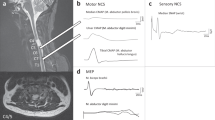
Abstract
Study design
Descriptive secondary analysis of two spinal cord injury (SCI) animal models.
Objectives
To compare the somatosensory evoked potential (SSEP) and motor behavioral (BBB) assessments of the two most used rodent SCI models (contusion and transection), to elucidate their functional similarity and differences over the acute phase of 3 weeks.
Setting
Neuro-electrophysiology SSEP and motor behavioral BBB assessments are used to provide a comparative analysis of the functional changes among various severities of contusion and transection SCI.
Methods
Adult male and female rats randomly grouped (n = 5) as following: mild (6.25 mm), moderate (12.5 mm), severe (25 mm), and very severe (50 mm) contusion as well as right T10 hemi-transection (RxI), left T8 and right T10 double hemi-transection (DxI), and T8 complete transection (CxI) injuries, plus the control group (laminectomy with no injury). Animal weight, body temperature, anesthesia, surgical procedures, electrophysiological SSEP monitoring, locomotion BBB scoring, and statistical analysis were identical among all animal groups.
Results
Statistical analysis of the SSEP and BBB data from both contusion and transection injury models indicate significant differences (P < 0.05). The results also show remarkable similarity for the severe and very severe contusion injuries to the complete transection, the moderate contusion injury to the double hemi-transection, and the mild contusion injury to the T10 hemi-transection injury.
Conclusion
Although contusion and transection spinal cord injuries have two completely different pathophysiologies, their injury progress during acute phase follow a similar trend.
Similar content being viewed by others
Introduction
It is critical to have standardized research models of spinal cord injuries (SCI) in order to characterize the onset as well as the progress of the various forms of injuries. Scientists must be able to reproduce SCI models [1, 2] to unify their studies and compare their results. In this multidisciplinary research, monitoring functional deficits [3, 4], examining neuroprotective effectiveness of hypothermia [5,6,7,8], evaluating long-term efficiency of stem cell replacement therapy [9,10,11,12], application of imaging techniques to detect anatomical changes [12], assessments of cortical plasticity, and reorganization of neuropathways within the spinal cord [4, 13,14,15] are few important fields of study to mention.
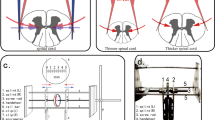
Although the spinal cord is well-protected by the strong bony structure of the vertebral arch, it is still vulnerable to various forms of insult, especially the impact injuries [1, 16]. Such injury could happen when high energy is delivered to a small parenchyma in a very short period. So-called contusive injury will not only contuse one or more segments of spinal cord that are directly underneath the site of impact, but also could cause either dislocation or fracture of the vertebrae causing laceration and partial or full transection of the spinal cord. Thus, contusion and transection injuries often happen simultaneously at and around the epicenter of the injury.
Researchers have successfully created reproducible and reliable contusion and transection models of SCI. Nevertheless, a true translational model, which could include key characteristics of both contusion and transection injuries, still is absent. Technical challenges such as difficulty in independently evaluating two variables in one research subject have long prevented the development of SCI models that carry both elements of injuries concurrently.
