Abstract
Study design
Cross-sectional study.
Objectives
To compare cardiac autonomic modulation of individuals with spinal cord injury (SCI) that practice different amounts of moderate to vigorous physical activity (PA) and able-bodied controls at rest and during a non-immersive Virtual Reality task.
Setting
Athletes with SCI of wheelchair basketball, wheelchair tennis, wheelchair handball, WCMX (wheelchair motocross), and para-swimming were assessed at the Faca na Cadeira Institute, ICEL and Clube Espéria in São Paulo, Brazil; non-athletes with SCI and able-bodied controls were assessed at the Acreditando Centro de Recuperação Neuromotora, São Paulo, Brazil.
Methods
One-hundred forty-five individuals were assessed: 36 athletes with traumatic SCI (41.1 ± 16.8 years old), 52 non-athletes with traumatic SCI (40.2 ± 14.1 years old), and 57 able-bodied individuals (39.4 ± 12.5 years old). Cardiac autonomic modulation was assessed through heart rate variability (HRV) measured in the sitting position at rest and during a VR game activity.
Results
We found significantly more favourable HRV for athletes with SCI when compared to non-athletes with SCI, but no differences between athletes with SCI and able-bodied controls. In addition, athletes and able-bodied controls showed adequate autonomic nervous system (ANS) adaptation (rest versus physical activity in VR), i.e., they experienced parasympathetic withdrawal during VR physical activity, which was not found in non-athletes with SCI.
Conclusion
The practice of moderate to vigorous physical activity is associated with healthier cardiac autonomic modulation in adults with SCI, which may lead to more favourable health outcomes.
Trial registration
ClinicalTrials.gov Identifier: NCT04618003, retrospectively registered.
Similar content being viewed by others
Introduction
One of the most pernicious consequences of a spinal cord injury (SCI) is impairment in cardiovascular function, which increases the risk for cardiovascular disease [1]. Cardiac impairments are among the major causes of death in people with SCI [2] and may be related to disruption of the autonomic nervous system (ANS) below the neurological level of the injury [3, 4]. This may also be related to the impact of reduced mobility on autonomic cardiac control as well as the sedentary lifestyle of individuals that become wheelchair users after SCI [5].
It is known that regular physical activity (PA) contributes positively to cardiovascular function, controlling the sympathovagal imbalance that occurs from the acute phase (i.e. days following the injury) and a chronic effect in cardiovascular function of the SCI combined with a sedentary lifestyle [4, 5] and improving cardiac vagal tone. Improvement in cardiac vagal tone may increase the chances of survival by decreasing the work and oxygen consumption of the heart, and even reducing the risk of lethal ventricular arrhythmias [1]. Despite these well-known benefits of PA, it is important to understand the influence of the amount of regular PA on HRV responses in individuals with SCI [6].
According to Vanderlei et al. [7], the ANS is widely and easily accessed through heart rate variability (HRV), which has been used to evaluate sympathetic and parasympathetic heart rate modulation. This is assessed by time in milliseconds between heart beats and is often an indicator of abnormal and insufficient ANS adaptation. HRV can be assessed at rest and during physical activity to measure the ANS autonomic adaptation to exercise, which is an important health marker.
Considering the above, the current study aimed to compare cardiac autonomic modulation of individuals with SCI that practice different amounts of moderate to vigorous PA and able-bodied controls at rest and during a non-immersive virtual reality (VR) task. We chose a VR task to promote PA, since VR has emerged as a new approach that can be implemented not only as a strategy to increase PA, but also as a tool to assess the individual’s performance in a safe and enjoyable way, with the possibility of being used as an exercise therapy [8, 9].
Thus, we analysed athletes with SCI representing the group that practice a high volume (>300 min/week) of moderate to vigorous PA and compare with non-athletes with SCI representing the group with ordinary volume of PA (between 150 and 300 min/week) [10, 11]. Moreover, we analysed a control group of able-bodied non-athletes in order to have a pattern of HRV behaviour at rest and during the VR task (i.e. this group was organised to establish a normal pattern of HRV).
