Abstract
Study design
This is a cross-sectional descriptive study.
Objectives
To quantify differences in hand muscle morphology between persons with cervical spinal cord injury (SCI) and uninjured adults.
Setting
The study was performed at the Guangdong Work Injury Rehabilitation Hospital.
Methods
We quantified hand muscle cross-sectional area (CSA), thickness, and echo intensity (EI) in 18 persons with subacute to chronic SCI and 23 controls using ultrasound imaging.
Results
Mean SCI abductor pollicis brevis (APB), abductor digiti minimi (ADM), and first dorsal interosseous (FDI) CSA were ~26%, 43%, and 37% smaller than the control means, the deficit in the APB being less than the ADM (P < 0.05). Muscle thickness was also smaller after SCI, but deficits in ADM (31%) and FDI (20%) thickness were less than the CSA deficits (P < 0.05). In five SCI persons, APB CSA and/or opponens pollicis (OP) thickness were normal despite complete motor paralysis. Mean longitudinal image EI was 40% higher in the OP and 15% higher in the flexor pollicis brevis (FPB) after SCI (P < 0.05), suggesting denervation-induced infiltration of fat and fibrous tissues. OP EI was related to OP thickness (r = −0.6, P = 0.007, n = 18). Mean axial image EI was 10% higher in the APB and ADM after SCI (P < 0.05). There were no significant correlations between muscle morphological properties and clinical features in the SCI participants.
Conclusion
Our results indicate significant SCI atrophy and elevated EI that are muscle dependent.
Similar content being viewed by others
Introduction
Of the many muscles that influence hand function, intrinsic hand muscles (referred to as “hand muscles” in this article) that move the thumb and fingers play a crucial role [1, 2]. For example, the thenar (thumb) muscles, consisting of the abductor pollicis brevis (APB), opponens pollicis (OP), and flexor pollicis brevis (FPB), work together with the first dorsal interosseous (FDI) to help generate pinch maneuvers between the thumb and index finger [3]. Traumatic cervical spinal cord injury (SCI) often impairs hand function; the ability to grasp and manipulate objects is difficult if not impossible, due in part to partial or complete hand muscle paralysis [4, 5]. A priority for persons living with tetraplegia is improvement in their hand function [6]. Hence, knowledge of factors underlying hand dysfunction is needed in order to improve rehabilitative treatments such as functional electrical stimulation (FES) [7,8,9].
SCI hand dysfunction may also reflect spinal motoneuron and/or ventral root damage (lower motoneuron lesion) in addition to paralysis that arises from disruption of spinal tracts (upper motoneuron lesion) [4, 10,11,12,13,14,15]. Muscles innervated by spinal roots over several segments caudal to the lesion epicenter may be completely or partially denervated [10, 16]. Nerve stimulation evoked hand muscle compound muscle action potentials (CMAP) may be absent or below normal within the first week of SCI [4]. Denervation has been confirmed based on the appearance of APB, adductor digiti minimi (ADM), and FDI fibrillation potentials during the initial months post injury [4, 10, 11], and lower estimated number of motor units after chronic SCI [11,12,13, 15]. Concurrent upper and lower motoneuron lesions would be expected to result in significant muscle atrophy, but morphology of the hand muscles after SCI has yet to be examined comprehensively [17].
The aforementioned studies provide little information about hand muscle morphology after SCI. Muscle ultrasound imaging can be used to examine muscle quantity by measuring muscle thickness or cross-sectional area (CSA) [18,19,20]. Thickness is often assessed because it is relatively simple to record and because CSA of some muscles are too large to be captured in a single image. However, CSA better represents muscle size and force-generating capacity [21]. Furthermore, CSA was found to be more sensitive than thickness at detecting lower limb muscle atrophy, but it is unknown whether this is also the case in hand muscles [22].
Muscle ultrasonography can also be used to examine muscle quality by measuring echo intensity (EI) (image brightness), which is related to the amount of fat and fibrous tissues in muscle [23, 24]. Partially denervated hand muscles in people with neurological disorders often have elevated EI [20, 25]. Furthermore, investigators found that EI was as sensitive as needle EMG at detecting partially denervated hand muscles in persons with focal neuropathies or lower motoneuron disorders [20, 26, 27]. Thus, it is conceivable that a rise in EI after SCI may indicate that some denervation has occurred [18, 20].
One might expect cervical SCI to cause hand muscle atrophy and elevated EI, particularly following complete injuries to the lower cord that may damage hand muscle motoneurons [10, 12, 15]. However, in a study of 17 adults with chronic complete C5–C7 SCI, 5 produced similar or larger maximal thenar forces evoked by 50 Hz median nerve stimulation compared to the mean force in uninjured adults [28]. This may indicate that thenar muscle size is preserved in some persons despite complete paralysis, not unlike reported cases of preserved lower limb muscles after SCI [29, 30]. Whether this apparent preservation in muscle size is unique to the thenar muscles or also occurs in other hand muscles is uncertain. It is possible that the magnitude of hand muscle atrophy may be muscle dependent because of differences in fiber type composition, motoneuron (axon) properties [31], and/or activity. For example, the APB may be better preserved compared to the ADM and FDI because of its higher type 1 fiber percentage (63%, 52%, and 57%, respectively) [32], and because SCI leads to preferential type 2 fiber atrophy [17, 33]. In addition, abundant APB involuntary activity in some persons after SCI may help to preserve APB size and strength [34].