Here, for the first time, we intend to present the results of two most prevalent rodent SCI models, contusion and transection, and provide a comparative analysis of their functional assessments. This article describes an analytical evaluation for the onset and progress of the two SCI models through neuro-electrophysiology by Somatosensory-evoked potential (SSEP) assessment and locomotion by Basso, Beattie, and Bresnahan (BBB score) examination. SSEP is the sensory response recorded from cortices after stimulating peripheral nerves, which has been adopted to measure the severity and evolution of spinal cord injuries objectively [17,18,19,20,21,22]. The BBB scoring is the traditional behavioral assessment of injury, which is based on 4 min observations of rats in a 90 cm diameter open field by two examiners [17, 23]. Briefly, during the contusion, most of the impact energy will be absorbed by the Gray Matter directly underneath of injury epicenter. Then, it will spread to the surrounding White Matter and extends to the proximal, distal, dorsal, and ventral adjacent areas over time, causing utmost irregular disruption of the spinal cord parenchyma [21, 24,25,26]. Clearly, the expansion of the damage depends on the severity of the impact. The NYU-Impactor is one of the most used devices to create contusive injuries in mice and rats [1, 25]. On the other hand, the transection model of SCI is produced by a clean transverse incision of the spinal cord, which generates a well-defined interruption of neuropathways with relatively consistent neuro-inflammation at the site of injury. A small scalpel (No. 11) is usually used to incise rodents’ spinal cord under microscope creating either complete or partial hemi-transection injury [2, 4, 27,28,29]. Due to the two very distinctive injury inductions (impact vs. incision), the histological data are not reported, though they were extensively presented in our previous publications [1, 2].
To be applicable for comparison purposes and future studies, the monitoring of the contusion and transection SCI should strictly be time dependent. This means the data collection and analysis for both SSEP signal and BBB scores should be from identical time-points post-injury. Other critical conditions, such as animal weight, body temperature, anesthetic drug, anesthesia induction methods, laminectomy to expose the spinal cord, other surgical procedures (except for the injury induction itself), post-surgery recovery care, recording instruments and statistical analysis must also be identical in both models [1, 2, 30, 31].
It is also important to mention that since these two injuries have different pathoanatomy and pathophysiology as well as natural history, we do not intend to correlate the contusion and transection SCI models. Our goal is to present the comparison (both similarity and contrast) among various severities of contusion and transection SCIs and provide a comparative analysis of the functional changes that start to happen immediately after onset of injury and the events that continue to progress during the acute and sub-acute phase of SCI.
Materials and methods
Experimental procedures
As we reported in detail previously [1, 2], equal number of adult (225–250 g) male and female rats were grouped randomly. Anesthesia induction and maintenance as well as body temperature of all subjects in all groups were kept identical and constant. Four screw electrodes corresponding to forelimbs and hindlimbs somatosensory areas of the left and right cortices and one near Lambda (as reference electrode) were carefully implanted on the skull of rats without rupturing the dura or penetrating the brain tissue [1, 2]. These five electrodes were connected to a data acquisition system for real-time monitoring and offline analysis. Four pairs of needle electrodes were placed near the left and right Median (forelimbs) and Tibial (hindlimbs) nerves (without touching the nerve bundles) to induce electrical stimulation. Each stimulation triggered recording from contralateral skull electrodes as well as three other electrode with one second pause between stimulations. Laminectomy in rats is a safe, secure, easy, and relatively fast (15 min) procedure to expose the dorsal part of the spinal cord, where either contusion under NYU-Impactor or clean transection under microscope was intended. Animal care post-SCI included s.c. saline injection (~10 cc/day), analgesic (4 days), antibiotic (3 days), temporary bladder expression twice a day for 3–5 days, adequate room temperature (18–23 °C) and humidity (40–60%), 12 h day/night cycle, free access to food and water at the bottom of the cage—all the conditions and procedures were identical for contusive and transection injuries.
Behavioral assessment
Few days before the injury, locomotion of all animals was assessed using the BBB rating scale [23]. The BBB scoring is a subjective 21-point scale used to assess the hindlimb joints movements of the animal in a 90 cm open-field by two observers independently. BBB score 0 is assigned no hindlimb motion, while simultaneous movement of the joints in hip, knee, and ankle in hindlimbs are given score up to 7. Scores 8 to 13 are given from motion with occasional plantar stepping without weight support to consistent front-hind limbs co-ordination. Advanced recovery with scores 14–20 is given to better recovery from plantar steps with parallel paw position, to hindlimb stepping with toe clearance, and fine paw coordination with tail balancing off the ground. The score 21 is reserved for animals with absolutely no motor behavior deficit.