We hypothesised that athletes with SCI, similarly to the able-bodied control group, would show more favourable HRV at rest and better adaptation of the ANS (i.e., higher sympathetic dominance/vagal withdrawal) during physical activity in a VR task when compared to non-athlete individuals with SCI. In addition, we hypothesised that the VR task could be used for assessment of ANS adaptation to PA in individuals with SCI.
Methods
This was a cross-sectional study, approved by the Ethical and Research Committee of the Medical School of the Federal University of São Paulo (UNIFESP), registered under CAAE 793517.1.0000.5505. The recruitment of non-athletes was carried out at the Acreditando Rehabilitation Center and the athletes were recruited at the Faca na Cadeira Institute, ICEL, and Clube Espéria. Participants were enroled in the study after signing the Free and Informed Consent Form. Convenience sampling was used, with data collected from March 2017 to October 2018
Participants
The study included athletes (AG) and non-athletes (NA) with a confirmed medical diagnosis of SCI, with voluntary extension of elbows and wrists, active trunk control, and who had already completed rehabilitation. For the athlete group, participants were required to fulfil the minimum criteria for defining an active athlete, described in the next section. In addition, a control group of able-bodied subjects, approximately paired by age and sex with the AG and non-athlete groups were recruited. Participants were excluded if they presented impairments in the upper limbs that interfered with performance during the VR game, were diagnosed with heart disease or other neurological disorders, were using medication that influenced the HRV analysis, and presented an error greater than 5% in the analysis of the trace made using Kubios software.
Assessment of function
The Functional Independence Measure (FIM) [12] and the Spinal Cord Independence Measure Version III (SCIM III) [13] were used to classify functional independence. The International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) [14] were employed to assess the level and severity of injury. To classify the physical activity levels of the participants, the Physical Activity in Athlete Questionnaire [15] and the Leisure Time Physical Activity Questionnaire for People with Spinal Cord Injury [16] were employed.
Assessment of heart rate variability
For the HRV assessment, participants were instructed not to consume alcohol or caffeine and not to smoke for 24 h before the test. The procedures to measure HRV were conducted according to the guidelines from the Task Force of the European Society of Cardiology and the North American Society of Stimulation and Electrophysiology [17] and were recorded using the Polar V800 electrocardiographic measuring device (Polar Electro, Finland), with a sampling rate of 1000 Hz [18, 19].
Determination of the linear indices of HRV for time and frequency was carried out using Kubios HRV software (v. 3.3.0) [20,21,22]. The indices used were: SDNN (standard deviation of the mean of all normal RR intervals) in milliseconds (ms); PNN50 (percentage of successive differences of the RR intervals with an absolute value greater than 50 ms); RMSSD (square root of the mean square of the differences between adjacent normal RR intervals); LF indices (low frequency spectral components, between 0.04 and 0.15 Hertz), recorded in ms2 and in normalised units (nu); HF (high frequency spectral components, between 0.15 and 0.4 Hertz), recorded in ms2 and nu; the ratio of LF/HF in ms2; the indices of the Poincaré graph with the SD1 (standard deviation of the instantaneous variability of the beat-to beat heart rate), SD2 (standard deviation of the long-term, continuous, RR interval variability), and the SD1/SD2 ratio-the ratio between the short- and long-term variations among the RR intervals [18, 20]; and Parassympathetic Nervous System index (PNS index), Sympathetic Nervous System index (SNS index), and Stress index given by HRV Kubios.
Protocol
For the three groups the HRV measures were captured in two instances: 1. For 5 min at rest in the seated position; and 2. During the VR activity, in which the individual executed movements according to a game in front of a computer for 8 min. The VR activity was performed using the game MoveHero, developed at the School of Arts, Sciences and Humanities of the University of São Paulo (EACH-USP). The game consists of virtual balls that fall at pre-determined time intervals and spatial distributions in four imaginary columns on a computer screen. The participant is required to move in the direction of the balls to virtually touch them as they reach four pre-determined targets placed to the sides of the player’s avatar (Fig. 1).
The game captures the participant’s movements with a webcam and does not require physical contact to perform the task. The participant is required to move their arms at their maximum lateral range. Before starting the task, the researcher positioned the participant at a distance from the screen that allowed him/her to achieve the maximum lateral reach range to each side (left and right) when reaching towards the two farthest balls (1 and 4). From that position, the distance between the computer table and the participant’s feet was measured and the participant was asked to move his/her wheelchair to 80% of this distance. During the task, the participant must wait for the balls to drop until they reach the pre-determined targets. When each ball reaches the target, the colour changes to green, which prompts the participant to reach for the ball. Therefore, the game requires the participant to have a strategy of planning their movement to reach the targets at the same time as the balls.
