Abstract
Study design
Clinical trial.
Objectives
We used a single-blind parallel-group design to test the feasibility and preliminary efficacy of a telehealth-based physical activity counseling intervention to increase physical fitness in people with SCI.
Setting
Seattle, Washington, United States.
Methods
We recruited under-active, manual wheelchair-using adults at least 1-year post-SCI who had at least two cardiometabolic risk factors/diseases. Participants underwent baseline tests of peak cardiorespiratory fitness; lipids, glucose and insulin; muscle and fat mass; self-reported physical activity, depression, pain and other factors. Participants were assigned 1:1 to treatment vs. usual care (UC) control conditions via concealed computerized randomization. Treatment was delivered via telephone and adapted from the 16-session Diabetes Prevention Program. All baseline tests were repeated at 6 months. Prespecified feasibility goals were to recruit at least nine participants/quarter and retain 85% with complete fitness testing at 6 months. Prespecified efficacy goals were to demonstrate at least a medium treatment effect size (0.50) on fitness, self-reported physical activity, and other outcomes.
Results
Seven participants were randomized to treatment, 8 to UC over 15 months. Maximum recruitment was only 5.4 participants/quarter. Thirteen (87%) of participants were retained. The effects of treatment on fitness and most cardiometabolic risk factors did not meet expectations, whereas the effects on self-reported physical activity, depression, and pain did meet expectations.
Conclusions
The study did not meet key efficacy and feasibility objectives, yet there were some promising effects on self-report measures and lessons to be learned for designing future trials.
Similar content being viewed by others
Introduction
Physical inactivity is a major problem facing people with SCI [1]. Approximately 50% of people with an SCI engage in no leisure-time physical activity such as sports, wheeling, or walking for pleasure, or therapeutic exercise [1]. SCI-related physical inactivity is influenced by injury level and severity, but also by mutable factors such as deconditioning, fatigue, reversible weakness, environmental barriers, and behavior patterns [2]. In people with physical disabilities, inactivity leads to a downward spiral involving loss of muscle mass, decreased resting energy expenditure, decreased total energy expenditure, and obesity [3]. Physical inactivity is linked with increased risks for cardiovascular disease, dyslipidemia, type-2 diabetes, insulin resistance, impaired cardiovascular structure and function, autonomic nervous system dysfunction [4], depression [5], pain, fatigue [6], and a poorer quality of life in persons with SCI [5]. One-quarter of healthy young persons with SCI do not have sufficient fitness to perform many essential activities of daily living, and people with paraplegia are barely more fit than persons with tetraplegia [7, 8].
Fortunately, clinic and laboratory-based aerobic conditioning and circuit training studies provide compelling evidence that people with SCI can improve their cardiorespiratory fitness and by doing so can partially reverse cardiovascular disease risk factors, enhance quality of life and improve elements of subjective well-being. The general paradigm is that structured exercise increases cardiorespiratory fitness (typically measured by changes in peak oxygen consumption--V̇O2 peak). A systematic review of 189 studies of people with chronic SCI show that 20–44 min of moderate-to-vigorous upper body aerobic exercise 2–5 times per week increases cardiorespiratory fitness, strength, body composition, and cardiovascular risk [9]. Increased fitness has a beneficial effect on atherogenic lipid profiles, elevated body fat and a cluster of other cardiometabolic risk factors such as glucose tolerance, insulin resistance, and hypertension [2]. Exercise training reduces low-density lipoprotein cholesterol (LDL), raises (good) high-density lipoprotein cholesterol (HDL) and can reverse the total cholesterol (TC):HDL ratio to near normal [10]. Exercise improves insulin sensitivity [11] as well as depression [12], pain [13], and quality of life [2, 5, 12, 14, 15].
Despite the apparent benefits of structured exercise, most people with SCI lack access to clinic-based conditioning programs [16]. At the time the study was conducted, no community-based physical activity intervention had demonstrated significant improvement in cardiorespiratory fitness. One relevant study may have been limited by not using an evidence-based counseling approach or experienced behavioral therapists [17]. Two randomized controlled trials of community-based physical activity promotion demonstrated increased self-reported physical activity, but lacked objective measures of fitness [18, 19].
This single-blind, parallel-group randomized controlled trial tested the feasibility and preliminary efficacy of a 6-month telehealth program to improve cardiorespiratory fitness via increased community-based moderate-to-vigorous physical activity (MVPA). The intervention was adapted from the evidence-based physical activity modules of the highly successful Diabetes Prevention Program (DPP), and was delivered by a psychologist via individual telephone counseling sessions [20]. Based on published advice regarding the conduct of pilot research [21, 22], our primary goals were to determine whether the study meets predetermined feasibility objectives. Our secondary goals were to estimate the effect size of the intervention on outcome measures.