Signal analysis
The SSEP signal has mainly four identifiable peaks. After stimulation, which appears within the first few milliseconds (ms), the first positive peak (P1) can be identified within the 5 ms, which is followed by first negative peak (N1) at 5–10 ms and the second positive peak (P2) appears at 10–20 ms, followed by second negative peak (N2) at 25–50 ms in rats. Note that the P1 peak could be absent often. Conventionally, identification of the N1 peak is used to analyze the latency and the amplitude of N1-P2 peaks is used for amplitude analyses and measurements [32,33,34,35,36,37,38,39,40,41,42]. In both contusion and transection experiments, the SSEPs contralateral to the side of limb stimulation were used for analysis. The signal-to-noise ratio was enhanced by using ensemble averaging of 100–700 sweeps. Peak detection was then applied to locate the N1 and P2 peaks of the averaged SSEP. The SSEP averaged signals were then normalized relative to the respective baseline signal. Relative here means that the amplitude of the SSEP was measured relative to the amplitude of the corresponding baseline SSEP signal. This was done by dividing the N1-P2 peak-to-peak amplitude of SSEPs by the N1-P2 peak-to-peak of the corresponding baseline. All signal processing was performed using MATLAB R2019a by The MathWorks, Inc.
Statistical analysis
Statistical analysis of the SSEP data was performed by a repeated measure analysis of variance over 5 time points: Baseline, Day 4, Week 1, Week 2, and Week 3. For the contusion experiments, the control group of rats with laminectomy but without any weight drop (injury) and the injury groups weight drop impact heights of 6.25 mm (mild), 12.5 mm (moderate), 25 mm (severe), and 50 mm (very severe) were analyzed. For the transection experiments, Right T10 hemi-transection injury (RxI); Left T8 and Right T10 double hemi-transection injury (DxI), and T8 complete transection injury (CxI) were analyzed. Pairwise multivariate “t” tests according to Fisher Least Significant Difference method and compensation for multiple comparisons on different days were performed. The null hypothesis was that the relative amplitudes are the same on different days before and after injury. Similar statistical analysis was also conducted for the motor behavioral scores.
Results
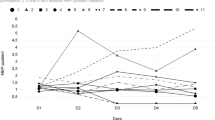
The relative amplitude of the SSEP signals normalized with respect to the baseline for the four contusion and three transection injury groups as well as the control group (which is identical for both injury models) is shown in Fig. 1. It is observed that the laminectomy only (control group) has no SSEP amplitude attenuation as expected. However, the relative SSEP amplitudes of the four contusion and three transection injury groups reduced corresponding to the increase in injury severity. In addition, the motor behavior BBB scores for both contusion and transection injury groups as well as the control group (which is identical for both injury models) is shown in Fig. 2. The BBB scores of the four contusion and three transection injury groups are reduced corresponding to the increase in injury severity and the BBB score of rats in laminectomy only (control group) showed no deficit as expected.
Statistical analysis of the SSEP data from both contusion and transection injuries indicated that a differential pattern for their progress of injuries began to appear after week 1 across all injury groups. Furthermore, significant differences between the pair-wise groups of the two higher (25 and 50 mm) and two lower (6.25 and 12.5 mm) injury severities (P < 0.05). Statistical analysis of the transection data also rejected the null hypothesis that all relative SSEP means are the same with (P < 0.05).
Discussion
The contusion model of spinal cord injury is the most relevant form of SCI in human and approximately one third of contusive SCIs have also equivalent transection injuries due to the blunt force that causes vertebral fractures and dislocations. Logically, in any research model, only one type of injury could be induced and investigated, while most of the times in real life, the two injuries happen concurrently.
