 1998;860:536–8.
O’Connor PJ. Forecasting of spinal cord injury annual case numbers in Australia. Arch Phys Med Rehabil. 2005;86:48–51.
DeVivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–72.
McCaughey EJ, Purcell M, McLean AN, Fraser MH, Bewick A, Borotkanics RJ, et al. Changing demographics of spinal cord injury over a 20-year period: a longitudinal population-based study in Scotland. Spinal Cord. 2016;54:270–6.
Yang JF, Musselman KE. Training to achieve over ground walking after spinal cord injury: a review of who, what, when, and how. J Spinal Cord Med. 2012;35:293–304.
Eisdorfer JT, Smit RD, Keefe KM, Lemay MA, Smith GM, Spence AJ. Epidural electrical stimulation: a review of plasticity mechanisms that are hypothesized to underlie enhanced recovery from spinal cord injury with stimulation. Front Mol Neurosci. 2020;13:163.
Milosevic M, Masugi Y, Sasaki A, Sayenko DG, Nakazawa K. On the reflex mechanisms of cervical transcutaneous spinal cord stimulation in human subjects. J Neurophysiol. 2019;121:1672–9.
Mayr W, Krenn M, Dimitrijevic MR. Epidural and transcutaneous spinal electrical stimulation for restoration of movement after incomplete and complete spinal cord injury. Curr Opin Neurol. 2016;29:721–6.
Ievins A, Moritz CT. Therapeutic stimulation for restoration of function after spinal cord injury. Physiology. 2017;32:391–8.
Calvert JS, Grahn PJ, Zhao KD, Lee KH. Emergence of epidural electrical stimulation to facilitate sensorimotor network functionality after spinal cord injury. Neuromodulation. 2019;22:244–52.
Gad PN, Kreydin E, Zhong H, Latack K, Edgerton VR. Non-invasive neuromodulation of spinal cord restores lower urinary tract function after paralysis. Front Neurosci. 2018;12:432.
Martin R. Impact of transcutaneous spinal cord stimulation on walking function in a patient with incomplete spinal cord injury. Arch Phys Med Rehabil. 2018;99:e49–e50.
Al’joboori Y, Massey SJ, Knight SL. Donaldson NdN, Duffell LD. The effects of adding transcutaneous spinal cord stimulation (tSCS) to sit-to-stand training in people with spinal cord injury: A pilot study. J Clin Med. 2020;9:2765.
Darrow D, Balser D, Netoff TI, Krassioukov A, Phillips A, Parr A, et al. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J Neurotrauma. 2019;36:2325–36.
Walter M, Lee AH, Kavanagh A, Phillips AA, Krassioukov AV. Epidural spinal cord stimulation acutely modulates lower urinary tract and bowel function following spinal cord injury: a case report. Front Physiol. 2018;9:1816.
Bourbeau D, Creasey G, French J, Grill WM, Howley S, Krassioukov A, et al. A roadmap for advancing neurostimulation approaches for bladder and bowel function after spinal cord injury. Spinal Cord. 2020;58:1227–32.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81.
Harkema SJ, Hillyer J, Schmidt-Read M, Ardolino E, Sisto SA, Behrman AL. Locomotor training: as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch Phys Med Rehabil. 2012;93:1588–97.
Sandler EB, Roach KE, Field-Fote EC. Dose-response outcomes associated with different forms of locomotor training in persons with chronic motor-incomplete spinal cord injury. J Neurotrauma. 2017;34:1903–8.
Yang JF, Musselman KE, Livingstone D, Brunton K, Hendricks G, Hill D, et al. Repetitive mass practice or focused precise practice for retraining walking after incomplete spinal cord injury? A pilot randomized clinical trial. Neurorehabil Neural Repair. 2014;28:314–24.
Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev. 2012;11:CD006676.
Harkema SJ, Behrman AL, Barbeau H. Locomotor training: principles and practice: Oxford University Press, USA; 2011.
Field-Fote EC, Lindley SD, Sherman AL. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J Neurol Phys Ther. 2005;29:127–37.
Rath M, Vette AH, Ramasubramaniam S, Li K, Burdick J, Edgerton VR, et al. Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury. J Neurotrauma. 2018;35:2540–53.
Hofstoetter US, Krenn M, Danner SM, Hofer C, Kern H, McKay WB, et al. Augmentation of voluntary locomotor activity by transcutaneous spinal cord stimulation in motor-incomplete spinal cord-injured individuals. Artif Organs. 2015;39:E176–86.
Megia Garcia A, Serrano-Muñoz D, Taylor J, Avendaño-Coy J, Gómez-Soriano J. Transcutaneous spinal cord stimulation and motor rehabilitation in spinal cord injury: a systematic review. Neurorehabil Neural Repair. 2020;34:3–12.
Elphick T, Bye E, Héroux M, Boswell-Ruys C, Butler J, McCaughey E, et al. Spinal stimulation and standing study (SSASSY). Australian and New Zealand Spinal Cord Society Annual Scientific Meeting; Virtual 2021.
Parhizi B, Barss TS, Mushahwar VK. Simultaneous cervical and lumbar spinal cord stimulation induces facilitation of both spinal and corticospinal circuitry in humans. Front Neurosci. 2021;15:379.
Inanici F, Samejima S, Gad P, Edgerton VR, Hofstetter CP, Moritz CT. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans Neural Syst Rehabil Eng. 2018;26:1272–8.