The purpose of this study is to develop an ultrasound imaging protocol to quantify and compare thenar, ADM, and FDI muscle morphology between persons with cervical SCI and a matched group of uninjured adults. The primary aims are to determine: (1) whether SCI–control group differences in muscle size and EI vary according to the muscle; (2) the relationships between muscle size, EI, and clinical features of the SCI; (3) if thickness and CSA quantify SCI atrophy (i.e., individual SCI thickness or CSA as a percentage of the control group means) to a similar extent. We tested the hypothesis that SCI atrophy is less in the thenar muscles compared to the ADM and FDI.
Methods
Participants
We studied 18 adults (2 female) of Chinese descent who had traumatic cervical SCI 15.2 (25.4) months previously (Table 1). Their mean (SD) age, height, weight, and hand circumference (measured across the knuckles of digits 2–5 with a tape measure) were 39.6 (13.7) (range 19–62) years, 1.68 (0.05) m, 57.7 (8.7) kg, and 198 (11) mm. Routine blood and urinary analysis was normal. We excluded persons who had diabetes, alcoholism, or symptoms (i.e., recent onset paresthesia) associated with peripheral neuropathy.
All SCI participants were involved in 5–6 days per week of in-patient rehabilitation, consisting of physiotherapy, occupational therapy, and traditional Chinese medicine treatments. As part of their treatment regimen, 3 persons (no. 2, 10, and 18) underwent direct neuromuscular electrical stimulation therapy of the APB and ADM (20 min, 6 days per week) for 2 months, 3 months, and 10 days, respectively, prior to ultrasound imaging. Four persons (no. 4, 15, 16, and 18) drove a manual wheelchair for daily activities.
Uninjured adults (n = 23) of Chinese descent were also tested and served as controls. Their mean (SD) age, height, weight, and hand circumference were 37.3 (12.9) (range, 21–64) years, 1.69 (0.07) m, 66.3 (8.7) kg, and 200 (10) mm. Age, height, and hand circumference were not significantly different between groups (P > 0.5). Weight was lower after SCI compared to the controls (P = 0.004). However, weight prior to SCI based on their recall, 63.8 (9.7) kg, was not different from the controls (P = 0.4, n = 17). All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed. The study was approved by the hospital ethics committee (no. AF/SC-07/2018.14) and each participant gave informed consent which conformed to the Declaration of Helsinki.
Clinical assessment of the SCI participants
The neurological level of injury and AIS classification were determined by a physiatrist according to the International Standards for the Neurological Classification of SCI. Neurological level of injury was at C2 (n = 1), C3 (n = 3), C4 (n = 7), C5 (n = 3), C6 (n = 2), or C7 (n = 2) and classified as AIS A (n = 8), AIS B (n = 2), AIS C (n = 4), AIS D (n = 3), or unclassified (n = 1). Disability was assessed according to the Spinal Cord Independence Measure (SCIM-III) [35] and right wrist flexor and extensor spasticity by the Modified Ashworth test. APB, ADM, and FDI motor power was determined by a manual muscle test (MMT). Key pinch force during a maximal voluntary contraction (MVC) was recorded using a pinch gauge (model PG-60, B & L Engineering, Santa Ana, CA).
At the time of imaging, four participants (no. 1, 6, 8, and 16) agreed to undergo a conventional EMG test; maximal surface CMAPs were recorded from the APB during median nerve stimulation and from the ADM and FDI during ulnar stimulation (see Supplementary Fig. S12 for details and the Discussion).
Ultrasound imaging procedures
We recorded images of the thenar muscles to represent median innervated muscles and the ADM and FDI to represent ulnar innervated muscles. Furthermore, the FDI was considered to be representative of the dorsal interossei. These muscles are commonly chosen for ultrasound or EMG examination in individuals with neurological conditions [20, 27]. For purposes of comfort and the rehabilitation schedule of the SCI participants, other muscles such as the adductor pollicis, palmar interossei, and lumbricals were not specifically imaged because the time to complete the imaging protocol would have gone beyond 1 h.
Imaging and analysis were done by a research physiotherapist (HL). A MyLabOne ultrasound system (Esaote, model 8100, Maastricht, the Netherlands) and a 6–13 MHz linear array probe (Esaote, model SL3323) was used. System settings were kept constant for all studies; peak frequency, 13 MHz; acoustic power, max; gain, 70%; image depth, 3 cm; focal point depth, 0.8 cm.
Participants were seated with the elbow flexed and forearm supported by a table. A thick layer of contact gel was used between the probe and skin. The probe was applied with minimal pressure to avoid overly compressing muscle.
Three consecutive axial images of the right APB, ADM, and FDI and three consecutive longitudinal images of the APB plus OP, FPB, ADM, and FDI were recorded, with the probe removed from the skin and repositioned after each acquisition. For axial APB, ADM, and FDI imaging, the probe was initially perpendicular to the skin surface and then tilted slightly to result in the best delineation of muscle border fascia and an echo of the first, fifth, and second metacarpal, respectively. For longitudinal images, the probe was tilted to yield the sharpest muscle border fascia. Immediately after each image was stored, the probe tilt angle was recorded with a digital inclinometer (San Liang Co.). These angles were found to differ little between the two groups (Supplementary Fig. S1).
In axial images, parts of the muscle border were not always distinct. Therefore, immediately after recording the last axial image for each of the APB, ADM, and FDI, we recorded 15 s video clips of the muscles contracting (passive or active) in all subjects. During off-line analysis of the static axial images, some muscle borders could be more easily determined by referring to the moving images. However, OP and FPB CSA could not be determined because of indistinct sections of the muscle border, even with the benefit of the videos. Further details of the imaging protocol according to the muscle examined are described in the following sections.