In this article, we intend to provide a comparative analysis of the functional assessment and define the contribution and role of the two major components (contusion and transection) in the overall progress of injury during the three weeks acute phase of SCI. Evidently, the anatomopathology and pathophysiology of the contusion and transection injuries are completely different and hence, realistically no correlation between the two injuries can be drawn. Contusion injury (transfer of high energy in a very short time) typically presents with irregular progress, extending proximally, distally, ventrally, dorsally, and laterally, within the spinal cord parenchyma over time. The injury always starts from the epicenter of contusion in Gray Matter, where the energy is primarily absorbed by higher density parenchyma. Then, it extends into the surrounding White Matter involving mostly larger diameter axons. This is because axons present both circumferential stress or tension and contractility. Hence, thicker and more rigid axons break easier than small-diameter axons. The transection injury, on the other hand, is a clean and precise incision with overall limited extension throughout the spinal cord parenchyma. The parenchymal transection injury mostly presents as acute axonal damage that consequently evolves into Wallerian degeneration. Obviously, the contrast between the extensive and irregular expansion of injury in contusion and the Wallerian degeneration in the transection injury is significant. Similarly, we do not attempt to correlate the BBB scores from the two injury models either, but only presenting their impact based on the severity of each injury group.
To avoid the indistinct and non-stable sub-acute phase of SCI, we focused on the comparative analysis of the relative amplitudes of SSEPs during the third-week post-SCI, as it reflects more steady state of pathophysiological conditions. In the case of mild (6.25 mm) contusion injury, the relative SSEP amplitude drops to 66%, which is nearly similar to the 55% relative amplitude changes of the right T10 hemi-transection injury. These percentage changes are comparable because of the similarity in propagation of action potential via ascending spared fibers (anatomically continued) through the injury site. However, in the case of moderate (12.5 mm) contusion and double (right T8 and left T10) hemi-transection injuries, the drop in SSEP amplitude signals is 53% and 48%, respectively. This relatively similar difference could be attributed to the signals that would still propagate through the parenchyma owing to the continuity of the axons in the moderate injury and otherwise healthy neuropathways of the opposite site of the double hemi-transection injuries. However, in the matter of severe (25 mm) and very severe (50 mm) contusion injury as well as complete T8 transection injury, there are substantial drop of SSEP amplitude to 11%, 2%, and 19%, respectively. These changes are due to considerable axonal interruption in the case of severe injury and practically no surviving axonal tracks with the injury expansion to a much larger surrounding parenchyma affecting higher number of neuropathways in the event of very severe injury. Wherein, still there is some form of continuity between the two transected edges of the spinal cord after complete transection injury at T8 (no insulating material was used), allowing spread of non-discriminatory electrical signal, which results in a major reduction of SSEP amplitude. Similar trend of analysis also confirms resemblance in results for the motor behavior (BBB scores) values.
In conclusion, the similarity of the SSEP amplitude changes and BBB scores for the severe and very severe contusion injuries to the complete transection, the moderate contusion injury to double hemi-transection, and the mild contusion injury to hemi-transection is noteworthy.
Data archiving and data availability statement
The data that support the findings of this study are available from the corresponding authors, H Al-Nashash and AH All, upon request.
References
Agrawal G, Kerr C, Thakor NV, All AH. Characterization of graded multicenter animal spinal cord injury study contusion spinal cord injury using somatosensory-evoked potentials. Spine. 2010;35:1122–7. https://doi.org/10.1097/BRS.0b013e3181be5fa7.
All AH, Al Nashash H, Mir H, Luo S, Liu X. Characterization of transection spinal cord injuries by monitoring somatosensory evoked potentials and motor behavior. Brain Res Bull. 2020;156:150–163. https://doi.org/10.1016/j.brainresbull.2019.12.012.
Sydekum E, Ghosh A, Gullo M, Baltes C, Schwab M, Rudin M. Rapid functional reorganization of the forelimb cortical representation after thoracic spinal cord injury in adult rats. Neuroimage. 2014;87:72–9. https://doi.org/10.1016/j.neuroimage.2013.10.045.
Vipin A, Thow XY, Mir H, Kortelainen J, Manivannan J, Al-Nashash H, et al. Natural progression of spinal cord transection injury and reorganization of neural pathways. J Neurotrauma. 2016;33:2191–201. https://doi.org/10.1089/neu.2015.4383.