Therefore, this pilot feasibility study had the following pre-specified objectives: (1) Enrollment of at least nine participants per quarter. This rate of enrollment is our benchmark for feasibility because it would yield 126 subjects in a 5-year grant, assuming an enrollment period of 3.5 years and 85% retention. This sample size would be sufficient to detect a moderate (0.5) effect size (80% power; alpha level p < 0.05, one-tailed) [23]; (2) at least 85% of randomized participants would be retained to reduce risk of bias [24]; (3) the psychologist would complete at least 80% of planned calls; (4) <10% of participants would report that they experienced injuries or other adverse events that they attribute to study-related procedures and there will be no serious adverse events; (5) among completers 95% of data would be complete; and (6) the effect size (Cohen’s d) of the intervention compared to usual medical care (UC) controls on cardiorespiratory fitness (primary outcome) as measured by V̇O2 peak from baseline to 6 months would be at least 0.50 to justify a single-site study and 0.30 to justify a multi-site trial (n = 278 subjects would be needed for a full trial assuming effect size = 0.30, power = 0.80, two-tailed test at p < 0.05). Lastly, without controlling for multiple comparisons we explored the effect of the intervention on multiple secondary outcomes such as cardiometabolic risk factors, MVPA, depression, pain, and quality of life and highlighted effect sizes of at least 0.50 (medium effect) in the expected direction.
Methods
Participants
The study was advertised via SCI newsletter subscribers (n = 2100), SCI outpatient clinic (n = 371 unique patients per year), research participant registry (n = 1635), and our website (>20,000 visits per month). People were invited to take part in 16-session telehealth program to increase physical activity. Inclusion criteria were: ages 18–70, at least 1 year post SCI, American Spinal Injury Association Impairment Scale A SCI at the C6 or below or incomplete injury at any level, uses a manual wheelchair for at least 50% of the time, engages in less than 150 min per week of MVPA [25], has at least two cardiometabolic risk factors (Body Mass Index greater than 21 kg/m2, fasting HDL cholesterol less than or equal to 40 mg/dL, fasting triglycerides greater than or equal to 150 mg/dL, fasting glucose greater than or equal to 100 mg/dL, and blood pressure greater than 119/79 mmHg). All participants had a physician approval to exercise.
Initially, we excluded people diagnosed and treated for diabetes, hyperlipidemia, or hypertension. After enrollment began, these criteria were found to be overly restrictive and eliminated. Other exclusion criteria were: BMI greater than 39, medically diagnosed ischemic heart disease; unstable angina, dysrhythmia or unstable autonomic dysreflexia; recent osteoporotic fracture, tracheostomy, already engaged in a structured diet or exercise program within 6 months, current pressure injury, current substance dependence, psychosis, severe chronic upper extremity pain, surgery pending within 6 months, recurrent infection or illness requiring hospitalization, or participation in another intervention study.
Procedures
The research study protocol was approved by the institutional review board at the University of Washington. This trial was registered on Clinicaltrials.gov (NCT02225028) prior to study enrollment. A trained research coordinator (MT) was in charge of recruitment, enrollment and assessments. She led prospective participants through a multi-step enrollment process including preliminary (self-report) eligibility screening, written informed consent and release of medical information, medical chart review, physician approval to exercise, and baseline examination.
The research coordinator oversaw the baseline assessment process which was conducted at the University of Washington Medical Center’s Clinical Research Center (CRC) and consisted of blood work, dual energy x-ray absorptiometry scan (DXA) scan, self-report measures, and peak fitness test. The baseline assessments were conducted in the morning due to fasting requirements. Participants were asked to abstain from caffeine and alcohol for 24 h before testing and from exercise for 48 h. The CRC nursing staff measured blood pressure, weighed the participant, drew blood for the lipid panel, blood glucose and insulin tests, and performed the DXA scan. Next, the research coordinator collected self-report data. Participants were given a light snack and a drink and then underwent cardiorespiratory fitness testing performed by the CRC exercise physiologist.
Immediately following the baseline assessment, the study biostatistician randomized participants 1:1 into treatment (6 months of physical activity counseling) vs. UC. Randomization was computer-generated, stratified by level of injury (paraplegia vs. tetraplegia), and concealed by using a permuted block randomization (blocks of 2 or 3). The biostatistician emailed group assignment to the psychologist (JD), while the research coordinator and CRC staff were kept blinded. The psychologist (JD) contacted treatment group members within one week of randomization to set-up physical activity counseling sessions. Those assigned to usual care were informed of their allocation via letter. After the 6-month treatment phase, participants returned to the medical center to repeat all the assessments conducted at baseline.
