Abstract
Study design
This is a double blind phase II/III placebo-controlled randomized trial of the safety and efficacy of GH treatment in incomplete chronic traumatic spinal cord injury.
Objective
The aim of this study was to investigate the possibility to use exogenous GH administration for motor recovery in chronic traumatic incomplete human SCI. The objectives were to establish safety and efficacy of a combined treatment of subcutaneous GH (or placebo) and rehabilitation in this population.
Setting
Hospital Nacional de Parapléjicos
Methods
The pharmacological treatment was a subcutaneous daily dose of growth hormone (GH, Genotonorm 0.4 mg, Pfizer Pharmaceuticals) or placebo for one year. The pharmacological treatment was performed, during the first six months under hospitalization and supervised rehabilitation.
Results
The main findings were that the combined treatment of GH plus rehabilitation treatment is feasible and safe, and that GH but not placebo increases the ISNCSCI motor score. On the other hand, the motor-score increment was marginal (after one-year combined treatment, the mean increment of the motor-score was around 2.5 points). Moreover, we found that intensive and long-lasting rehabilitation program per se increases the functional outcome of SCI individuals (measured using SCIM III and WISCI II).
Conclusions
It is important to highlight that our aim was to propose GH as a possible treatment to improve motor functions in incomplete SCI individuals. At least with the doses we used, we think that the therapeutic effects of this approach are not clinically relevant in most subjects with SCI.
Similar content being viewed by others
Introduction
Recovery or improving motor abilities is one of the main goals of patients after traumatic spinal cord injury (SCI), being associated with quality of life and satisfaction [1]. More than 50% of people with SCI have motor-incomplete lesions. The proportion of incomplete SCI has been increasing [1, 2], and most of the motor recovery occurs within months after injury. After 12–18 months, usually no further spontaneous motor recovery is possible [2]. Many different strategies have been proposed to recover motor functions beyond the spontaneous recovery. Nowadays, not even one is considered effective.
Growth hormone (GH), also known as somatotropin, is a peptide hormone that is synthesized and secreted by the somatotrophs of the anterior pituitary gland [3].
Its secretion is mainly regulated by the hypothalamic GH‐releasing hormone (GHRH) as a stimulator and by somatostatin as inhibitor [4]. Once released in the bloodstream, GH reaches the target organs. Classically, the effect of GH includes hyperglycemia, lipolysis and protein anabolism, and it has direct effects on cellular proliferation and differentiation. The anabolic effects of GH are mediated by insulin-like growth factor-I (IGF1), which stimulates whole-body protein synthesis, including skeletal muscle and collagen proteins. The stimulation of muscle protein anabolism and growth by GH has led to the hypothesis that GH use would increase muscle strength and power. Indeed, starting from the early 1980s, GH became increasingly used as a doping agent by athletes, subsequently entering the list of banned substances [5].
IGF1 is produced primarily in the liver, and in various tissues throughout the body. In response to GH [6], IGF1 regulates growth, glucose uptake, and protein metabolism (i.e., IGF1‐dependent GH effects). IGF1‐independent GH effects include stimulation of insulin secretion, lipolysis, and gluconeogenesis [7]. GH can cross both the blood [8] and CSF–brain barriers [9, 10], as does the IGF1 [11]. The GH/IGF1 axis has been implicated in physiological brain functioning, neurogenesis, myelination, and synaptic plasticity [12, 13]. Moreover, GH and IGF1 play a role in muscle metabolism [14]. For the combined effects over muscle and central nervous system, GH and IGF1 can be considered as potential drugs to improve motor functions in SCI, even in a chronic stage. Some case report supported this hypothesis [15,16,17].
Several promising therapies to improve motor functions in SCI have been translated into clinical trials, but none have yet proven to be of significant benefit in humans nor in the acute or in the chronic stage [18]. Failure of translation may be attributed to several factors, among the most relevant ones is the greater heterogeneity of human SCI when compared with experimental animal models with subsequent variability in spontaneous neurological recovery [2]. Furthermore, it must be acknowledged that animal models do not fully represent the human condition and secondary-injury mechanisms may vary in importance and timing among species.
Since exogenous GH administration is generally considered safe, we decided to test the effects of GH administration concomitantly with rehabilitation [19,20,21]. The aim of this study was to investigate the possibility to use GH exogenous administration for motor recovery in chronic traumatic incomplete human SCI. Our objectives were to establish the safety and efficacy of a combined treatment with subcutaneous GH (or placebo) and rehabilitation in this population. To guarantee a homogeneous rehabilitation and compliance with the treatment, we decided that during the first six months, all participants were hospitalized.