Data analysis
Descriptive statistics were performed. Categorical data are reported as absolute and relative frequencies, while continuous data are reported as mean and standard error on the graphs and as mean, standard deviation, and confidence interval (CI) in Table S1 (Supplementary Material). The normality of the data was assessed by a histogram with analysis of the normality curve. Linear mixed models (LMM) were used to determine the mean changes in HRV indices in the three groups (athletes with SCI, non-athletes with SCI, and able-bodied) for two repeated measurements (Rest and during VR activity). The Least Significance Difference (LSD) was used as a post-hoc test in order to find the differences pointed out in the LMM effects and interactions. We employed Linear Regression to test associations between HRV indices (RMSSD and SDNN) and age, sex, Neurological Level (transformed into continuous data, classified as follows: C1 = 1; C2 = 2; C3 = 3; C4 = 4; C5 = 5; C6 = 6; C7 = 7; C8 = 8; T1 = 9; T2 = 10; T3 = 11; T4 = 12; T5 = 13; T6 = 14; T7 = 15; T8 = 16; T9 = 17; T10 = 18; T11 = 19; T12 = 20; L1 = 21; L2 = 22; L3 = 23, L4 = 24 and L5 = 25), severity of injury (A to D, as classified by International Standards for Neurological Classification of SCI (ISNCSCI)), SCIM-III, and FIM. Values of p < 0.05 were considered significant. All analyses were conducted with the Statistical Package for Social Sciences (SPSS; IBM, Chicago, Illinois, USA), version 26.0.
Results
A total of 194 potential participants were invited to take part in this study and 145 patients were included in the data analysis. The reasons for exclusions are shown in Fig. 2.
Participants were of both sexes, with 36 athletes with SCI (age 41 ± 17 years, mean ± SD), including 20 from wheelchair basketball, 6 from wheelchair tennis, 5 from wheelchair handball, 3 from WCMX (wheelchair motocross), and 2 from para-swimming. The athletes were defined as persons who performed moderate to vigorous physical activity for more than 10 h/week. In addition, 52 individuals, non-athletes with SCI (age 40 ± 14 years) and 57 able-bodied non-athletes (age 39 ± 13 years) participated in this study.
Table 1 presents the characteristics of the included participants. When comparing the three groups, there were no significant differences in age and sex (Table 1). The athletes and non-athletes were similar in neurological level and severity of injury according to the ISNCSCI for A and B classifications. The non-athletes presented a significantly higher number of subjects with Grades C and D. When comparing the FIM and SCIM-III, the athletes presented significantly greater independence than the non-athletes.
Heart rate variability
Time domain (TD)
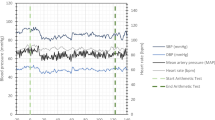
There were significant effects for Groups for mean RR (p < 0.001), SDNN (p < 0.001), RMSSD (p < 0.001), pNN50 (p < 0.001), and mean HR (p < 0.001). non-athletes with SCI presented lower mean RR, SDNN, RMSSD, and pNN50 and higher mean HR when compared to athletes with SCI and able-bodied controls. No differences between athletes with SCI and able-bodied controls were found (Fig. 3 and Table S1 in the Supplementary Material).
SDNN, standard deviation of the mean of all normal RR intervals in milliseconds (ms); PNN50, percentage of successive differences of the RR intervals with an absolute value greater than 50 ms; RMSSD, square root of the mean square of the differences between adjacent normal RR intervals; LF, low frequency spectral components, between 0.04 and 0.15 Hertz; HF, high frequency spectral components, between 0.15 and 0.4 Hertz; SD1, standard deviation of the instantaneous variability of the beat-to beat heart rate; SD2, standard deviation of the long-term, continuous, RR interval variability; SD1/SD2, ratio-the ratio between the short- and long-term variations among the RR intervals; SNS, Sympathetic Nervous System index; PNS, Parasympathetic Nervous System index.