Imaging thenar (FPB, APB, OP) morphology
The forearm was supinated, with the fingers adducted and slightly flexed. The thumb was abducted 45° and attached to a vertical post with tape. A line was drawn connecting the volar aspects of the scaphoid bone and the first metacarpophalangeal joint, and a mark was made at the mid-point. (i) FPB thickness (superficial plus deep head) in longitudinal images. The probe was positioned in the palm to lay over the flexor pollicis longus (FPL) tendon, which served as a landmark. The probe tilt angle relative to the horizontal plane was adjusted to result in a sharp image of the FPL tendon and the superficial FPB (Supplementary Fig. S2). The probe was then shifted laterally until the FPL tendon disappeared and the superficial and deep FPB appeared together. (ii) APB and OP thickness in longitudinal images. Thickness of the APB and OP were captured together in the same longitudinal image. For these images, the probe was placed on the line connecting the scaphoid and the first metacarpophalangeal joint, centered over the mid-point, and then rotated counter-clockwise about 10–20°. (iii) APB CSA in axial images. For these images, the probe was centered on the mid-point mark over the APB, perpendicular to the FPL tendon (the tendon path was marked in the palm with a piece of tape); the FPL tendon and the proximal half of the APB run approximately parallel [1].
Imaging ADM morphology
The forearm was supinated with the hand in a relaxed natural position. A line was drawn over the bulk of the ADM connecting the pisiform bone to the level of the metacarpophalangeal joint. (i) ADM thickness in longitudinal images. The probe was placed on the line drawn over the ADM, centered on the mid-point, and tilted upward and downward to give the sharpest ADM border and presence of the flexor digitorum tendon which served as an additional landmark (Supplementary Fig. S3). (ii) ADM CSA in axial images. The probe was held perpendicular to the drawn line at the mid-point. Note, the ADM was not recorded in six SCI participants due to time constraints.
Imaging FDI morphology
The forearm was prone, with the fingers slightly flexed and the thumb adducted. A line was drawn over and parallel to the second metacarpal, and a line perpendicular to this was drawn at the level of the first metacarpophalangeal joint. (i) FDI thickness in longitudinal images. The probe was positioned about 5 mm medial to the line over the second metacarpal (Supplementary Fig. S4). The probe tilt angle was altered to give the sharpest FDI border and presence of the flexor digitorum tendon. (ii) FDI CSA in axial images. The probe was positioned perpendicular to the second metacarpal at the level of the first metacarpophalangeal joint.
Image analysis
Images were analyzed using ImageJ software (National Institutes of Health, Bethesda, Maryland). Thickness of the APB, FPB, OP, ADM, and FDI was the largest distance between the superior and inferior muscle border (excluding border fascia), drawn perpendicular to the longitudinal muscle axis, using an electronic caliper (Fig. 1). For determination of APB, ADM, and FDI CSA, the freehand tool was used to trace the entire muscle border. The mean EI within the traced CSA was determined by computer-assisted gray scale analysis; 8-bit scale; black = 0; white = 255. Similarly, EI in longitudinal images was determined in a rectangular region of interest drawn to cover about one-third or more of visible muscle. Each of the three recorded longitudinal and axial images of the different muscles was analyzed once, and means of the three images were used for statistical analysis.
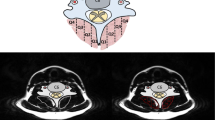
Each column depicts, from top to bottom, APB, ADM, and FDI axial images, and FPB, APB (OP), ADM, and FDI longitudinal images. Thickness and CSA measurements are symbolized by yellow lines. Note the smaller thickness and CSA in both SCI participants. Echo intensity (EI) was abnormally elevated across muscles in B and normal in D (about 10% lower than the control EI means).
Reliability of imaging and analysis procedures
Reliability of the image acquisition plus analysis procedures was done by repeated acquisition of images from uninjured adults on different days and analyzing the images using ImageJ. Intra-rater and inter-rater reliability (intraclass correlation coefficient) for CSA and thickness was high and this was generally the case for EI, consistent with other studies (Supplementary Table S1) [20, 36]. Bland and Altman plots reveal that differences (y-axis) in muscle CSA, thickness, and EI between days (intra-rater), or between raters, were usually less than 10% of the means (x-axis) (Supplementary Figs S5–S8).
Statistics
Data were analyzed using SPSS for Windows V. 22 (IBM, SPSS Inc. Chicago, IL). CSA, thickness, and EI data in both groups were found to be normally distributed (Shapiro–Wilk test). A linear mixed model was used, where group (SCI, control) and muscle were set as fixed factors and participant (subject ID) as a random factor. All pairwise comparisons were adjusted for multiple comparisons using the Bonferroni procedure. Associations between measures were determined using Spearman’s rank correlation. Data are presented as means (SD), and differences were considered significant when P < 0.05.
Results
Representative images of two SCI participants and two matched controls are shown (Fig. 1A–D; also see Supplementary Fig. S9 for additional comparisons). Atrophy is apparent in all muscles in both SCI persons. In one SCI participant, EI is similar to the matched control (Fig. 1D). In the other, EI in all muscles is abnormally elevated, a possible indication of ongoing widespread denervation (Fig. 1B).