Methods
A total of 54 SCI participants were enrolled for the clinical trial (mean age 36.3 ± 9.9, range 21–71 years). The clinical trial was approved by the local ethical committee and by the Spanish Drug Agency (AEMPS) and registered on ClinicalTrials.gov (NCT01329757). To favor the recruitment, the clinical trial was announced on the webpage of the Hospital Nacional de Parapléjicos (HNP) and on the Spanish media.
Demographic and clinical data for screening and enrollment purpose
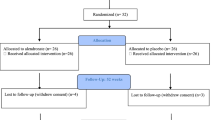
The first step was an assessment for eligibility initially made by a phone interview. The flowchart is reported in Fig. 1. After this step, participants were screened immediately before or after the hospitalization. We used the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) [22] to classify subjects using the ASIA Impairment Scale (AIS); neurological level of SCI (the more caudal neurological level with normal neurological function) and the following clinical data were also collected: age, cause of the lesion, and time since SCI lesion. Moreover, we performed interviews, revised clinical records, obtained vital signs, ECG, and blood and urine samples to evaluate the general medical condition of the patients. Blood sample was also used to evaluate basal levels of IGF1. ISNCSCI [22] is the standard evaluation of the SCI patients: it includes muscle function grading, sensory grading, neurological level, and AIS. AIS is used to classify the patients in five main groups A–E. Only patients with AIS B and C were included in the study. All these examinations and procedures were performed by a physical medicine and rehabilitation specialist. All subjects with traumatic SCI were assessed and screened for this trial. Those who met the inclusion criteria were offered enrolment (Table 1). Following written informed consent and prior to randomization patients were hospitalized at the HNP. A sample size of 62 subjects, 31 in each arm, was estimated to be sufficient to detect a clinical difference of five points between groups in increasing motor score assuming a standard deviation of seven using a two-tailed t-test of the difference between means with 80% power and a 5% level of significance. Considering a dropout rate of 25%, the sample size required is 76 (38 per group). After the screening, we finally enrolled and randomized 54 SCI patients (the main clinical and demographic and characteristics are reported in Table 2). The whole period for recruitment and follow-up lasted six years, that was longer than expected (so we decided to end the trial).
Study design
This is a double-blind phase II/III placebo-controlled randomized trial of the safety and efficacy of HGH treatment in incomplete chronic traumatic spinal cord injury. The pharmacological treatment was GH (subcutaneous daily dose of Genotonorm 0.4 mg, Pfizer Pharmaceuticals) or placebo for one year. The pharmacological treatment was associated, during the first six months, to hospitalization and supervised rehabilitation. After six months, the patients were discharged and continued the treatment at home (not supervised). Physical therapy was individualized, depending on neurological level and AIS of the participants of both the GH and placebo groups. The physical therapy was individualized to take into account the SCI individual difference (e.g., neurological level). On the other hand, the professional prescribing the physical therapy was blinded to the pharmacological therapy received (GH or placebo). Therapy time was 1 h every morning and 2 h every afternoon for 5 days per week (Monday to Friday) for 6 months. The afternoon therapy included sport activity. Patients were allowed to practice sport activity during the weekend. The clinical trial goal was to test the safety and efficacy of the combined treatment.
Randomization and masking
Subjects were randomized (parallel assignment, 1:1) to receive GH (Genotonorm 0.4 mg, Pfizer Pharmaceuticals) or placebo (equal volume of normal saline using the same syringe system). For this purpose, sets of 20 envelopes containing a card indicating the placebo or GH kits were prepared. The cards were randomly inserted into the envelopes and then the envelopes were sequentially numbered and sealed. The cards and the envelopes were prepared by an independent individual not otherwise involved in the patient selection and evaluation. Different 20 envelope kits were prepared. For this study, we used three kits (allowing a randomization of up to 60 patients). When enrolled, patients were administered the next available envelope code, corresponding to a placebo or GH kit. All subjects and medical and research personnel (including nurses and therapists) were blinded to treatment until the end of the study. To test if masking was effective at 6- and 12-month follow-up, subjects and evaluators were given a forced-choice question about whether GH or placebo was received to verify the correct masking.
Data collection
Safety and clinical variables were collected at Days zero and 15, and Months six and 12. Trial monitoring to ensure data quality was internally performed. After the drug administration started, participants were evaluated for adverse events daily while in hospital and at each clinical evaluation (and on demand) subsequently. All serious adverse events were reviewed promptly by clinicians and clinician researchers not otherwise involved in this study. A summary of all serious adverse events was reviewed every 6 months.