Treatment and control groups
Physical activity intervention
The treatment group received a multi-component intervention consisting of a free home exercise tool-kit and a 16-session physical activity counseling curriculum delivered by the psychologist via telephone calls. We mailed participants a free home exercise tool-kit that included a set of exercise bands with soft grips, ankle strap and door anchor (Black Mountain Products) as well as a DVD that provided verbal instructions and videos in which people with SCI demonstrated stretching, aerobic exercise, and strength training routines designed specifically for people with paraplegia or tetraplegia [26]. The treatment group received up to 16 physical activity telephone counseling sessions spread over 24 weeks. The content of the counseling sessions was based on the community-based physical activity component of the widely-replicated Diabetes Prevention Program (DPP) [20]. The exercise physiologist (PB) and psychologist (JD) adapted the DPP program for persons with SCI. (See Supplementary Appendix 1 for description of adapted counseling sessions.) The psychologist met with participants at mutually convenient times and used motivational interviewing techniques [27] as well as SMART (specific, measurable, attainable, realistic, and timely) [28] goals setting to promote adherence to the physical activity program. The ultimate goal of the overall program was to have the participant achieve at least 150 min of MVPA per week (or higher if their baseline was already near 150 min) [25]. Participants could exercise in their home or at community facilities, if available.
Usual care
This group received a letter informing them of their test results and randomization status. They were advised to seek medical care to make lifestyle changes such as diet and exercise to address their cardiometabolic disease or risk factors.
Outcome measures
Cardiorespiratory fitness
The primary outcome was cardiorespiratory fitness defined as peak oxygen consumption (V̇O2 peak). We used open-circuit spirometry during a continuous graded exercise test at 60 RPM on a Monark Rehab Trainer Model 881-E arm crank ergometer to measure V̇O2 peak. The test was conducted by an exercise physiologist and monitored by a cardiologist for safety, using 12-lead EKG. Testing began at a workload of 10 Watts (W) for persons with tetraplegia or 20 W for those with paraplegia and was increased by 10 or 20 W, respectively every 3 min until volitional exhaustion was reached. In accordance with the American College of Sports Medicine Guidelines for Exercise Testing and Training, 7th Edition [29], peak work was defined as volitional exhaustion, inability to maintain targeted workload, or the point at which increasing workload failed to further increase V̇O2.
Cardiometabolic risk factors
We calculated BMI via height and weight and measured waist circumference. We used DXA (GE Lunar Prodigy) to measure grams of lean and fat body mass. Insulin sensitivity index (ISI) was measured during an oral glucose tolerance test and calculated as: 1002/[(FPG x FPI)x (GMEAN x IMEAN)], where FPG = fasting plasma glucose, FPI = fasting plasma insulin, and GMEAN and IMEAN reflect the 2-hour (75 g) OGTT averages for glucose and insulin, respectively. From a standard lipid panel, we calculated TC:HDL and Low Density Lipoprotein:HDL ratios.
Patient-reported outcomes
These measures were obtained via structured interviews conducted by the research coordinator. We used the Physical Activity Recall Assessment for SCI (PARA-SCI) which is a reliable and valid measure of participant-reported minutes per week of leisure time MVPA [30]. In order to determine whether any weight loss or changes in body composition were attributable to changes in diet, we collected data from a 3-day food log to measure total calories as well as grams of fat, carbohydrates, and protein consumed. To assess whether the physical activity intervention resulted in changes in pain, pain interference, or shoulder pain specifically we administered the Brief Pain Inventory intensity and interference scales [31] and the Wheelchair User’s Shoulder Pain Index [32]. We used the Patient Health Questionnaire-9 [33], the Medical Outcomes Study Short Form-12 (SF-12) [34], and the World Health Organization Quality of Life Scale (WHOQOL-BREF) [35] to determine whether the intervention resulted in changes in depressive symptoms or health-related quality of life. Finally, we measured participant confidence (1 not at all confident to 7 completely confident) in their ability to schedule physical activity (4 items) and to be active despite general barriers (6 items) and facility barriers (6 items). These measures have played an important role in prior theory-based research on physical activity in SCI and have demonstrated reliability and validity [18, 36].