All groups presented decreased mean RR (p < 0.001), SDNN (p = 0.002), RMSSD (p = 0.010), and pNN50 (p = 0.007) and an increased mean HR (p < 0.001) during VR compared to rest. However, although the Fixed Effects showed no significant interactions for all time domain indices, the post-hoc analysis showed that only athletes with SCI and able-bodied controls presented significant differences between rest and during VR. No significant differences were observed in non-athletes between rest and during VR. In addition, when comparing groups in each condition, the post-hoc analysis showed no significant differences between athletes and non-athletes with SCI, nor between athletes and able-bodied controls during VR. The only difference found was between non-athletes and able-bodied controls (Fig. 3 and Table S1 in the Supplementary Material).
Frequency Domain (FD)
There were significant effects for Groups for LF ms2 (p < 0.001), HF ms2 (p < 0.001), and the LH/HF ratio (p = 0.048), in which non-athletes with SCI presented lower LF ms2 and HF ms2 when compared to athletes with SCI and able-bodied controls. In addition, athletes showed a lower LF/HF ratio than able-bodied controls (Fig. 3 and Table S1 in the Supplementary Material).
A decrease in LF ms2 (p = 0.001) and HF ms2 (p = 0.002) was observed between Rest and during VR in all groups (no effect for LF/HF ratio). However, although the Fixed Effects showed no significant interactions, the post-hoc analysis demonstrated that only athletes with SCI and able-bodied controls presented significant effects for LF ms2 and HF ms2 between the two conditions. There were no significant differences between rest and during VR for non-athletes. When comparing Groups in each condition, the post-hoc analysis showed no significant difference between athletes and non-athletes with SCI, nor between athletes and able-bodied controls during VR. The only difference found was between non-athletes and able-bodied controls. The LF ms2 at rest was also significantly different between athletes with SCI and able-bodied controls (Fig. 3 and Table S1 in the Supplementary Material).
Poincaré graph, sympathetic, parasympathetic and stress indices
There were significant effects for Groups for SD1 (p < 0.001), SD2 (p < 0.001), the PNS index (p < 0.001), SNS index (p < 0.001) and Stress index (p < 0.001), with no effect for SD1/SD2. Non-athletes with SCI presented lower SD1 and PNS indices and a higher SNS index when compared to athletes with SCI and able-bodied controls. Furthermore, non-athletes with SCI presented a higher Stress index and lower SD2 when compared to athletes and able-bodied controls. athletes with SCI also presented a higher Stress index and lower SD2 than able-bodied controls (Fig. 3 and Table S1 in the Supplementary Material).
The main effect for rest and during VR showed that all groups presented a decrease in SD1 (p = 0.009), SD2 (p = 0.001), and the PNS index (p < 0.001), and an increase in the SNS index (p < 0.001) and Stress index (p < 0.001) between Rest and during VR. However, although the Fixed Effects showed no significant interactions, the post-hoc analysis demonstrated significant effects in athletes with SCI and able-bodied controls. There were no significant differences between Rest and during VR in non-athletes.
The post-hoc analysis comparing the two conditions showed no significant difference between athletes and non-athletes with SCI, nor between athletes and able-bodied controls for SD2 during VR. There was a difference between non-athletes and able-bodied controls for SD1 and the PNS index (Fig. 3 and Table S1 of the Supplementary Material).
Regression analysis
To understand which factors may influence HRV response, two regression analyses were performed between RMSSD and pNN50 (as dependent variables) and sex, age, neurological level (continuous data), ISNCSCI, SCIM-II, and FIM. The analysis revealed significant regression models for RMSSD: FIM, F(1, 49) = 7.4, p = 0.009, r2 = 0.134, resulting in the following equation: = 0.239 × RMSSD. The analysis for pNN50 revealed: FIM F(1, 49) = 4.9, p = 0.031, r2 = 0.094, resulting in the following equation: = 0.150 × pNN50. In other words, higher scores on the FIM increases the RMSSD index by 0.24 times and the pNN50 index by 0.15 times. No influence was found for the other variables.