Safety variables
Vital signs, ECG, and blood and urine samples were collected at each visit for safety. Moreover, we collected info about pain and spasticity. Pain was graded with a numeric rating scale (NRS), the NRS is a pain rating from zero, no pain, and 10, maximum pain. Spasticity was graded using Modified Ashworth Scale (MAS) and spasms were measured using Penn Spasm Frequency Scale (PSFS) [23, 24]. Neurological level and AIS score were also considered safety variables to detect possible neurological worsening.
IGF1
The main effects of the GH exogenous administration are mediated by the subsequent IGF1 increment. For this reason, we monitored the baseline value and the time course of IGF1.
Clinical outcome measures
Neurological function was assessed at intervals using the American Spinal Cord Injury Association (ASIA) and International Spinal Cord Society (ISCoS) standardized neurological examination, including the motor and sensory composites. We used the ISNCSCI [22] to evaluate the SCI subjects and the following clinical data were collected: cause of the lesion, AIS, neurological level of SCI, motor score (muscle function grade sum for key muscle strength in upper and lower extremities), sensory scores (sensory grade sum of pin prick and of light-touch sensations in each key sensory point), and time since SCI lesion. The Motor Score uses standard manual muscle testing on a six-grade scale: 0: total paralysis; 1: visible or palpable contraction; 2: active movement through range of motion with gravity eliminated; 3: active movement through range of motion against gravidity; 4: active movement through range of motion against gravity and moderate resistance in specific position; 5: active movement through range of motion against full resistance in specific position; 5*: normal if inhibiting factors were not present and NT = not testable. The key muscles/functions included in the Motor Score are elbow flexors, wrist extensors, elbow extensors, finger flexors, finger abductors, hip flexors, knee extensors, ankle dorsi flexors, long-toe extensors, and ankle plantar flexors, whereas a total motor score of 100 is possible. In this clinical trial, the main variable was the motor score of the ISNCSCI.
Secondary-outcome variables were Patient Global Impression of Change (PGIC), AIS conversion, ISNCSCI sensory scores (pin prick and light touch), Spinal Cord Independence Measure (SCIM III), Walking Index for Spinal Cord Injury (WISCI II), and EQ-5D. The Spinal Cord Independence Measure third version (SCIM III) is a scale for the assessment of achievements of daily function of patients with spinal cord lesions. It contains 19 tasks organized in three subscales: self-care, respiration and sphincter management, and mobility. A total score out of 100 is achieved, with the subscales weighted as follows: self-care: scored 0–20; respiration and sphincter management: scored 0–40; and mobility: scored 0–40 [25].
The Walking Index for Spinal Cord Injury (WISCI II) is a functional capacity scale developed to measure improvements in ambulation in persons with spinal cord injury, by evaluating the amount of physical assistance, braces or devices required to walk 10 meters. A score from 0 to 20 is assigned. Level 0: the patient is unable to stand and/or participate in walking to level 20: ambulates with no devices, with no brace and no assistance [26].
EQ-5D is an instrument that evaluates the generic quality of life, with one question for each of five dimensions that includes mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [27].
The self-report measure PGIC reflects a patient’s belief about the efficacy of treatment. PGIC is a 7-point scale depicting a patient’s rating of overall improvement. Patients rate their change as “very much improved,” “much improved,” “minimally improved,” “no change,” “minimally worse,” “much worse,” or “very much worse” [28,29,30,31].
Statistical analyses
Data are reported as mean ± standard deviation for parametrical variables and median and range for nonparametrical variables. The first step of our statistical analysis was to compare the demographic and clinical data of the two groups. Male/female and neurological-level (cervical or thoracic) ratios were compared using a χ2 test. A Student t-test was used to estimate the between group difference of age, time since SCI, and IGF1. AIS (A–E), motor score, sensory scores (pin prick and light touch), SCIM III, WISCI II, pain (NRS), spasticity (MAS and PSFS) and EQ-5D were compared using Mann–Whitney test. To test if masking was effective at 6- and 12-month follow-up, subjects and evaluators were given a forced-choice question about whether GH or placebo was received and compared using a χ2 test.
The second step of our statistical analysis was to compare the safety-variable changes over time of both groups. Vital signs, ECG, blood (including thyroid hormones) and urine results were used to identify clinical and subclinical safety concerns. AIS conversion and neurological level were evaluated at 15 days at 6 and 12 months. after the start of treatment (Mann–Whitney test). The number of adverse events between groups was compared (Mann–Whitney test). Normalized NRS, MAS, and PSFS changes produced by the treatment were evaluated using a repeated-measures ANOVA. These variables were evaluated by the Kolmogorov–Smirnov normality test and were consistent with a normal distribution. Data were entered into separate repeated-measures ANOVA, with TIME (baseline, 15 days, 6 months, and 12 months) as within-subject’s factors and GROUP (GH or placebo) as between-subject’s factors. In case of significant effects, Fisher’s Least significant difference (LSD) test was used for post hoc comparisons. During ANOVA execution, the degrees of freedom were corrected with Greenhouse coefficients if sphericity could not be assumed.