Statistical methodology
Differences in patient and injury characteristics (Table 1) were assessed for statistical imbalance between the two treatment groups at baseline using Mann-Whitney tests (continuous variables) and Fisher’s exact tests (categorical variables). Treatment effects on outcome (Tables 2 and 3) were assessed using t-tests on the change score from baseline to 6 months, with the two subjects who were not followed for outcome excluded from the analysis. The distributions of the change scores were assessed for normality using the Shapiro-Wilk test, with the four measures failing this test noted. Unstandardized effect sizes were reported as the difference in the mean change scores, and standardized effect sizes were reported as Cohen’s d. All statistical analyses used a two-sided alpha of 0.05 as the significance threshold, and all primary analyses were interpreted in the context of multiple comparisons per Benjamini–Hochberg.
Results
We enrolled participants from November 2014 until January of 2016. Enrollment was cut short by the unexpected closing of the fitness testing lab. Fifteen persons, eleven males (73%) and four females participated in the trial (see Table 1). Eighty percent of the participants were White, 13% reported mixed racial background, and 7% reported “Other” racial background. Two persons (7%) reported being Hispanic. Mean age was 52 (±13) years old and participants were on average 16 (±13) years post-SCI. Two persons (13%) had cervical injuries and the remainder had paraplegia. Eight persons were randomly assigned to usual care and seven to the exercise condition. There were no statistically significant imbalances between the two groups on demographic or clinical variables or on any of the outcome measures at baseline. Mean rating of perceived exertion (RPE) during the baseline fitness test was significantly higher in the UC (18.4 ± 2.3) vs. exercise condition (17.1 ± 1.2; p = 0.029; data not shown).
Feasibility and safety outcomes
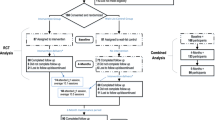
We did not meet our primary feasibility benchmark, a recruitment rate of at least 9 participants per quarter. We recruited only 2.0 persons per quarter with our original exclusion criteria and 5.4 persons per quarter after we relaxed our eligibility criteria to include people with diabetes, hyperlipidemia, or hypertension. Of the 122 persons referred or self-referred to the study, nearly 38% did not return our screening calls. Approximately 12% of the total referred were enrolled and randomized (see Fig. 1).
We exceeded our goal of retaining at least 85% of randomized participants. Fourteen of 15 participants (93%) completed at least one 6-month measure and 13 (87%) completed the primary outcome, the 6-month cardiorespiratory fitness exam. The psychologist completed on average 10 out of the planned 16 counseling sessions per participant (62.5%; range 3–16) vs. our goal of 80%. Information on participant level-counseling is presented in Supplementary Appendix 2. There was one adverse event attributable to the study and no serious adverse events. The adverse event involved the arm of the DXA scanner striking a participant’s knee as it was returning to the start position. Physician examination detected no injury. Among the 14 who finished the study, 95% of all data was complete. Thirteen participants completed 100% of all measures. One participant completed 34% of the measures.
Preliminary efficacy outcomes
The effect size for the primary efficacy outcome, cardiorespiratory fitness as measured by V̇O2 peak, was very small (0.06; see Table 1) and nonsignificant. According to our pre-specified study aims, an effect size below 0.30 indicates that the intervention is not potentially effective as a means of improving fitness and needs to be redesigned.
Among the secondary cardiorespiratory and cardiometabolic measures, only the effect of the intervention on systolic blood pressure met the medium effect size threshold of 0.50 in the expected direction (see Table 2). In general, the physical activity intervention had no clear pattern of effects on these variables.
Among the participant self-reported outcomes, the intervention had a medium or larger effect on six variables (see Table 3). There was a medium effect of the intervention in the expected direction on self-reported minutes per week of MVPA relative to the UC group. The intervention group reported a large drop in depression severity and likelihood of meeting criteria for probable major depressive disorder, relative to UC controls. Pain intensity increased in both groups, but significantly less in the treatment group. Pain interference declined in the intervention group, but increased among UC. Finally, the intervention had an unexpected negative effect in the area of facility barriers. The treatment group reported significantly decreased confidence in their abilities to use exercise equipment and access help at a community fitness center relative to UC controls.
Discussion
The purpose of this pilot study was to determine whether the tele-health intervention was feasible and had an effect on cardiorespiratory fitness (V̇O2 peak) that was large enough to justify a fully powered efficacy study. The pilot study did not meet key efficacy and feasibility objectives, yet there were some promising results and useful lessons to be learned from the trial.