Group mean muscle CSA and thickness after SCI
Mean CSA was smaller in the SCI compared to the control group, F (1, 37.4) = 44.3, P < 0.0001, Fig. 2A. There was also a group by muscle interaction, F (2, 36.1) = 13.5, P < 0.0001; pairwise comparisons indicate that the controls had larger ADM CSA than APB CSA (P = 0.001, 95% confidence interval (CI) for the difference, 9.8 to 42.9 mm2) consistent with previous reports in uninjured adults including cadaver muscle [19, 37]. However, there was no difference in CSA between the ADM and APB after SCI (P = 1.0, 95% CI, −26.8 to 15.5 mm2). These data suggest that the APB may be relatively preserved, most apparent in four SCI persons (Fig. 5F and Supplementary Fig. S10A). The fact that SCI atrophy seems to be muscle dependent is unlikely explained by differences in sample size between the SCI ADM (n = 12) and APB or FDI (n = 18) (Supplementary Fig. S11).
A SCI (white symbols) and control (black symbols) mean CSA in the APB, ADM, and FDI. B SCI and control mean thickness in the APB, OP, FPB, ADM, and FDI. For clarity, only one-half SD are shown. C Mean SCI CSA and thickness as a percentage of the control group mean. TK thickness. * significantly different from control group (P < 0.05).
Thickness was also smaller after SCI compared to the control group, F (1, 37.4) = 54.3, P < 0.0001, Fig. 2B. There was also a group by muscle interaction, F (4, 188) = 2.6, P = 0.04; pairwise comparisons indicate that the controls had larger FPB than OP thickness (P < 0.0001, 95% CI, 1.0 to 3.7 mm), but they were similar after SCI (P = 1.0, 95% CI, −0.78 to 2.3 mm).
To summarize the effects of SCI on muscle size, mean SCI CSA and thickness of the different muscles are presented as a percentage of the control means (Fig. 2C).
SCI atrophy based on thickness versus CSA
In the groups combined, individual thickness and CSA were significantly associated in the APB (n = 41), ADM (n = 35), and FDI (n = 41) (r = 0.81, 0.88, 0.84, P < 0.0001, Spearman correlation, two-tailed, Fig. 3 upper panels). In the bottom panels of Fig. 3 are individual SCI thickness versus CSA, expressed as a percentage of the control group muscle means. If individual SCI relative (% control) thickness and CSA are identical, they should fall on the line of unity slope (dotted line). Except for one-third of the APB data, the results show that most data points fall below unity slope, suggesting that atrophy is underestimated when based on thickness compared to CSA. Mean relative CSA was lower than mean relative thickness in the ADM 56.7 (16.8)% versus 69.4 (15.7) %, P = 0.005, n = 12, and FDI 62.6 (17.1)% versus 80.5 (15.2)%, P < 0.0001, n = 18, (paired t-test, Fig. 2C).
Top three panels: individual CSA and thickness plotted together for each muscle in the SCI and control group. Bottom three panels: individual SCI CSA and thickness, each expressed as a percentage of the control group means. The dotted line is the line of unity slope. The gray symbols correspond to the female participants.
Echo intensity (EI) after SCI
Group mean EI in axial images
Mean SCI axial EI was higher than control, F (1, 39.7) = 5.1, P = 0.03, Fig. 4A, and there was a group by muscle interaction, F (1, 35.3) = 3.8, P = 0.03; pairwise comparisons indicate a small but significant elevation in EI in the APB and ADM after SCI (P = 0.02 and 0.003, 95% CI, 1.1–13.0 and 4.7–21.3, respectively), but not in the FDI (P = 0.5).
Group mean EI in longitudinal images
Longitudinal EI after SCI was also higher than control, F (1, 39.0) = 7.9, P = 0.008, Fig. 4B, and there was a group by muscle interaction, F (1, 37.1) = 9.0, P = 0.0003; pairwise comparisons indicate a large elevation in EI in the OP and a modest elevation in the FPB after SCI (P < 0.0001 and 0.01, 95% CI, 21.9–51.3 and 2.8–20.4, respectively), but no group differences in APB, ADM, or FDI EI (P = 0.09, 0.1, and 0.7).
Individual axial and longitudinal EI
The range of EI was wider after SCI compared to the controls. This is apparent in plots of individual axial versus longitudinal EI in the APB, ADM, and FDI (Fig. 5A–C) and longitudinal OP EI versus longitudinal FPB EI (Fig. 5D). The most obvious group difference is the abnormally high EI in the OP and FPB in eight SCI participants (no. 1, 3, 5–7, 12–14), three of whom also had elevated EI in all other muscles (no. 6, 7, 12). SCI axial EI was strongly associated with longitudinal EI in the APB, ADM, and FDI; partial correlations after controlling for age: r = 0.82, r = 0.76, r = 0.92, P < 0.0001, P = 0.007, P < 0.0001), whereas the corresponding control values were more modest (r = 0.46, r = 0.62, r = 0.57, P = 0.03, P = 0.002, P = 0.006). Also, OP and FPB EI were strongly correlated (r = 0.91, P < 0.0001) after SCI, but not in the controls (r = 0.17, P = 0.4). Elevated SCI EI was unrelated to ultrasound probe angle during image capture (i.e., Fig. 5E and Supplementary Fig. S1).
A–C Axial versus longitudinal EI in the APB, ADM, and FDI. D OP versus FPB longitudinal EI. E Ultrasound probe angle (degrees) during image capture versus OP longitudinal EI. F–H CSA versus mean EI (average of longitudinal and axial EI) in the APB, ADM, and FDI. I FPB thickness versus FPB longitudinal EI. J OP thickness versus OP longitudinal EI. Lines of best fit are shown in for each group when correlations are significant.
EI versus muscle CSA and thickness
There was a significant inverse relationship between SCI OP EI and OP thickness (partial r = −0.6, P = 0.007, Fig. 5J). SCI FPB EI and FPB thickness were similarly related, but the correlation was not significant (r = −0.43, P = 0.08, Fig. 5I). Mean EI (average of longitudinal EI and axial EI) of the APB, ADM, and FDI was not significantly correlated with the corresponding muscle CSA (Fig. 5F–H, P > 0.05).