The third step of our statistical analysis was to evaluate the time course of IGF1. IGF1 values were evaluated by the Kolmogorov–Smirnov normality test and were consistent with a normal distribution. Data were entered into a repeated-measures ANOVA, with TIME (baseline, 15 days, 6 months, and 12 months) as within-subject’s factors and GROUP (GH or placebo) as between-subject’s factors. In case of significant effects, Fisher’s Least significant difference (LSD) test was used for post hoc comparisons. During ANOVA execution, the degrees of freedom were corrected with Greenhouse coefficients if sphericity could not be assumed. As IGF1 is age-dependent, this analysis was also repeated incorporating age as a covariate (ANCOVA).
The last step of our statistical analysis was to compare the clinical variable changes after one year of treatment. Data were normalized by dividing each value for the baseline mean. As the principal variable (Motor Score) concerns, we evaluated the changes after one year of treatment (baseline and 12 months) and over time (baseline, 15 days, 6 months, and 12 months). Normalized ISNCSCI motor scores were evaluated by the Kolmogorov–Smirnov normality test and were consistent with a normal distribution. Motor-score changes produced by the treatment were evaluated using a repeated-measures ANOVA with TIME as within-subject’s factors and GROUP (GH or placebo) as between-subject’s factors. The following covariates (baseline values) were added to the model to correct for all the possible confounding factors (ANCOVA): age, sex, time since injury, AIS, motor and sensory levels, motor and sensory scores (pin prick and light touch), SCIM III, WISCI II, pain (NRS) and spasticity (MAS and PSFS). The degrees of freedom were corrected with Greenhouse coefficients if sphericity could not be assumed. Fisher’s Least significant difference (LSD) test was used for post hoc comparisons.
Normalized ISNCSCI sensory scores, SCIM III, WISCI II and EQ-5D, were similarly evaluated, with the exception that for SCIM III, WISCI II and EQ-5D, no covariates were added to the model. PGIC was evaluated only at 6 and 12 months after the start of treatment. We used a Mann–Whitney test to compare GH and placebo groups. AIS conversion was evaluated at 15 days, 6, and 12 months after the start of treatment (Mann–Whitney test).
For significant variables, we also calculated the standardized difference (Cohen’s D), considering a small-effect size d~0.20; medium-effect size d~0.50; large-effect size d~0.80; very-large-effect size d~1.30.
All statistical analyses were performed with the software STATISTICA. The results were considered significant at p < 0.05.
Results
Subjects
The flowchart shows how the 54 SCI individuals were enrolled. Twenty eight were assigned to the GH group and twenty six to the placebo group. The study was terminated upon recruitment of 54 motor-incomplete subjects, a recruitment target lower than the a priori-defined (n = 76) due to technical reasons. Baseline demographic and clinical characteristics, including IGF1 baseline value for the GH and placebo groups, are summarized in Table 2. There were four dropouts (two from GH and two from placebo arm). One subject dropout at day three after treatment started (GH group) due to femur fracture that was considered as a severe adverse event not related to the medication. One subject dropout at day 188 after treatment started (placebo group) due to anal fistula surgery that was considered as a severe adverse event not related to the medication. The other two subjects’ dropouts due to personal decision (consent withdrawn) in the first two weeks after treatment started. There was one protocol violation related to the interventions. A subject stopped medication intake at day 342 but was evaluated at day 365, so he was included in the final analysis. No other relevant adverse events occurred and there were no differences between GH and placebo groups with respect to dropouts and adverse events. Evaluators and subjects were not able to identify any difference between patient membership to GH and placebo group at six (Evaluators: Pearson chi-squared=1.01, p = 0.3128; Subjects: Pearson chi-squared=0.102, p = 0.7495) and 12 months (Evaluators: Pearson chi-squared=0.98, p = 0.3221; Subjects: Pearson chi-squared=3.10, p = 0.0773).
Safety variables
Vital signs, ECG, and blood and urine did not show any change that was a concern for safety. No subjects worsened the AIS or the neurological level during the study. Spasticity and spasms were stable during the study. A tendency to increment of pain over time was observed only in the placebo group (ANOVA: F = 2.568, p = 0.0567), that after one-year treatment had a mean NRS of 3.75 ± 3.3 (e.g., approximately one point higher than at baseline). No differences were observed when compared with the GH-treated group, so we will not consider this finding as relevant for this study.