Discussion
In the current study, we compared HRV in athletes with SCI, non-athletes with SCI and an able-bodied control group at rest and during a VR task. As hypothesised, athletes with SCI presented more favourable cardiac autonomic modulation than non-athletes with SCI, with HRV indices similar to able-bodied controls in both conditions. In addition, the VR game promoted an increase in heart rate, with ANS adaptation, i.e., higher sympathetic dominance/vagal withdrawal, which is physiologically expected, only for athletes with SCI and able-bodied controls, but not for non-athletes with SCI.
-
Comparisons between groups and HRV in rest position.
Initially, our results showed that despite including different levels and severities of injury, the individuals were similar across groups (athletes and non-athletes) for all demographic variables, as presented in Table 1. There was no statistical difference between the groups for age and sex or neurological level, although the non-athlete group included a higher number of individuals with less severe injuries according to the ISNCSC (less body segments affected by the injury and more functional capacity). However, the functional assessments (FIM and SCIM-III) showed that the athlete group presented higher levels of functional independence when compared to the non-athlete group. This is an interesting result, showing that, despite having a significant motor impairment, athletes with SCI were able to adapt to their impairments and present greater functionality in their daily lives. These results corroborate studies [22,23,24] that showed that subjects with SCI who regularly perform moderate to vigorous physical activity have greater functional independence in the domains of motor function, self-care, and transfers, with higher FIM and SCIM III scores.
Considering HRV, the cardiac autonomic modulation of athletes with SCI and able-bodied individuals was more favourable in both conditions (rest and physical activity) when compared to non-athletes with SCI. During rest, our results showed several HRV variables representing higher parasympathetic activity in athletes with SCI were significantly different from non-athletes with SCI. This result may be explained by studies that showed a decrease in the efferent sympathetic activity on the sinoatrial node and an increase in the parasympathetic control of the heart at rest as a result of the chronic effect of training [25,26,27,28,29].
Moreover, our regression analysis pointed out that higher scores on functional scales (FIM) influence the parasympathetic response analysed during rest (i.e., individuals with SCI that presented a higher functional score also presented higher parasympathetic activity at rest). This indicates more favourable health, corroborating results demonstrating that a programme of regular exercise of moderate to vigorous PA, focusing on better functionality for individuals with SCI, promotes healthier physiological responses that favour vagal dominance at rest.
Another important finding that we would like to emphasise is that HRV indices in athletes with SCI were not significantly different from those of able-bodied individuals. At rest, there were no differences, while non-athletes with SCI presented lower HRV. This result shows that the regular practice of a high volume of moderate to vigorous PA in individuals with SCI promotes ANS modulation similar to able-bodied controls, which represents better homoeostasis in these groups [30].
-
Physical activity and HRV in VR task.
The use of VR games is growing as a PA strategy that allows a progressive increase in the levels of aerobic activity, according to the individual’s cardiovascular and functional capacity. To date, the intensity and duration of exercises in individuals with SCI have been very difficult to control in conventional environments, but in VR environments, these factors can be easily customised by changing the phases or type of games.
Thus, our results show that besides the more favourable HRV at rest in athletes with SCI and able-bodied controls, these groups also presented autonomic adaptation to acute exercise, i.e., increases in Mean HR, the SNS index and Stress index, and decreases in global indices such as SDNN, LF ms2, the LF/HF ratio, SD2, and the SD2/SD1 ratio, as well as in parasympathetic indices such as RMSSD, pNN50, HF ms2, and the PNS index. These findings corroborate studies [8, 31] that stated that during exercise, there is an expected decrease in the parasympathetic tone and an increase in the sympathetic response, which is maintained by mechanisms related to baroreceptors, and this response was not present in non-athletes with SCI. This result not only verifies the adaptation of athletes to physical activity but also demonstrates the possibility of using VR tasks as a potential tool to promote enough movement to change heart rate and autonomic indices in able-bodied controls and athletes with SCI. However, we can speculate that for non-athlete individuals with SCI, the VR activity should be intensified to promote more movement and be more challenging in order to produce autonomic changes, as no adaptations to PA in VR were observed for this group.
The limitation of this study was that we did not evaluate individuals with acute or subacute SCI, which could bring important information about the effects of physical activity in this period. Hence, we believe that future studies should analyse the long-term effect of high intensity activities (in both VR and conventional environments) and adapted sports in sedentary individuals with SCI, and also in individuals with acute SCI. We believe that this could provide better evidence on the use of VR to promote improved health in people with SCI.