Correlation between muscle morphology and clinical measures after SCI
Muscle morphology (CSA, thickness, and EI) was not significantly associated with clinical measures, including injury duration, injury level, SCIM-III, and MAS.
Discussion
We have developed an ultrasound imaging protocol to quantify the effects of cervical SCI on hand muscle morphology. It is unlikely that the observed muscle-dependent differences between the SCI and control groups are explained by imaging errors or artifacts because we standardized the imaging and analysis procedures. The thumb (hand) was stabilized in the same position across participants, thus minimizing any differences in muscle length on muscle morphology. In addition, the ultrasound probe was placed over muscle according to the same surface landmarks and tilted according to the same imaged structures. Muscle size and EI were found to be unrelated to the probe inclination angles, which were similar between groups (Supplementary Fig. S1). We established good day-to-day and inter-investigator reliability of the imaging and analysis procedures (Supplementary Figs S5–S8 and Supplementary Table S1). Finally, mean thickness of the APB (5.8 mm), ADM (10.4 mm), and FDI (13.5 mm) in the 21 males of the control group are similar to healthy male means in a previous study (4.9, 10.7, and 12.1 mm, respectively, n = 24, 49 years) [38].
SCI atrophy based on thickness versus CSA
The measurement to characterize muscle size is an important matter to consider when investigating muscle morphology [39]. In ultrasonography, thickness is often determined because CSA can be difficult to assess [20]. However, thickness may be less sensitive than CSA at detecting muscle atrophy (hypertrophy) [22]. In the present study, apparent ADM and FDI atrophy after SCI was more pronounced based on CSA compared to thickness (Figs 2C and 3). An underestimate of CSA atrophy based on a single linear dimension (thickness) is not entirely unexpected. However, the magnitude of the underestimate may depend on the longitudinal image plane (i.e., sagittal or coronal) recorded for the thickness measurement and whether atrophy occurs evenly in the muscle cross-section. Investigators examining hand muscle atrophy over time should consider assessing whether there is an optimal longitudinal image plane and thickness measurement that best quantifies CSA atrophy.
Muscle CSA and thickness after SCI
Muscle size was significantly smaller in the SCI group compared to the control group, implying that atrophy occurred after SCI. Furthermore, the extent of CSA atrophy differed between muscles; it was least in the APB, most in the ADM, and intermediate in the FDI, providing some support for our hypothesis (Fig. 2C). Also, normal (≥control mean) APB CSA and OP thickness was evident in four and six SCI persons, respectively (Fig. 5F, J), whereas ADM CSA, FDI CSA, and FPB thickness were <93% of the control mean in all cases (Fig. 5G–I). The muscle atrophy described here has implications for the selection of rehabilitation therapy such as FES [7,8,9] (see “Implications”).
It is noteworthy that the opposite pattern of atrophy, i.e., the ADM is relatively preserved compared to the APB, occurs in motoneuron diseases such as ALS (referred to as the “split-hand”) [40]. The explanation for the apparent different pattern of muscle atrophy after SCI compared to motoneuron disease may be multifactorial, possibly reflecting different pathophysiology in spinal tracts and motoneurons
Changes in muscle size after cervical SCI may reflect the interaction of numerous factors, including disuse (i.e., muscle activity), denervation/reinnervation, trophic factors, and inherent muscle (motoneuron) properties [41]. In the SCI group as a whole, muscle size was not significantly associated with clinical features of the injury. However, normal APB (no. 17, 18) or OP (no. 11, 12, 14, 18) size was evident in persons with apparent complete thenar motor paralysis (MMT = 0), suggesting that factors other than residual volitional muscle activity (i.e., reflex activity, passive forces, trophic factors) helped to preserve muscle size [41]. Furthermore, abundant involuntary thenar motor unit activity and spasms may help to minimize fiber atrophy and even cause type I fiber hypertrophy [34]. Stilwill and Sahgal [17] reported larger type I fibers and smaller type II fibers in the OP in persons with chronic complete SCI (n = 4) compared to controls (n = 2). Also, type 1 fiber percentage is higher in the APB than the ADM and FDI that may be a factor in the relative preservation of the APB [32].
Echo intensity (EI) after SCI
EI is an indirect marker of fat and fibrous tissue in muscle and is often elevated in partially denervated muscles [20, 25]. It is known from electrophysiological studies that cervical SCI may cause one or more hand muscles to become partially or completely denervated [4, 10,11,12]. Thus, Berman et al. recorded absent or reduced CMAP and diffuse fibrillation potentials in persons with C2–C6 SCI [10]. Our findings expand on these earlier results to suggest that the extent of partial denervation may be muscle dependent. Relative to the controls, OP EI was much higher after SCI (most apparent in eight SCI persons, Figs 4B and 5J), whereas the elevations in the APB, FPB, and ADM EI were modest. Furthermore, SCI participants with smaller OP thickness tended to have higher OP EI (Fig. 5J). This association could mean that denervation contributed to the most severe cases of OP atrophy, although this notion is speculative. Others have reported that denervation of more than 75% of a muscle’s motor unit pool leads to atrophy (i.e., denervation atrophy) [42]. Alternatively, elevated OP EI could reflect a volume effect; more ultrasound beam tissue transitions per surface area in atrophied (smaller) muscles. This would seem to be a less likely explanation for the rise in EI because (i) OP EI was elevated in some SCI persons despite having the same OP thickness as a healthy control (Fig. 5J) and (ii) in some SCI persons, multiple muscles did not have elevated EI despite evident atrophy.