IGF1
The main effects of the GH exogenous administration are mediated by the subsequent IGF1 increment. For this reason, we monitored the baseline value (all participants) and the time course of IGF1 (in a subgroup of participants). ANCOVA (age included as a covariate) showed interaction TIME per GROUP (ANOVA: F = 7.087, p = 0.0002). Post hoc confirmed a significant effect at all times (p < 0.05). The Cohen’s d showed that the treatment had a large-/very-large-effect size (six months, d = 1.6; one year, d = 0.95).
Neurological recovery
Primary-outcome measure
Significant effects of treatment after one-year treatment are summarized in Fig. 2, Fig. 3, and Table 3. The primary end point (ISNCSCI motor score recovery after one-year treatment). Motor recovery (significant increment of motor-score compared with baseline) was present in the GH group but not in the placebo group (ANCOVA: F = 5.910, p = 0.0205). ANCOVA showed interaction TIME per GROUP (F = 2.919, p = 0.0377). Post hoc confirmed a significant effect between baseline and one year (p < 0.05) and between 15 days and one year (p < 0.01). Even if significant, the Cohen’s d showed that the treatment had a small-effect size (d = 0.15).
Secondary-outcome measures
No significant effects between groups and over time were observed comparing normalized ISNCSCI sensory scores (pin prick and light touch). After one-year treatment, AIS conversion was similar in both groups (Mann–Whitney, p = 0.4281). Very few subjects experienced AIS conversion both in GH (n = 4/26; B, C = 1, B–D = 1, and C, D = 2) and placebo (n = 2/24; B, C = 1, C, D = 1) groups.
SCIM III and WISCI II
Functional recovery (significant increment of SCIM III and WISCI II compared with baseline) was present in both groups (ANOVA: SCIM III: F = 23.532, p < 0.0001; WISCI II: F = 8.493, p = 0.0054). Repeated-measures ANOVA confirmed no interaction TIME per GROUP (all p > 0.2), but showed a strong effect of treatment on SCIM III (F = 10.009, p < 0.0001) and on WISCI II (F = 10.575, p < 0.0001). Post hoc confirmed a significant effect between baseline and 15 days for SCIM III (p < 0.05) and for both SCIM III and WISCI II when comparing the baseline and six months (p < 0.001) and the baseline and one year (p < 0.001). The Cohen’s d showed that six-month and one-year treatment had a medium-effect size (SCIM III, d = 0.3–0.35; WISCI II, d = 0.4–0.6).
EQ-5D
Quality-of-life nonsignificant increment (EQ) was observed one year after treatment in the GH group but not in the placebo group (ANOVA: F = 3.447, p = 0.0695). This result was not confirmed by the repeated-measures ANOVA (all p > 0.05).
PGIC
After one-year treatment, PGIC was similar in both groups (Mann–Whitney, p = 0.6873). Most of the subjects referred to improvement both in GH (n = 21/26) and placebo (n = 16/24) groups.
Discussion
The purpose of this single-center study was to demonstrate the safety and efficacy of a combined treatment of a drug (GH) and rehabilitation in incomplete SCI subjects in a chronic stage and of traumatic origin. As a therapeutic strategy, GH presents several advantages for possible clinical use. From a pharmacological perspective, it is clinically available for human use and can be safely administered over long periods. From a mechanistic perspective, it has an influence directly or indirectly (via IGF1) on multiple biochemical pathways potentially useful for motor recovery. As far as efficacy concerns, we focused on the potential effects on ISNCSCI motor score (principal variable) and other functional outcome scores for the treatment of human SCI. Our results demonstrate high protocol compliance (few dropouts even with a very demanding clinical trial), and that the dose used appears well-tolerated in human SCI subjects (no safety concerns). The main findings we report here can be summarized as follows: (1) the combined treatment (GH plus rehabilitation treatment is feasible and safe); (2) as in healthy subjects and in other disorders, GH exogenous administration increases the blood levels of IGF1 (one of GH biological effectors) also in SCI population; (3) GH but not placebo increases the ISNCSCI motor-score in incomplete, traumatic, and chronic SCI individuals; (4) intensive and long-lasting rehabilitation program per se increases the functional outcome of SCI individuals (measured using SCIM III and WISCI II). Moreover, it is possible that GH but not placebo increases the quality of life in incomplete, traumatic, and chronic SCI individuals. On the other hand, we consider that ISNCSCI motor-score increment (even if statistically significant) is rather modest and of little clinical relevance (as demonstrated by size effect estimation). Moreover, the motor score improvement, is not paralleled by a sensory improvement making unreliable a neurological recovery, and points to a more probable muscular strength improvement (e.g., a sort of doping effect). After one-year combined treatment, the average increment of the motor score was around 2.5 points. Thus, only a marginal proportion of chronic SCI individuals may functionally benefit from GH therapy. We would like to remark that this change is below the value that can be considered a clinically significant change [32, 33].