Our original study goal was to target persons at risk for cardiometabolic diseases and exclude those already diagnosed with diabetes, hypertension, or hyperlipidemia. We quickly found that these exclusions would severely limit our ability to recruit interested participants. Moreover, the scientific literature indicates that exercise is a safe and potentially effective intervention for people with these diagnoses [37, 38]. After relaxing these exclusion criteria our recruitment rate more than doubled from 2.0 to 5.4 participants recruited per quarter. Future researchers should be cautious not to adopt overly restrictive eligibility criteria for reasons having to do with obtaining a sufficient sample size and in order to benefit the greatest possible fraction of people with SCI.
Despite extensive website, email, and clinic-based advertising and having access to a fairly large population of persons with SCI, only 122 persons were referred or self-referred to the trial and of those, 46 persons did not respond to screening attempts, seven declined to participate and 15 were randomized. These numbers suggest much less interest in or greater barriers to physical activity than we anticipated. Our experience is not unique. Indeed, the average study sample size derived from a scoping review of 14 SCI exercise studies was only 24 persons [39]. Survey research suggests that the vast majority of people with SCI either do exercise somewhat or report that they want to exercise [40]. Yet, among individuals with SCI negative beliefs and perceived external barriers likely contribute to low exercise participation such as thinking exercise is too difficult, not safe, and not of interest, or not knowing how to exercise as well as not having exercise equipment or not knowing of an accessible fitness center [40].
These findings in conjunction with general health behavior change models such as the Health Action Process Approach (HAPA) [41] and can guide recruitment efforts. For example, the HAPA model highlights how beliefs about one’s ability to exercise, the benefits of exercise, and the risks of exercising or not exercising determine whether people form intentions to exercise or take action. We may have improved study recruitment if we created study ads that highlighted important benefits of exercise, explained how the study would make exercising easier (i.e., provide free exercise equipment, boost motivation and focus on ways to exercise in their home environment), and provided evidence that exercise is safe for people with SCI [42]. We also could have described the risks of not exercising for people with SCI such as developing cardiometabolic syndrome [43].
Among those persons whom we were able to screen, our study procedures generally performed well compared to prior research. Compared to the scoping review [39], we a had similar rate of people declining to participate (6 vs. 5%) and lower rate of drop-out (7 vs. 16%). We had a higher rate of exclusions (62 vs. 35%) mostly due to having screened 24 persons who were interested in the study, but lived too far from our site to undergo fitness testing. To avoid this problem, future researchers who use telehealth to promote physical activity should consider objective outcome measures that can be obtained without requiring the participant come to the research center. Enrollment into our study was also limited by the fact our only on-campus fitness-testing center closed unexpectedly for financial reasons. Therefore, recruitment rate estimates from this study may underestimate the potential reach of a telerehabilitation approach to physical activity promotion in people with SCI.
The intervention did not produce a sufficient dose of physical activity to have an effect on cardiorespiratory fitness (V̇O2 peak). Nevertheless, there was a moderate size effect (0.62) on a valid measure of self-reported MVPA. The size of the effect on self-reported MVPA is similar to the effect size produced by other telehealth interventions in SCI [18, 19]. In order to produce higher quality research in this area, we need more objective indicators of physical activity frequency, intensity, and duration that are valid in SCI. Research on the reliability and validity of wearable accelerometry in people who use manual wheelchairs is emerging, including the use of individually tailored cut-points for defining MVPA [44]. The use of wearable accelerometry has the advantage of permitting investigators to monitor (and potentially influence) the frequency, intensity, and duration of participant physical activity remotely in real-time via devices that can be connected to the internet.
The effects of the intervention on depression and pain are promising and consistent with prior research. Hicks et al. [12] reported that a supervised exercise program three times per week for 9 months resulted in sustained euthymia and decreased pain compared to increased depression and pain among wait-list controls. A small controlled trial of Iyengar yoga resulted in decreased depression compared to wait-list controls [45]. Multiple studies have demonstrated decreased shoulder pain as a result of exercise interventions [46]. The efficacy of exercise interventions for major depression in the general population is now quite compelling [47].
We found that this community-based physical activity intervention was safe. In addition, participant retention rates and data completeness also exceeded expectations. However, the average number of completed calls (10 of 16) did not meet our goal. Anticipating and planning to cope with facility and other barriers to physical activity was contained in sessions 9–12 of our curriculum. These topics are important components of successful physical activity interventions [18] and most participants missed some or all of this material. This may explain why the intervention group reported decreased confidence in their ability to use exercise facilities and resources relative to the UC group. Decreased confidence in their ability to use exercise facilities and resources also me be attributable to the fact that most participants chose to be more active in their home environment rather than in a gym.