The reason for the lack of elevation in EI in any muscle of five SCI participants (no. 4, 10, 15, 16, and 18) is uncertain, although three of them generated pinch forces. Possibly, denervation did not occur or was not severe enough to result in detectable increases in EI [20]. Previously, studies of focal neuropathies revealed normal EI in 17% of muscles assessed as having pathological changes in both MMT and EMG [27].
Case studies with CMAP recordings
In the present study we focused on characterizing group differences in muscle morphology rather than comprehensively examining possible determinants for these differences. Nevertheless, we speculate on disuse versus denervation atrophy by considering the EMG (Supplementary Fig. S12) and morphological data in the subset of four SCI participants. In three of these four persons, CMAP and conduction velocity were above the lower limit of normal (except for ADM and FDI CMAP in participant no. 8), and CSA in all muscles was ≥60% of the control means. These data imply that atrophy may be related more to disuse than to denervation [43], and an example of this is presented in more detail in the following section (participant no. 16). In contrast to these three persons, there is evidence of significant denervation atrophy in participant no. 1, and her morphological and EMG responses are also described in detail below.
Participant no. 16: this individual represents a clinically significant case of muscle-dependent atrophy. CMAP recordings indicated that innervation was essentially intact in the APB, ADM, and FDI. FDI CSA was 60% of control and EI was 10% above normal. These observations imply that FDI atrophy may be caused more by disuse than denervation. In contrast, APB CSA (and OP thickness) and EI in all thenar muscles were normal. The relative preservation of APB compared to the FDI CSA may be related to the higher residual strength in the former (MMT score of 4 versus 1), although his pinch MVC was only 17% of normal strength. This result suggests that a large residual strength is not a necessary precondition for preserving APB CSA. The normal APB and OP size may be explained by greater use of the thenar muscles (i.e., active and passive forces associated with driving a manual wheelchair daily) combined with the higher type I fiber percentage of the APB.
Participant no. 1: this person also demonstrated clinically significant muscle-dependent atrophy. There was little voluntary control of the hand muscles. Absent ADM and FDI CMAP indicate that these muscles were completely denervated (MMT scores of 1 in the ADM and FDI may reflect activation of non-ulnar innervated finger extensor muscles in the forearm [5]). In previous studies, hand muscle CMAPs were absent in about 10–15% of SCI participants [4, 10, 44]. FDI CSA was only 25% of the healthy female mean FDI CSA (181.8 mm2, 28.7 ± 7 years, N = 15, our unpublished data using the same imaging protocol as the present study) (Supplementary Fig. S9B). Also, CSA of the FDI (45 mm2) and APB (50.7 mm2) were similar, resulting in an FDI/APB CSA ratio of 0.9, much lower than the mean ratio in the control (2.0) and the SCI (1.8) groups. These data suggest that FDI atrophy may have been due to the combined effects of denervation (i.e., direct trauma to motoneurons and/or C8–T1 spinal roots) and disuse. Her FDI EI was normal, however. The response in her FDI resembles the first-year responses in completely denervated vastus lateralis due to conus cauda lesion; severe atrophy without major fat and fibrous tissue accumulation [7, 45]. In contrast to the absent ulnar responses, an abnormally small APB CMAP (2 mV) was elicited. The APB CSA was 55% of the healthy female mean APB CSA (92.7 mm2, our unpublished data, n = 15) and APB EI was 31% higher than the control mean. These data imply that the APB was partially denervated, but collateral reinnervation may have been sufficient to prevent denervation atrophy. Thus, in the case of the APB, much of the atrophy may stem from disuse.
Correlation between muscle morphology and clinical measures after SCI
Muscle CSA was severely diminished (i.e., below average for the SCI group), in the two SCI participants (no. 1 and 3) with C7 injury. In these cases, the injury may have resulted in more severe damage to the hand muscle motoneurons, possibly resulting in denervation atrophy [16]. In the SCI group as a whole, however, muscle size or EI was not significantly correlated with injury level, completeness of the injury, or other clinical measures. Previous work regarding level of the lesion on hand muscles properties is mixed; one study reported fibrillation potentials and absent or pathologically reduced CMAP regardless of the level of the lesion (C3–C6) [10], whereas another noted absent or pathologically reduced CMAP only in persons with low level (C6–C7/T1) injuries [4]. In the present study, a number of factors may be responsible for the lack of significant correlations between muscle morphology and clinical measures. The SCI participants were not a homogenous group, with injuries low and high in the cord and of various durations. The impact of these factors on the extent of atrophy would be further complicated by varying degrees of reinnervation and involuntary muscle activity. However, others have reported significant correlations between hand muscle thickness and clinical measures in larger cohorts made up of persons with different neuromuscular disorders (i.e., polyneuropathy, ALS, spinal muscular atrophy, among others, i.e., r2 = 0.62) [46], or a single disorder (ALS, i.e., r2 = 0.16) [25].
Limitations
SCI “atrophy” and EI were approximated by comparing the data of the SCI group to the control group. Thus, the reported mean differences in muscle morphology between the groups may overestimate or underestimate the actual morphological changes that occurred in an individual SCI participant since their injury date. EI as an indicator of partially denervated muscle has been validated using EMG examinations in persons with neurological disorders [20, 26, 27], but this has not been done in the SCI population. Damage to peripheral nerves (i.e., compression) cannot be completely ruled out as contributing to the observed findings. However, previous data indicate that direct trauma to motoneurons and/or spinal roots (or trans-synaptic degeneration) may be the main cause for fibrillation potentials, particularly in the initial months after SCI [10, 44, 47].