Maybhate A, Hu C, Bazley FA, Yu Q, Thakor NV, Kerr CL, et al. Potential long term benefits of acute hypothermia after spinal cord injury: assessments with somatosensory evoked potentials. Crit Care Med. 2012;40:573. https://doi.org/10.1097/CCM.0b013e318232d97e.
Teh DBL, Chua SM, Prasad A, Kakkos I, Jiang W, Yue M, et al. Neuroprotective assessment of prolonged local hypothermia post contusive spinal cord injury in rodent model. Spine J. 2018;18:507–14. https://doi.org/10.1016/j.spinee.2017.10.066.
Bazley F, Pashai N, Kerr C, All A. The effects of local and general hypothermia on temperature profiles of the central nervous system following spinal cord injury in rats. Ther Hypothermia Temp Manag. 2014;4:115–24. https://doi.org/10.1089/ther.2014.0002.
Vipin A, Kortelainen J, Al-Nashash H, Chua S, Thow X, Manivannan J, et al. Prolonged local hypothermia has no long-term adverse effect on the spinal cord. Ther Hypothermia Temp Manag. 2015;5:152–62. https://doi.org/10.1089/ther.2015.0005.
Kerr C, Letzen B, Hill C, Agrawal G, Thakor N, Sterneckert J, et al. Efficient differentiation of human embryonic stem cells into oligodendrocyte progenitors for application in a rat contusion model of spinal cord injury. Int J Neurosci. 2010;120:305–13. https://doi.org/10.3109/00207450903585290.
All AH, Bazley FA, Gupta S, Pashai N, Hu C, Pourmorteza A, et al. Human embryonic stem cell-derived oligodendrocyte progenitors aid in functional recovery of sensory pathways following contusive spinal cord injury. PloS One. 2012;7:e47645. https://doi.org/10.1371/journal.pone.0047645.
All A, Gharibani P, Gupta S, Bazley F, Pashai N, Chou B, et al. Early intervention for spinal cord injury with human induced pluripotent stem cells oligodendrocyte progenitors. Plos One. 2015 Jan 30;10:e0116933. https://doi.org/10.1371/journal.pone.0116933.
Bazley FA, Pourmorteza A, Gupta S, Pashai N, Kerr C, All AH. DTI for assessing axonal integrity after contusive spinal cord injury and transplantation of oligodendrocyte progenitor cells. Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:82-5. https://doi.org/10.1109/EMBC.2012.6345876.
Bazley FA, All AH, Thakor NV, Maybhate A. Plasticity associated changes in cortical somatosensory evoked potentials following spinal cord injury in rats. Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:2005–8. https://doi.org/10.1109/IEMBS.2011.6090564.
Blesch A, Tuszynski M. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–7. https://doi.org/10.1016/j.tins.2008.09.008.
Ghosh A, Haiss F, Sydekum E, Schneider R, Gullo M, Wyss M, et al. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci. 2010;13:97–U266. https://doi.org/10.1038/nn.2448.
McDonald J, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–25. https://doi.org/10.1016/S0140-6736(02)07603-1.
Agrawal G, Thakor N, All A. Evoked potential versus behavior to detect minor insult to the spinal cord in a rat model. J Clin Neurosci. 2009;16:1052–5. https://doi.org/10.1016/j.jocn.2008.08.009.
Fatoo N, Mirza N, Ahmad R, Al-Nashash H, Naeini H, Thakor N. Detection and assessment of spinal cord injury using spectral coherence. Annu Int Conf IEEE Eng Med Biol Soc. 2007;2007:1426-9. https://doi.org/10.1109/IEMBS.2007.4352567.
Dawson G. Cerebral responses to electrical stimulation of peripheral nerve in man. J Neurol Neurosurg Psychiatry. 1947;10:134.
Mir H, Al-Nashash H, Kerr D, All A, Thakor N. Using variations of somatosensory evoked potentials to quantify spinal cord injury level. Engineering. 2013;5:99–102. https://doi.org/10.4236/eng.2013.510B020.