To put the results of this study in the context of recent research, at least three trials have now been published that used objective outcome measures to assess the impact of community-based physical activity or exercise interventions in people with SCI. One study used three wirelessly synched wearable activity monitors to show that a 6-month intervention consisting of motivational interviewing, feedback goals setting, home visit and information delivered by physical or occupational therapists resulted in greater wheeled physical activity at 6 and 12 months after discharge from inpatient rehabilitation compared to no treatment controls [48]. Another project demonstrated that inactive adults with chronic paraplegia exposed to a home-based graded arm ergometry program four times per week for 6 weeks had improved cardiorespiratory fitness, decreased serum fasting insulin, and insulin resistance compared to “lifestyle maintenance” group [49]. The third study used a theory-based intervention consisting of a personal training session followed by eight weekly behavioral coaching sessions to support adherence to a tailored exercise program delivered in-person or via Skype by the investigator who is also an experienced personal trainer [50]. Compared to wait-list controls, the intervention group had 17% more accelerometer-based physical activity, 19% higher V̇O2 peak during fitness testing and five-times greater self-reported MVPA. Features of these studies that contrast with the current study and may have contributed to their success include: (1) using shorter training periods (6–8 weeks vs. 6 months), (2) offering participants a graded in-home arm ergometry program, (3) having the intervention delivered by physical trainers, physical therapists or occupational therapists rather than a psychologist, and (4) tailoring the use of behavior change techniques to the participant rather than providing them in a fixed order [50]. Recent studies that have focused on mechanisms of increased exercise in people with SCI have emphasized the importance of increasing exercise self-efficacy, anticipating problems and barriers and making plans to cope with them proactively, reducing pain disability, and reducing helplessness [50, 51]. Strategies that seem crucial to the success of exercise interventions in general are using sound behavior change theory [41] and applying evidence-based behavior change methods [50, 51]. Taken together, these studies show that a wide range of people with SCI who are sedentary and have upper extremity function can become significantly more active, fit, and healthy via community-based interventions that combine expertise from exercise science and behavioral science.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ginis KA, Arbour-Nicitopoulos KP, Latimer AE, Buchholz AC, Bray SR, Craven BC, et al. Leisure time physical activity in a population-based sample of people with spinal cord injury part ii: Activity types, intensities, and durations. Arch Phys Med Rehabil. 2010;91:729–33.
Nash MS. Exercise as a health-promoting activity following spinal cord injury. J Neurol Phys Ther. 2005;29:87–103.
Rimmer JH, Schiller W, Chen MD. Effects of disability-associated low energy expenditure deconditioning syndrome. Exerc Sport Sci Rev. 2012;40:22–29.
Jacobs PL, Nash MS. Exercise recommendations for individuals with spinal cord injury. Sports Med. 2004;34:727–51.
Ginis KAM, Jetha A, Mack D, Hetz S. Physical activity and subjective well-being among people with spinal cord injury: a meta-analysis. Spinal Cord. 2010;48:65–72.
Tawashy AE, Eng JJ, Lin KH, Tang PF, Hung C. Physical activity is related to lower levels of pain, fatigue and depression in individuals with spinal-cord injury: a correlational study. Spinal Cord. 2009;47:301–6.
Dearwater SR, LaPorte RE, Robertson RJ, Brenes G, Adams LL, Becker D. Activity in the spinal cord-injured patient: an epidemiologic analysis of metabolic parameters. Med Sci Sports Exerc. 1986;18:541–4.
Noreau L, Shephard RJ, Simard C, Pare G, Pomerleau P. Relationship of impairment and functional ability to habitual activity and fitness following spinal cord injury. Int J Rehabil Res. 1993;16:265–75.
van der Scheer JW, Martin Ginis KA, Ditor DS, Goosey-Tolfrey VL, Hicks AL, West CR, et al. Effects of exercise on fitness and health of adults with spinal cord injury: a systematic review. Neurology. 2017;89:736–45.
Nash MS, Jacobs PL, Mendez AJ, Goldberg RB. Circuit resistance training improves the atherogenic lipid profiles of persons with chronic paraplegia. J Spinal Cord Med. 2001;24:2–9.
de Groot P, Hjeltnes N, Heijboer A, Stal WBK. Effect of training intensity on physical capacity, lipid profile and insulin sensitivity in early rehabilitation of spinal cord injured individuals. Spinal Cord. 2003;41:673–9.
Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, et al. Long-term exercise training in persons with spinal cord injury: Effects on strength, arm ergometry performance and psychological well-being. Spinal Cord. 2003;41:34–43.
Mulroy SJ, Thompson L, Kemp B, Hatchett PP, Newsam CJ, Lupold DG, et al. Strengthening and optimal movements for painful shoulders (STOMPS) in chronic spinal cord injury: a randomized controlled trial. Phys Ther. 2011;91:305–24.