Clinical implications
The most important result of this study is that moderate to vigorous physical activity should be recommended for adults with SCI, since these activities promote healthier cardiac autonomic modulation, which may lead to a better health outcome. Furthermore, VR may be used for assessment of ANS adaptation to physical activity in athletes with SCI and able-bodied individuals but should be used with caution for this aim in non-athletes with SCI, as they did not show ANS adaptation considering their low HRV even at rest.
Data availability
Supporting datasets and software code can be obtained from Talita Dias da Silva: write at ft.talitadias@gmail.com.
References
Cragg JJ, Noonan VK, Krassioukov A, Borisoff J. Cardiovascular disease and spinal cord injury: results from a national population health survey. Neurology. 2013;81:723–8.
Buch AN, Coote JH, Townend JN. Mortality, cardiac vagal control and physical training—what’s the link? Exp Physiol. 2002 Jul;87:423–35.
Sahota IS, Ravensbergen HRJC, McGrath MS, Claydon VE. Cerebrovascular responses to orthostatic stress after spinal cord injury. J Neurotrauma. 2012;29:2446–56.
Serra-Añó P, Magraner LM, Morales J, Gomis M, Garcia-Massó X, González LM, et al. Heart rate variability in individuals with thoracic spinal cord injury. Spinal Cord. 2015;53:59–63.
Myers J. Exercise and cardiovascular health. Circulation. 2003;107:e2–e5.
Buker DB, Oyarce CC, Plaza RS. Effects of spinal cord injury in heart rate variability after acute and chronic exercise: a systematic review. Top Spinal Cord Inj Rehabil. 2018;24:167–76.
Vanderlei LCM, Pastre CM, Hoshi RA, de Carvalho TD, de Godoy MF. Noções básicas de variabilidade da frequência cardíaca e sua aplicabilidade clínica. Braz J Cardiovasc Surg. 2009;24:205–17.
Dimbwadyo-Terrer I, Trincado-Alonso F, de Los Reyes-Guzmán A, Aznar MA, Alcubilla C, Pérez-Nombela S, et al. Upper limb rehabilitation after spinal cord injury: a treatment based on a data glove and an immersive virtual reality environment. Disabil Rehabil: Assistive Technol. 2016;11:462–7.
Silva TDD, Oliveira PMD, Dionizio JB, Santana APD, Bahadori S, Dias ED, et al. Comparison between conventional intervention and non-immersive virtual reality in the rehabilitation of individuals in an inpatient unit for the treatment of COVID-19: a study protocol for a randomized controlled crossover trial. Front Psychol. 2021;12:178.
IPAQ Research Committee et al. Guidelines for the data processing and analysis of the International Physical Activity Questionnaire. 2005. 2016.
Matsudo, S, Araújo, T, Marsudo, V, Andrade, D, Andrade, E and Braggion, G. Questinário internacional de atividade fisica (IPAQ): estudo de validade e reprodutibilidade no Brasil. Rev. Bras. Ativ. fís. Saúde, 2001. pp. 5–18.
Da Silva GA, Schoeller SD, Gelbcke FL, De Carvalho ZMF. Avaliação funcional de pessoas com lesão medular: utilização da escala de independência funcional-MIF. Texto Contexto Enferm. 2012;21:929–36.
Catz A, Itzkovich M, Steinberg F, Philo O, Ring H, Ronen J, et al. The Catz-Itzkovich SCIM: a revised version of the spinal cord independence measure. Disabil Rehabil. 2001;23:263–8.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34:535–46.
Araújo CG, Scharhag J. Athlete: a working definition for medical and health sciences research. Scand J Med Sci Sports. 2016;26:4–7. Jan
Ginis KAM, Phang SH, Latimer AE, Arbour-Nicitopoulos KP. Reliability and validity tests of the leisure time physical activity questionnaire for people with spinal cord injury. Arch Phys Med Rehabil. 2012;93:677–82.
TFESC (Task Force of the European Society of Cardiology) and the NASPE (North American Society of Pacing and Electrophysiology). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65.