It is well known that in adults with GH deficiency, a normalization of muscle strength [14] and positive effects on aerobic exercise capacity [34] are observed following long-term GH-replacement therapy. Despite some report suggesting a GH deficiency in SCI population [35], we did not formally assess this in our cohort, and this can be identified as a study limitation. On the other hand, the IGF1 levels of our cohort at baseline were within normal range.
GHD has been studied in neurological disorders affecting central nervous systems, such as multiple sclerosis, amyotrophic lateral sclerosis [36, 37], and traumatic brain injury, and it has been confirmed in SCI. In SCI individuals with GHD, GH combined with physical therapy has been reported to improve quality of life and sensory deficit (subclinically) of complete SCI individuals [35].
From a clinical point of view, it seems more important the observation that rehabilitation and physical therapies are able to improve functional outcome even in a chronic stage. This suggests that not all the SCI individuals in the chronic stage are using all their potential. The hospitalization (first six months) may guarantee correct drug management, and a supervised lifestyle. Moreover, the individuals probably benefit from rehabilitation and sport activities. It has been pointed out that intense exercise promotes activity-induced neuronal plasticity. Similar results were reported by other authors during replacement therapy in SCI with GHD [35].
We found that GH but not placebo increases the quality of life that cannot be due to the increased functional independence gained along the clinical trial, as it is not present in the placebo group. On the other hand, placebo group showed a tendency of pain worsening. We do not have a clear explanation why pain tends to be worse in the placebo group.
The present study was specifically designed to assess the patients when no spontaneous recovery of either motor or sensory functions is supposed to happen (minimum 18 months after injury), to avoid interference with spontaneous recovery mechanisms. The results of this clinical trial have to be considered valid in this stage of the SCI, as we cannot guarantee the safety of GH therapy in the early stage (hyperacute, acute, or subacute) of the SCI. Moreover, we cannot speculate that in earlier stages, more efficacy is expected. We avoided to assess the patients in the worst-possible clinical situation, which is a complete AIS-A injury, and we preferred to design the trial for incomplete SCI (AIS B and C). To obtain motor improvement in AIS-A injury, neural repair and/or regeneration are probably required. As GH/IGF1 may have different mechanisms that may favor motor improvement, we decided to start with incomplete SCI individuals that may benefit from neural repair and/or regeneration, but also from nervous system and muscle better functioning.
We conclude, as a result of this study, that GH given subcutaneously for one year is well-tolerated and safe. In a randomized, multiple-blind manner, the treatment was associated with a marginal improvement in neurological (motor) outcomes compared with placebo. Moreover, we observed that the rehabilitation program can improve functional outcome even in a chronic stage of SCI, warranting further formal investigations of rehabilitation strategy.
It is important to highlight that our aim was to propose GH as a possible treatment to improve motor functions in incomplete SCI individuals. At least with the doses we used (and with the increment of IGF1 we found), we think that this approach is not useful. We consider important to remark this aspect to avoid any expectations that could lead to massive unlabeled use in the SCI community.
Data availability
All data and the full-trial protocol will be provided upon reasonable request.
References
Wyndaele M, Wyndaele J-J. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord [Internet]. 2006;44:523–9. http://www.nature.com/articles/3101893
Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord [Internet]. 2007;45:190–205. http://www.nature.com/articles/3102007
Melmed S. New therapeutic agents for acromegaly. Nat Rev Endocrinol [Internet]. 2016;12:90–8. http://www.nature.com/articles/nrendo.2015.196
Morishita M, Iwasaki Y, Onishi A, Asai M, Mutsuga N, Yoshida M, et al. The effects of GH-releasing hormone/somatostatin on the 5′-promoter activity of the GH gene in vitro. J Mol Endocrinol [Internet]. 2003 [cited 2020 May 3]: 441–8. Available from: https://jme.bioscientifica.com/view/journals/jme/31/3/441.xml.