Agrawal G, Sherman D, Maybhate A, Gorelik M, Kerr D, Thakor N, et al. Slope analysis of somatosensory evoked potentials in spinal cord injury for detecting contusion injury and focal demyelination. J Clin Neurosci. 2010;17:1159–64. https://doi.org/10.1016/j.jocn.2010.02.005.
Agrawal G, Sherman D, Walczak P, Bulte J, Thakor NV, Kerr D, et al. Shape analysis of somatosensory evoked potentials to detect a focal spinal cord lesion. Conf. Proc. IEEE Northeast Bioeng., Boston, MA, 3–5 April 2009, pp. 1–2. https://doi.org/10.1109/NEBC.2009.4967710.
Basso D, Beattie M, Bresnahan J. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. https://doi.org/10.1089/neu.1995.12.1.
Bazley F, Hu C, Maybhate A, Pourmorteza A, Pashai N, Thakor N, et al. Electrophysiological evaluation of sensory and motor pathways after incomplete unilateral spinal cord contusion laboratory investigation. J Neurosurg-Spine. 2012;16:414–23. https://doi.org/10.3171/2012.1.SPINE11684.
Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231-55. https://doi.org/10.1016/s0079-6123(02)37019-5.
Bazley F, Maybhate A, Tan C, Thakor N, Kerr C, All A. Enhancement of bilateral cortical somatosensory evoked potentials to intact forelimb stimulation following thoracic contusion spinal cord injury in rats. IEEE Trans Neural Syst Rehabil Eng. 2014;22:953–64. https://doi.org/10.1109/TNSRE.2014.2319313.
Bellardita C, Marcantoni M, Löw P, Kiehn O. Sacral spinal cord transection and isolated sacral cord preparation to study chronic spinal cord injury in adult mice. Bio Protoc. 2018;8:e2784. https://doi.org/10.21769/BioProtoc.2784.
Al-Nashash H, Luo S, Liu X, All AH. Trading baseline with forelimbs somatosensory evoked potential for longitudinal analysis in thoracic transection spinal cord injury. J Neurosci Methods. 2020;343:108858. https://doi.org/10.1016/j.jneumeth.2020.108858.
Mir H, Al-Nashash H, Yuan TX, Kortelainen J, Min CS, Manivannan J, et al. Assessment of bilateral SSEP signals enhancement following transectional spinal cord injury using linear modeling. World Congress on Medical Physics and Biomedical Engineering, June 7–12, 2015, Toronto, Canada pp 1219–1219. https://doi.org/10.1007/978-3-319-19387-8_296.
Kortelainen J, Al-Nashash H, Vipin A, Thow X, All A. The effect of anaesthesia on somatosensory evoked potential measurement in a rat model. Lab Anim. 2016;50:63–6. https://doi.org/10.1177/0023677215589514.
Kortelainen J, Vipin A, Yuan TX, Mir H, Thakor N, Al-Nashash H, et al. Effect of isoflurane on somatosensory evoked potentials in a rat model. Annu Int Conf IEEE Eng Med Biol Soc. 2014;2014:4286–9. https://doi.org/10.1109/EMBC.2014.6944572.
Al-Nashash H, Fatoo NA, Mirza NN, Ahmed RI, Agrawal G, All AH. Spinal cord injury detection and monitoring using spectral coherence. IEEE Trans Biomed Eng. 2009 Aug;56(8):1971–9. https://doi.org/10.1109/TBME.2009.2018296.
All AH, Agrawal G, Walczak P, Maybhate A, Bulte JWM, Kerr DA. Evoked potential and behavioral outcomes for experimental autoimmune encephalomyelitis in Lewis rats. Neurol Sci. 2010 Oct;31:595–601. https://doi.org/10.1007/s10072-010-0329-y.