Implications
SCI muscle atrophy established here would be expected to reduce maximal electrically evoked force and may contribute to volitional strength loss, depending on whether fibers under voluntary control have atrophied. However, even weak MVC forces (<10% control MVC) after SCI may enable useful hand function, and may help to facilitate activity-dependent neural plasticity [48]. Hence, rehabilitation to promote fiber hypertrophy in hand muscles may be crucial for producing functional voluntary or FES forces in some persons after SCI, even in completely denervated muscles [7,8,9].
Additional longitudinal SCI studies related to impairment and rehabilitation of neuromuscular function are needed. Examination of hand muscles are ideal for such studies; muscle morphology by ultrasound can be measured relatively easily in the clinic or at the bedside, together with electrophysiological examination of whole muscle/motor units and measures of hand function. Studies of this nature may help determine how much of weak voluntary forces is due to paralysis, disuse atrophy, and denervation atrophy. Furthermore, although therapies such as neuromuscular electrical stimulation and FES have been shown to improve hand function in persons with tetraplegia, it is uncertain how much of the improvement is related to neural plasticity, muscle morphology, and muscle contractility [9, 49]. Also, in the future, this knowledge may help in the selection of the most appropriate treatments, i.e., whether therapies should be predominantly cellular or activity based and fine-tuned according to the muscle.
Conclusions
We found that ultrasound can be used to detect differences in hand muscle morphology between persons with cervical SCI and a matched group of healthy adults. The results suggest that the extent of SCI atrophy and elevated EI can vary widely between muscles and individuals. Differences of this nature should be considered in the planning and implementation of hand muscle rehabilitation after SCI.
Data availability
The individual data that support the findings of this study are included in the published article and its Supplementary information file.
References
Gupta S, Michelsen-Jost H. Anatomy and function of the thenar muscles. Hand Clin. 2012;28:1–7.
Pasquella JA, Levine P. Anatomy and function of the hypothenar muscles. Hand Clin. 2012;28:19–25.
Lee SK, Wisser JR. Restoration of pinch in intrinsic muscles of the hand. Hand Clin. 2012;28:45–51.
Curt A, Dietz V. Neurographic assessment of intramedullary motoneurone lesions in cervical spinal cord injury: consequences for hand function. Spinal Cord. 1996;34:326–32.
Harvey LA, Batty J, Jones R, Crosbie J. Hand function of C6 and C7 tetraplegics 1–16 years following injury. Spinal Cord. 2001;39:37–43.
Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–83.
Kern H, Boncompagni S, Rossini K, Mayr W, Fanò G, Zanin ME, et al. Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): a role for myofiber regeneration? J Neuropathol Exp Neurol. 2004;63:919–31.
Panisset MG, Galea MP, El-Ansary D. Does early exercise attenuate muscle atrophy or bone loss after spinal cord injury? Spinal Cord. 2016;54:84–92.
Peckham PH, Keith MW, Kilgore KL, Grill JH, Wuolle KS, Thrope GB, et al. Efficacy of an implanted neuroprosthesis for restoring hand grasp in tetraplegia: a multicenter study. Arch Phys Med Rehabil. 2001;82:1380–8.
Berman SA, Young RR, Sarkarati M, Shefner JM. Injury zone denervation in traumatic quadriplegia in humans. Muscle Nerve. 1996;19:701–6.
Brandstater ME, Dinsdale SM. Electrophysiological studies in the assessment of spinal cord lesions. Arch Phys Med Rehabil. 1976;57:70–4.
Gorman PH, Kikta DG, Peckham PH. Neurophysiologic evaluation of lower motor neuron damage in tetraplegia. Muscle Nerve. 1998;21:1321–3.
Li L, Li X, Liu J, Zhou P. Alterations in multidimensional motor unit number index of hand muscles after incomplete cervical spinal cord injury. Front Hum Neurosci. 2015;9:238.
Peckham PH, Mortimer JT, Marsolais EB. Upper and lower motor neuron lesions in the upper extremity muscles of tetraplegics. Paraplegia. 1976;14:115–21.
Yang JF, Stein RB, Jhamandas J, Gordon T. Motor unit numbers and contractile properties after spinal cord injury. Ann Neurol. 1990;28:496–502.
Grumbles RM, Thomas CK. Motoneuron death after human spinal cord injury. J Neurotrauma. 2017;34:581–90.
Stilwill EW, Sahgal V. Histochemical and morphologic changes in skeletal muscle following cervical cord injury: a study of upper and lower motor neuron lesions. Arch Phys Med Rehabil. 1977;58:201–6.
Miller J, Gollee H, Purcell M. Ultrasound imaging as a diagnostic tool to assess the functional status of muscles after a spinal cord injury. Ultrasound Med Biol. 2021;47:386–97.
Mohseny B, Nijhuis TH, Hundepool CA, Janssen WG, Selles RW, Coert JH. Ultrasonographic quantification of intrinsic hand muscle cross-sectional area; reliability and validity for predicting muscle strength. Arch Phys Med Rehabil. 2015;96:845–53.
Simon NG, Ralph JW, Lomen-Hoerth C, Poncelet AN, Vucic S, Kiernan MC, et al. Quantitative ultrasound of denervated hand muscles. Muscle Nerve. 2015;52:221–30.
Fukunaga T, Roy RR, Shellock FG, Hodgson JA, Edgerton VR. Specific tension of human plantar flexors and dorsiflexors. J Appl Physiol. 1996;80:158–65.