Hicks AL, Martin Ginis KA, Pelletier CA, Ditor DS, Foulon B, Wolfe DL. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: a systematic review. Spinal Cord. 2011;49:1103–27.
Nash M, van de Ven I, van Elk N, Johnson B. Effects of circuit resistance training on fitness attributes and upper-extremity pain in middle-aged men with paraplegia. Arch Phys Med Rehabil. 2007;88:70–75.
Rimmer JH, Riley B, Wang E, Rauworth A, Jurkowski J. Physical activity participation among persons with disabilities: barriers and facilitators. Am J Prev Med. 2004;26:419–25.
Froehlich-Grobe K, Aaronson LS, Washburn RA, Little TD, Lee J, Nary DE, et al. An exercise trial for wheelchair users: project workout on wheels. Contemp Clin Trials. 2012;33:351–63.
Arbour-Nicitopoulos KP, Ginis KA, Latimer AE. Planning, leisure-time physical activity, and coping self-efficacy in persons with spinal cord injury: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:2003–11.
Latimer A, Martin Ginis K, Arbour K. The efficacy of an implementation intention intervention for promoting physical activity among individuals with spinal cord injury: a randomized controlled trial. Rehabil Psychol. 2006;51:273–80.
The Diabetes Prevention Program Research Group. The diabetes prevention program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–71.
Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiat Res. 2011;45:626–9.
Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1.
Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet. 2002;359:781–5.
Physical Activity Guideline Writing Committee. Physical activity guidelines for Americans. Washington DC: Office of Disease Prevention and Health Promotion; 2008.
National Center on Physical Activity and Disability. Exercise program for individuals with spinal cord injuries: Tetraplegia VHS/DVD & Quick Series Booklet. National Center on Physical Activity and Disability; 2006. https://www.nchpad.org/369/2068/Exercise~Program~for~Individuals~with~Spinal~Cord~Injuries~~Tetraplegia~VHS~DVD.
Miller WR, Rollnick S. Motivational interviewing: helping people change. Third ed., New York, NY: The Guilford Press; 2012.
Bovend’Eerdt TJ, Botell RE, Wade DT. Writing smart rehabilitation goals and achieving goal attainment scaling: a practical guide. Clin Rehabi.l 2009;23:352–61.
Whaley MH, Brubaker PH, Otto RM, Armstrong LE. ACSM’s guidelines for exercise testing and prescription. 7th ed. Philadelphia, PA; Lippincott Williams & Wilkins; 2006.
Latimer AE, Ginis KA, Craven BC, Hicks AL. The physical activity recall assessment for people with spinal cord injury: validity. Med Sci Sports Exerc. 2006;38:208–16.
Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap. 1994;23:129–38.
Curtis KA, Roach KE, Applegate EB, Amar T, Benbow CS, Genecco TD, et al. Development of the wheelchair user’s shoulder pain index (WUSPI). Paraplegia. 1995;33:290–3.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13.
Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83.
Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the whoqol group. Qual Life Res. 2004;13:299–310.
Ginis KA, Tomasone JR, Latimer-Cheung AE, Arbour-Nicitopoulos KP, Bassett-Gunter RL, Wolfe DL. Developing physical activity interventions for adults with spinal cord injury. Part 1: a comparison of social cognitions across actors, intenders, and nonintenders. Rehabil Psychol. 2013;58:299–306.
Park S, Kim J, Lee J. Effects of exercise intervention on adults with both hypertension and type 2 diabetes mellitus: a systematic review and meta-analysis. J Cardiovasc Nurs. 2020. https://doi.org/10.1097/JCN.0000000000000651.
Rijal A, Nielsen EE, Hemmingsen B, Neupane D, Gaede PH, Olsen MH, et al. Adding exercise to usual care in patients with hypertension, Type 2 diabetes mellitus and/or cardiovascular disease: a protocol for a systematic review with meta-analysis and trial sequential analysis. Syst Rev. 2019;8:330.
Lai B, Cederberg K, Vanderbom KA, Bickel CS, Rimmer JH, Motl RW. Characteristics of adults with neurologic disability recruited for exercise trials: a secondary analysis. Adapt Phys Act Q. 2018;35:476–97.
Cowan RE, Nash MS, Anderson KD. Exercise participation barrier prevalence and association with exercise participation status in individuals with spinal cord injury. Spinal Cord. 2013;51:27–32.