Agrawal G, Sherman D, Thakor N, All AH. A novel shape analysis technique for somatosensory evoked potentials. Annu Int Conf IEEE Eng Med Biol Soc. 2008;2008:4688–91. https://doi.org/10.1109/IEMBS.2008.4650259.
Mir H, Al-Nashash H, Kerr D, Thakor N, All A. Histogram based quantification of spinal cord injury level using somatosensory evoked potentials. Annu Int Conf IEEE Eng Med Biol Soc. 2010;2010:4942–5. https://doi.org/10.1109/IEMBS.2010.5627238.
Väyrynen E, Noponen K, Vipin A, Thow XY, Al-Nashash H, Kortelainen J, et al. Automatic parametrization of somatosensory evoked potentials with chirp modelling. IEEE Trans Neural Syst Rehabil Eng. 2016;24:981–92. https://doi.org/10.1109/TNSRE.2016.2525829.
Mir H, Al-Nashash H, Kerr D, All AH, Thakor N. Spinal cord injury evaluation using morphological difference of somatosensory evoked potentials. 5th International Conference on Bioinformatics and Biomedical Engineering. 1–4. https://doi.org/10.1109/icbbe.2011.5780408.
Sherman DL, Wuyyuru V, Brooke MJ, Zhang HX, Sepkuty JP, Thakor NV, et al. Spinal cord integrity monitoring by adaptive coherence measurement. J Neurosci Methods. 2010 Oct 30;193:90–9. https://doi.org/10.1016/j.jneumeth.2010.07.035.
Mir H, Al-Nashash H, Kortelainen J, All A. Novel modelling of somatosensory evoked potentials for the assessment of spinal cord injury. in IEEE Transactions on Biomedical Engineering, vol. 65, no. 3, pp. 511–520, March 2018, https://doi.org/10.1109/TBME.2017.2700498.
Mir HS, Al-Nashash H, All AH, Thakor NV Quantification of spinal cord injury level using somatosensory evoked potentials. 2010 4th International Conference on Bioinformatics and Biomedical Engineering, 2010, pp. 1–4, https://doi.org/10.1109/ICBBE.2010.5515579.
Mir H, Al-Nashash H, Kortelainen J, All A. Assessment of spinal cord injury via sparse modeling of somatosensory evoked potential signals. 2017 Asia Modelling Symposium (AMS), 2017, pp. 13–17, https://doi.org/10.1109/AMS.2017.11.
All AH, Luo S, Liu X, Al-Nashash H. Effect of thoracic spinal cord injury on forelimb somatosensory evoked potential. Brain Res Bull. 2021;173:22–27. https://doi.org/10.1016/j.brainresbull.2021.05.005
Acknowledgements
The authors would like to thank colleagues and students in our both contusion and transection SCI teams for their collaboration and contribution with the experimental procedures, electrophysiology recordings, motor behavioral scoring, and care for animals as well as for their administrative support.
Funding
This project received funding from the following sources at the Hong Kong Baptist University: Start-Up Tier 1 Fund # 21.4531.162640 (PI: AH All), Century Club Fund # 11.42.4531.135462.00.00 (PI: AH All), Faculty Seed Fund # 31.4531.179234 (PI: AH All), Initiation Grant Faculty Niche Research Areas Fund RC-FNRA-IG/20-21/SCI/02 (PI: AH All), HKBU Matching Equipment Fund RC-EMF/20-21/07 (PI: AH All), and 2021 Hong Kong Government UGC—General Research Fund (GRF) (PI: AH All).
Author information
Authors and Affiliations
Contributions
Angelo All (AA) and Hasan Al Nashash (HA) contributed to the conception and experimental design. AA supervised the experimental procedures and provided critical revision. HA completed data analysis. AA and HA drafted the paper.
Corresponding authors
Ethics declarations
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
ALL, A.H., Al-Nashash, H. Comparative analysis of functional assessment for contusion and transection models of spinal cord injury. Spinal Cord 59, 1206–1209 (2021). https://doi.org/10.1038/s41393-021-00698-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00698-2