Holt RIG, Ho KKY. The use and abuse of growth hormone in sports. Endocr Rev [Internet]. 2019;40:1163–85. https://academic.oup.com/edrv/article/40/4/1163/5512652
Bikle DD, Tahimic C, Chang W, Wang Y, Philippou A, Barton ER. Role of IGF-I signaling in muscle bone interactions. Bone [Internet]. 2015;80:79–88. https://europepmc.org/articles/PMC4600536
Dominici FP, Argentino DP, Muñoz MC, Miquet JG, Sotelo AI, Turyn D. Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm IGF Res [Internet]. 2005;15:324–36. https://linkinghub.elsevier.com/retrieve/pii/S1096637405000754
Pan W, Yu Y, Cain CM, Nyberg F, Couraud PO, Kastin AJ. Permeation of growth hormone across the blood-brain barrier. Endocrinol [Internet]. 2005;146:4898–904. https://academic.oup.com/endo/article-lookup/doi/10.1210/en.2005-0587
Lai Z, Emtner M, Roos P, Nyberg F. Characterization of putative growth hormone receptors in human choroid plexus. Brain Res [Internet]. 1991;546:222–6. https://linkinghub.elsevier.com/retrieve/pii/000689939191485J
Johansson J-O, Larson G, Andersson M, Elmgren A, Hynsjö L, Lindahl A, et al. Treatment of growth hormone-deficient adults with recombinant human growth hormone increases the concentration of growth hormone in the cerebrospinal fluid and affects neurotransmitters. Neuroendocrinol [Internet]. 1995;61:57–66. https://www.karger.com/Article/FullText/126813
Carro E. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci [Internet]. 2005;25:10884–93. http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.2909-05.2005
Martín‐Rodríguez JF, Ramos‐Herrero VD, Parras GG, Flores‐Martínez Á, Madrazo‐Atutxa A, Cano DA, et al. Chronic adult‐onset of growth hormone/IGF‐I hypersecretion improves cognitive functions and LTP and promotes neuronal differentiation in adult rats. Acta Physiol [Internet]. 2019 [cited 2020 May 3]: e13293. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/apha.13293.
Özdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci [Internet]. 2006;9:1371–81. http://www.nature.com/articles/nn1789
Chikani V, Ho KKY. Action of GH on skeletal muscle function: molecular and metabolic mechanisms. J Mol Endocrinol [Internet]. 2014;52:R107–23. https://doi.org/10.1530/JME-13-0208.
Arce VM, Devesa P, Devesa J. Role of growth hormone (GH) in the treatment on neural diseases: from neuroprotection to neural repair. Neurosci Res [Internet]. 2013;76:179–86. https://linkinghub.elsevier.com/retrieve/pii/S0168010213001053
Devesa J, Reimunde P, Devesa P, Barberá M, Arce V. Growth hormone (GH) and brain trauma. Horm Behav [Internet]. 2013;63:331–44. https://linkinghub.elsevier.com/retrieve/pii/S0018506X12000530
Reimunde P, Quintana A, Castañón B, Casteleiro N, Vilarnovo Z, Otero A, et al. Effects of growth hormone (GH) replacement and cognitive rehabilitation in patients with cognitive disorders after traumatic brain injury. Brain Inj [Internet]. 2011;25:65–73. http://www.tandfonline.com/doi/full/10.3109/02699052.2010.536196
Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, et al. Traumatic spinal cord injury—repair and regeneration. Neurosurg [Internet]. 2017;80:S9–22. https://academic.oup.com/neurosurgery/article/80/3S/S9/3045001
Winkler T, Sharma HS, Stålberg E, Badgaiyan RD, Westman J, Nyberg F. Growth hormone attenuates alterations in spinal cord evoked potentials and cell injury following trauma to the rat spinal cord. Amino Acids [Internet]. 2000;19:363–71. https://doi.org/10.1007/s007260070067.
Muresanu DF, Sharma A, Lafuente JV, Patnaik R, Tian ZR, Nyberg F, et al. Nanowired delivery of growth hormone attenuates pathophysiology of spinal cord injury and enhances insulin-like growth factor-1 concentration in the plasma and the spinal cord. Mol Neurobiol [Internet]. 2015;52:837–45. http://link.springer.com/10.1007/s12035-015-9298-8
Heredia M, Fuente A, Criado J, Yajeya J, Devesa J, Riolobos AS. Early growth hormone (GH) treatment promotes relevant motor functional improvement after severe frontal cortex lesion in adult rats. Behav Brain Res [Internet]. 2013;247:48–58. https://linkinghub.elsevier.com/retrieve/pii/S0166432813001459
Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference for the 2011 revision of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med [Internet]. 2011;34:547–54. http://www.tandfonline.com/doi/full/10.1179/107902611X13186000420242
Bohannon RW, Smith MB. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys Ther [Internet]. 1987;67:206–7. https://academic.oup.com/ptj/article/2728158/Interrater
Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, et al. Intrathecal baclofen for severe spinal spasticity. N. Engl J Med [Internet]. 1989;320:1517–21. http://www.nejm.org/doi/abs/10.1056/NEJM198906083202303
Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC, et al. The spinal cord independence measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil Rehabil [Internet] . 2007;29:1926–33. https://doi.org/10.1080/09638280601046302.