Schwarzer R, Lippke S, Luszczynska A. Mechanisms of health behavior change in persons with chronic illness or disability: the Health Action Process Approach (HAPA). Rehabil Psychol. 2011;56:161–70.
Warms CA, Backus D, Rajan S, Bombardier CH, Schomer KG, Burns SP. Adverse events in cardiovascular-related training programs in people with spinal cord injury: a systematic review. J Spinal Cord Med. 2014;37:672–92.
Nash MS, Groah SL, Gater DR, Dyson-Hudson TA, Lieberman JA, Myers J, et al. Identification and management of cardiometabolic risk after spinal cord injury. J Spinal Cord Med. 2019;42:643–77.
Lankhorst K, Oerbekke M, van den Berg-Emons R, Takken T, de Groot J. Instruments measuring physical activity in individuals who use a wheelchair: a systematic review of measurement properties. Arch Phys Med Rehabil. 2020;101:535–52.
Curtis K, Hitzig SL, Bechsgaard G, Stoliker C, Alton C, Saunders N, et al. Evaluation of a specialized yoga program for persons with a spinal cord injury: A pilot randomized controlled trial. J Pain Res. 2017;10:999–1017.
Cratsenberg KA, Deitrick CE, Harrington TK, Kopecky NR, Matthews BD, Ott LM, et al. Effectiveness of exercise programs for management of shoulder pain in manual wheelchair users with spinal cord injury. J Neurol Phys Ther. 2015;39:197–203.
Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42–51.
Nooijen CF, Stam HJ, Bergen MP, Bongers-Janssen HM, Valent L, van Langeveld S, et al. A behavioural intervention increases physical activity in people with subacute spinal cord injury: a randomised trial. J Physiother. 2016;62:35–41.
Nightingale TE, Walhin JP, Thompson D, Bilzon JLJ. Impact of exercise on cardiometabolic component risks in spinal cord-injured humans. Med Sci Sports Exerc. 2017;49:2469–77.
Ma JK, West CR, Martin Ginis KA. The effects of a patient and provider co-developed, behavioral physical activity intervention on physical activity, psychosocial predictors, and fitness in individuals with spinal cord injury: a randomized controlled trial. Sports Med. 2019;49:1117–31.
Nooijen CF, Stam HJ, Schoenmakers I, Sluis TA, Post MW, Twisk JW, et al. Working mechanisms of a behavioural intervention promoting physical activity in persons with subacute spinal cord injury. J Rehabil Med. 2016;48:583–8.
Acknowledgements
We extend gratitude to Danielle Nacamuli, Chris Garbaccio, the UW Institute of Translational Health Sciences grant number UL1 TR002319, and the UW Medical Center Clinical Research Center staff.
Funding
This research was funded by the Craig H. Neilsen Foundation, Psychosocial Research Grant Number 290122. The funder had no role in the conduct, interpreting or reporting the results of the study.
Author information
Authors and Affiliations
Contributions
CHB was responsible for study design and concept, interpreted results, drafted the manuscript, approved final version, is accountable for the overall integrity of the work. He had full access to the data in the study and final responsibility for the decision to submit for publication. JRD contributed to treatment design, acquired data, interpreted results, revised the manuscript, approved final version, agrees to be accountable for the work, especially intervention delivery. PB contributed to treatment design, revised the manuscript, approved final version, agrees to be accountable for the work. DAC contributed to study design, revised the manuscript, approved final version, agrees to be accountable for the work. MMT acquired data, interpreted results, revised manuscript, approved of final version, agrees to be accountable for the work, especially data collection and integrity. JB analyzed data and interpreted results, revised the manuscript, approved final version, agrees to be accountable for the work. MSN contributed to study concept and design, interpreted results, revised the manuscript, approved final version, agrees to be accountable for the work.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Bombardier is a paid motivational interviewing trainer. The other authors declared no conflicts of interest.
Ethics
The study was approved by the University of Washington Human Subjects Review Board, Committee G, Application number 47327. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bombardier, C.H., Dyer, J.R., Burns, P. et al. A tele-health intervention to increase physical fitness in people with spinal cord injury and cardiometabolic disease or risk factors: a pilot randomized controlled trial. Spinal Cord 59, 63–73 (2021). https://doi.org/10.1038/s41393-020-0523-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-020-0523-6
This article is cited by
-
Effectiveness and feasibility of the workout on wheels internet intervention (WOWii) for individuals with spinal cord injury: a randomized controlled trial
Spinal Cord (2022)
-
Current Approaches in Telehealth and Telerehabilitation for Spinal Cord Injury (TeleSCI)
Current Physical Medicine and Rehabilitation Reports (2022)