Palakshappa JA, Reilly JP, Schweickert WD, Anderson BJ, Khoury V, Shashaty MG, et al. Quantitative peripheral muscle ultrasound in sepsis: Muscle area superior to thickness. J Crit Care. 2018;47:324–30.
Pillen S, Tak RO, Zwarts MJ, Lammens MM, Verrijp KN, Arts IM, et al. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol. 2009;35:443–6.
Reimers K, Reimers CD, Wagner S, Paetzke I, Pongratz DE. Skeletal muscle sonography: a correlative study of echogenicity and morphology. J Ultrasound Med. 1993;12:73–7.
Ríos-Díaz J, Del Baño-Aledo ME. Quantitative neuromuscular ultrasound analysis as biomarkers in amyotrophic lateral sclerosis. Eur Radio. 2019;29:4266–75.
Gunreben G, Bogdahn U. Real-time sonography of acute and chronic muscle denervation. Muscle Nerve. 1991;14:654–64.
Schwennicke A, Bargfrede M, Reimers CD. Clinical, electromyographic, and ultrasonographic assessment of focal neuropathies. J Neuroimaging. 1998;8:136–43.
Thomas CK. Contractile properties of human thenar muscles paralyzed by spinal cord injury. Muscle Nerve. 1997;20:788–99.
Castro MJ, Apple DF Jr., Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80:373–8.
Malisoux L, Jamart C, Delplace K, Nielens H, Francaux M, Theisen D. Effect of long-term muscle paralysis on human single fiber mechanics. J Appl Physiol. 2007;102:340–9.
Bae JS, Sawai S, Misawa S, Kanai K, Isose S, Kuwabara S. Differences in excitability properties of FDI and ADM motor axons. Muscle Nerve. 2009;39:350–4.
Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. Autops study J Neurol Sci. 1973;18:111–29.
Castro MJ, Apple DF Jr., Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999;86:350–8.
Zijdewind I, Thomas CK. Spontaneous motor unit behavior in human thenar muscles after spinal cord injury. Muscle Nerve. 2001;24:952–62.
Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee MT, et al. A multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord. 2007;45:275–91.
Misirlioglu TO, Ozyemisci Taskiran O. Reliability of sonographic muscle thickness measurements of the thenar and hypothenar muscles. Muscle Nerve. 2018;57:E14–E17.
Jacobson MD, Raab R, Fazeli BM, Abrams RA, Botte MJ, Lieber RL. Architectural design of the human intrinsic hand muscles. J Hand Surg Am. 1992;17:804–9.
Abraham A, Drory VE, Fainmesser Y, Lovblom LE, Bril V. Quantitative sonographic evaluation of muscle thickness and fasciculation prevalence in healthy subjects. Muscle Nerve. 2020;61:234–8.
Close PJ, Stokes MJ, L’Estrange PR, Rowell J. Ultrasonography of masseter muscle size in normal young adults. J Oral Rehabil. 1995;22:129–34.
Abraham A, Fainmesser Y, Drory VE, Bril V. Split-hand phenomenon in motor neuron diseases: sonographic assesment of muscle thickness. Clin Neurophysiol. 2020;131:1721–5.
Edgerton VR, Roy RR, Allen DL, Monti RJ. Adaptations in skeletal muscle disuse or decreased-use atrophy. Am J Phys Med Rehabil. 2002;81:S127–47.
Gordon T, Tyreman N. Sprouting capacity of lumbar motoneurons in normal and hemisected spinal cords of the rat. J Physiol. 2010;588:2745–68.
Kern H, Hofer C, Mödlin M, Mayr W, Vindigni V, Zampieri S, et al. Stable muscle atrophy in long-term paraplegics with complete upper motor neuron lesion from 3- to 20-year SCI. Spinal Cord. 2008;46:293–304.
Krasilovsky G. Nerve conduction studies in patients with cervical spinal cord injuries. Arch Phys Med Rehabil. 1980;61:204–9.
Kern H, Carraro U, Adami N, Biral D, Hofer C, Forstner C, et al. Home-based functional electrical stimulation rescues permanently denervated muscles in paraplegic patients with complete lower motor neuron lesion. Neurorehabil Neural Repair. 2010;24:709–21.
Abraham A, Drory VE, Fainmesser Y, Algom AA, Lovblom LE, Bril V. Muscle thickness measured by ultrasound is reduced in neuromuscular disorders and correlates with clinical and electrophysiological findings. Muscle Nerve. 2019;60:687–92.
Aisen ML, Brown W, Rubin M. Electrophysiologic changes in lumbar spinal cord after cervical cord injury. Neurology. 1992;42:623–6.
Needham-Shropshire BM, Klose KJ, Tucker ME, Thomas CK. Manual muscle test score and force comparisons after cervical spinal cord injury. J Spinal Cord Med. 1997;20:324–30.
Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI. Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord. 2006;44:143–51.
Funding
This study was supported by the Guangzhou Science and Technology Program (Grant nos 201904010256 and 201704030039).
Author information
Authors and Affiliations
Contributions
CSK, HL, CNZ, and XY conceived the idea of the study and contributed to the writing of the protocol. HL, CSK, and CNZ acquired and analyzed the data; HL and XY recruited the participants. XY provided all medical support. CSK wrote the first version of the manuscript. All authors read and contributed to the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Klein, C.S., Liu, H., Zhao, C.N. et al. Quantitative ultrasound imaging of intrinsic hand muscles after traumatic cervical spinal cord injury. Spinal Cord 60, 199–209 (2022). https://doi.org/10.1038/s41393-021-00653-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00653-1