Musselman KE, Yang JF. Spinal cord injury functional ambulation profile: a preliminary look at responsiveness. Phys Ther [Internet]. 2014;94:240. [cited 2020 May 3] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3912625/
Johnson JA, Coons SJ, Ergo A, Szava-Kovats G. Valuation of EuroQOL (EQ-5D) health states in an adult US sample. Pharmacoeconomics [Internet] 1998;13:421–33. https://doi.org/10.2165/00019053-199813040-00005.
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry. 2007;4:28–37.
Kretzer RM. A clinical perspective and definition of spinal cord injury. Spine (Philos Pa 1976) [Internet] 2016;41:S27. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00007632-201604017-00018.
Aguilar-Rodríguez M, Peña-Pachés L, Grao-Castellote C, Torralba-Collados F, Hervás-Marín D, Giner-Pascual M. Adaptation and validation of the Spanish self-report version of the spinal cord independence measure (SCIM III). Spinal Cord [Internet] 2015;53:451–4. http://www.nature.com/articles/sc2014225.
Ansari NN, Naghdi S, Arab TK, Jalaie S. The interrater and intrarater reliability of the Modified Ashworth Scale in the assessment of muscle spasticity: limb and muscle group effect. NeuroRehabilitation. 2008;23:231–7.
Scivoletto G, Tamburella F, Laurenza L, Molinari M. The spinal cord independence measure: how much change is clinically significant for spinal cord injury subjects. Disabil Rehabil. 2013;35:1808–13.
Scivoletto G, Tamburella F, Laurenza L, Molinari M. Distribution-based estimates of clinically significant changes in the International Standards for Neurological Classification of Spinal Cord Injury motor and sensory scores. Eur J Phys Rehabil Med. 2013;49:373–84.
Widdowson WM, Gibney J. The effect of growth hormone replacement on exercise capacity in patients with GH deficiency: a metaanalysis. J Clin Endocrinol Metab [Internet]. 2008;93:4413–7. https://academic.oup.com/jcem/article/93/11/4413/2627280
Cuatrecasas G, Kumru H, Coves MJ, Vidal J. GH deficiency in patients with spinal cord injury: efficacy/safety of GH replacement, a pilot study. Endocr Connect [Internet]. 2018;7:1031 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6198193/
Morselli LL, Bongioanni P, Genovesi M, Licitra R, Rossi B, Murri L, et al. Growth hormone secretion is impaired in amyotrophic lateral sclerosis. Clin Endocrinol [Internet]. 2006;65:385–8. http://doi.wiley.com/10.1111/j.1365-2265.2006.02609.x
Gironi M, Solaro C, Meazza C, Vaghi M, Montagna L, Rovaris M, et al. Growth hormone and disease severity in early stage of multiple sclerosis. Mult Scler Int [Internet]. 2013 [cited 2020 May 10]: [1–5]. Available from: http://www.hindawi.com/journals/msi/2013/836486/.
Acknowledgements
The authors thank Prof. Jesus Devesa and Prof. Jesus Tresguerres for inspiring the study. The authors thank Dr. Pasqualetti and Dr. Jose Luis Rodriguez Martín for valuable methodological support. Moreover, the authors want to thank all the staff of the “Hospital Nacional de Parapléjicos” that collaborated to the study (physiotherapists, occupational therapists, nurses, physicians, etc.). This research was funded by the “Ministerio de Sanidad y Politicas Sociales” of Spain and the “Fondo Europeo de Desarrollo Regional – FEDER” (project: TRA-173).
Author information
Authors and Affiliations
Contributions
AER, MR, and AO were responsible for clinical trial design, funding and trial management. IRC, SCA, RPG, FAL, CCL, MAM, RMCL, FTD, FJRG, and JFV were responsible for screening, evaluations, rehabilitation planning, and patient management. VSL, MC, and AO were responsible for randomization, data management, and analysis. VSL, MR, and AO were responsible for writing the data report, extracting and analyzing data, and interpreting the results. DGM and AO were responsible for drug management (pharmacy and drug logistics). AER, MR, and AO wrote the first draft of the paper. All the authors reviewed the paper and approved the final version.
Corresponding author
Ethics declarations
Competing interests
This is a no-sponsor study and none of the authors or collaborators had any competing interests.
STATEMENT OF ETHICS
We certify that all applicable institutional and governmental regulations were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Esclarin-Ruz, A., Rodríguez-Carrión, I., Ceruelo-Abajo, S. et al. Phase II/III placebo-controlled randomized trial of safety and efficacy of growth hormone treatment in incomplete chronic traumatic spinal cord injury. Spinal Cord 59, 917–924 (2021). https://doi.org/10.1038/s41393-021-00662-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00662-0