Hernando D, Garatachea N, Almeida R, Casajús JA, Bailón R. Validation of heart rate monitor Polar RS800 for heart rate variability analysis during exercise. J Strength Conditioning Res. 2018;32:716–25.
Pumprla J, Howorka K, Groves D, Chester M, Nolan J. Functional assessment of heart rate variability: physiological basis and practical applications. Int J Cardiol. 2002;84:1–14.
Achten J, Jeukendrup AE. Heart rate monitoring. Sports Med. 2003;33:517–38.
Vanderlei LCM, Pastre CM, Hoshi RA, de Carvalho TD, de Godoy MF. Noções básicas de variabilidade da frequência cardíaca e sua aplicabilidade clínica. Braz J Cardiovasc Surg. 2009;24:205–17.
Magnani PE, Cliquet Junior A, Abreu DCCD. Postural control assessment in physically active and sedentary individuals with paraplegia. Acta Ortopedica Bbras. 2017;25:147–50.
Da Silva MCR, de Oliveira RJ, Conceição MIG. Effects of swimming on the functional independence of patients with spinal cord injury. Rev Bras Med Esport. 2005;11:237–41.
Franz M, Richner L, Wirz M, von Reumont A, Bergner U, Herzog T, et al. Physical therapy is targeted and adjusted over time for the rehabilitation of locomotor function in acute spinal cord injury interventions in physical and sports therapy. Spinal Cord. 2018;56:158.
Santos RA, Pires FO, Bertuzzi R, Lima-Silva AE, de Oliveira O. Autonomic modulation during incremental exercise with upper limbs in individuals with spinal cord injury. Rev Brasileira De Med Do Esport. 2011;17:409–12.
Dixon EM, Kamath MV, McCartney N, Fallen EL. Neural regulation of heart rate variability in endurance athletes and sedentary controls. Cardiovasc Res. 1992;26:713–9.
Gregoire J, Tuck S, Hughson RL, Yamamoto Y. Heart rate variability at rest and exercise: influence of age, gender, and physical training. Can J Appl Physiol. 1996;21:455–70.
Millar PJ, Rakobowchuk M, Adams MM, Hicks AL, McCartney N, MacDonald MJ. Effects of short-term training on heart rate dynamics in individuals with spinal cord injury. Autonomic Neurosci. 2009;150:116–21.
DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–9. Nov
Fouradoulas M, von Känel R, Schmid JP. Heart rate variability—state of research and clinical applicability. Prax. 2019;108:461–8. https://doi.org/10.1024/1661-8157/a003206. German
Houssiere A, Najem B, Ciarka A, Velez-Roa S, Naeije R, van de Borne P. Chemoreflex and metaboreflex control during static hypoxic exercise. Am J Physiol-Heart Circ Physiol. 2005;288:H1724–H1729.
Acknowledgements
We would like to thank Amanda Orasmo Simcsik, Joyce Alves de Lima and Thais Nogueira for the kindly assistance during data collection. ED was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 88882.45912/2019-01. LM was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001. TS was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP)–Finance Code: 2016/08358-0.
Author information
Authors and Affiliations
Contributions
ED collected patient data and drafted the article. LM collected patient data, drafted the article and revised the manuscript critically for intellectual content. TS designed and coordinated the study, performed the statistical analyses, interpreted the data and revised the manuscript critically for intellectual content. NS, PV and BB collected patient data and drafted the article. NP, RG, SS and JA provided assistance on patient data collection and revised the manuscript. CM, AD, CO, JD, BR VB revised the manuscript critically for intellectual content. VB coordinated the study, drafted the article, and revised the manuscript critically for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Written informed consent to participate in this study was provided by the participants.
Consent for publication
The participants provided written informed consent for the publication of any associated data, since it does not show their personal data.
Ethics approval
This study was reviewed and approved by the Ethical and Research Committee of the Medical School of the Federal University of São Paulo (UNIFESP), registered under CAAE 793517.1.0000.5505.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Dias, E.D., de Menezes, L.D.C., da Silva, T.D. et al. Comparison of cardiac autonomic modulation of athletes and non-athletes individuals with spinal cord injury at rest and during a non-immersive virtual reality task. Spinal Cord 59, 1294–1300 (2021). https://doi.org/10.1038/s41393-021-00722-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00722-5