Gerasimenko YP, Lu DC, Modaber M, Zdunowski S, Gad P, Sayenko DG, et al. Noninvasive reactivation of motor descending control after paralysis. J Neurotrauma. 2015;32:1968–80.
Ward AR. Electrical stimulation using kilohertz-frequency alternating current. Phys Ther. 2009;89:181–90.
Ward AR, Lucas-Toumbourou S. Lowering of sensory, motor, and pain-tolerance thresholds with burst duration using kilohertz-frequency alternating current electric stimulation. Arch Phys Med Rehabil. 2007;88:1036–41.
Manson GA, Calvert JS, Ling J, Tychhon B, Ali A, Sayenko DG. The relationship between maximum tolerance and motor activation during transcutaneous spinal stimulation is unaffected by the carrier frequency or vibration. Physiol Rep. 2020;8:e14397.
Benavides FD, Jo HJ, Lundell H, Edgerton VR, Gerasimenko Y, Perez MA. Cortical and subcortical effects of transcutaneous spinal cord stimulation in humans with tetraplegia. J Neurosci Res. 2020;40:2633–43.
Freyvert Y, Yong NA, Morikawa E, Zdunowski S, Sarino ME, Gerasimenko Y, et al. Engaging cervical spinal circuitry with non-invasive spinal stimulation and buspirone to restore hand function in chronic motor complete patients. Sci Rep. 2018;8:1–10.
Serrano-Muñoz D, Gómez-Soriano J, Bravo-Esteban E, Vázquez-Fariñas M, Taylor J, Avendaño-Coy J. Intensity matters: therapist-dependent dose of spinal transcutaneous electrical nerve stimulation. PLoS One. 2017;12:e0189734.
Gerasimenko Y, Gorodnichev R, Moshonkina T, Sayenko D, Gad P, Edgerton VR. Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehabil Med. 2015;58:225–31.
Jilge B, Minassian K, Rattay F, Pinter MM, Gerstenbrand F, Binder H, et al. Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp Brain Res. 2004;154:308–26.
Awosika OO, Sandrini M, Volochayev R, Thompson RM, Fishman N, Wu T, et al. Transcutaneous spinal direct current stimulation improves locomotor learning in healthy humans. Brain Stimul. 2019;12:628–34.
Murray LM, Tahayori B, Knikou M. Transspinal direct current stimulation produces persistent plasticity in human motor pathways. Sci Rep. 2018;8:1–11.
Serrano-Muñoz D, Gómez-Soriano J, Bravo-Esteban E, Ávila-Martín G, Galán-Arriero I, Taylor J, et al. Soleus H-reflex modulation following transcutaneous high-and low-frequency spinal stimulation in healthy volunteers. J Electromyogr Kinesiol. 2019;46:1–7.
Deyo RA, Walsh NE, Martin DC, Schoenfeld LS, Ramamurthy S. A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. N. Engl J Med. 1990;322:1627–34.
Petrie J, Hazleman B. Credibility of placebo transcutaneous nerve stimulation and acupuncture. Clin Exp Rheumatol. 1985;3:151–3.
Dittuno P, Dittuno J Jr. Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39:654–6.
Musselman KE. Clinical significance testing in rehabilitation research: what, why, and how? Phys Ther Rev. 2007;12:287–96.
Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference for the 2011 revision of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2011;34:547–54.
Baunsgaard C, Nissen U, Christensen K, Biering-Sørensen F. Modified Ashworth scale and spasm frequency score in spinal cord injury: reliability and correlation. Spinal Cord. 2016;54:702–8.
Krogh K, Christensen P, Sabroe S, Laurberg S. Neurogenic bowel dysfunction score. Spinal cord. 2006;44:625–31.
Oppe M, Devlin NJ, van Hout B, Krabbe PF, de Charro F. A program of methodological research to arrive at the new international EQ-5D-5L valuation protocol. Value Health. 2014;17:445–53.
Van Hedel H, Wirz M, Curt A. Improving walking assessment in subjects with an incomplete spinal cord injury: responsiveness. Spinal Cord. 2006;44:352–6.
Black J, Baharestani MM, Cuddigan J, Dorner B, Edsberg L, Langemo D, et al. National Pressure Ulcer Advisory Panel’s updated pressure ulcer staging system. Adv Ski Wound Care. 2007;20:269–74.
Acknowledgements
The authors acknowledge Professor Rob Herbert/Peter Humburg for their statistical advice; SpinalCure Australia, Spinal Cord Injuries Australia and Paraquad for their assistance with an advertisement for this trial; and the support of local Spinal Cord Injury Units at each site.
Funding
Funding for this study has been received from SpinalCure Australia and Catwalk NZ.
Author information
Authors and Affiliations
Contributions
Conceptualisation: EAB, MEH, CLB, BBL, EJM, JEB, and SCG; Methodology: EAB, MEH, CLB, BBL, EJM, JEB, and SCG; Writing—original draft: EAB, EJM, and MEH; Writing—review & editing: All authors; Project administration: EAB, MEH, CLB, BBL, EJM, JEB, and SCG; Funding acquisition: SG and JB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bye, E.A., Héroux, M.E., Boswell-Ruys, C.L. et al. Transcutaneous spinal cord stimulation combined with locomotor training to improve walking ability in people with chronic spinal cord injury: study protocol for an international multi-centred double-blinded randomised sham-controlled trial (eWALK). Spinal Cord 60, 491–497 (2022). https://doi.org/10.1038/s41393-021-00734-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00734-1