Abstract
Locomotor training holds tremendous appeal to people with spinal cord injury who are wheelchair dependent, as the reacquisition of gait remains one of the most coveted goals in this population. For the last few decades this type of training has remained primarily in the clinical environment, as it requires the use of expensive treadmills with bodyweight support or complex overhead suspension tracks to facilitate overground walking. The development of powered exoskeletons has taken locomotor training out of the clinic, both improving accessibility and providing a potential option for community ambulation in people with lower limb paralysis. A question that has yet to be answered, however, is whether or not locomotor training offers a sufficiently intense stimulus to induce improvements in fitness or health. As inactivity-related secondary health complications are a major source of morbidity and mortality in people with SCI, it would be important to characterize the potential of locomotor training to not only improve functional walking ability, but also improve health-related fitness. This narrative review will summarize the key literature in this area to determine whether locomotor training challenges the cardiovascular, muscular or metabolic systems enough to be considered a viable form of exercise.
Similar content being viewed by others
Introduction
It is widely recognized that physical activity positively contributes to overall health and well-being, and evidence-based physical activity guidelines (PAGs) are now available for numerous populations, including those living with spinal cord injury (SCI) [1]. These guidelines indicate the minimum amounts of both aerobic and resistance-based exercise needed to improve both fitness and health outcomes in the SCI population. Clearly stated within the PAGs are both the time factor, i.e. how long one must exercise in each session, and the intensity of the effort needed in order to obtain fitness and/or health benefits. Briefly, the minimum threshold to improve fitness is 20 min of moderate-to-vigorous aerobic activity twice per week plus three sets of ten repetitions of strengthening exercises of major muscle groups twice per week. The minimum threshold to improve cardiometabolic health is 30 min of moderate-to-vigorous aerobic activity three times per week [1].
The terms physical activity and exercise are often used interchangeably, but there are differences between the two terms. Physical activity is a broader concept, describing any bodily movement carried out by skeletal muscles that requires metabolic energy, whereas exercise is considered to be a subcomponent of physical activity, and is characterized by more intentional movement that is planned, structured, and repetitive [2]. Both physical activity and exercise can be used to improve aspects of physical fitness (e.g. cardiovascular endurance, muscle strength and body composition). For non-athletic persons with SCI, any improvement in fitness is particularly beneficial, as this population is purportedly one of more inactive sectors of society [3], and many of the secondary co-morbidities associated with SCI, such as hyperlipidemia, glucose intolerance, type 2 diabetes, cardiovascular disease, are related to this inactive lifestyle [4, 5]. Thus, for people living with SCI, an improvement in physical fitness not only will enhance ability to carry out activities of daily living, it could potentially carry a host of important health-related benefits that may decrease the risk of secondary health complications [6].
Wheelchair treadmills, arm ergometers, recumbent elliptical machines and resistance training equipment are perhaps the most common modes of exercise for people with SCI who are unable to ambulate. In the last 20 years, there has been a tremendous emergence of training devices that can be used to facilitate upright locomotion in this population, but whether or not these devices are considered as “exercise training” equipment (with the goal of improving fitness) is still up to debate.
Modes of locomotor training
Recovery of locomotion is recognized as one of main priorities for people living with SCI [7], and numerous devices have been developed to assist with locomotor training in people with lower limb paralysis. Bodyweight-supported (BWS) treadmills allow for ambulatory training while a portion of one’s bodyweight is off-loaded. This type of intervention may or may not be augmented by electrical stimulation of the leg muscles to enhance muscle activation. The addition of a robotic orthosis (i.e. Lokomat™) to the treadmill eliminates the need for manual therapists and provides additional options related to various aspects of gait kinematics. In the over two decades that these devices have been utilized in the clinical setting, their efficacy in improving locomotor function has been validated, but it is still not clear whether they are superior to overground walking training [8, 9].
More recently, powered exoskeletons that can be used for overground locomotor training have received a great deal of attention, as they have the potential to be used in the community and/or home setting. These devices (e.g. Ekso™, ReWalk™, Indego™) are positioned over the paralyzed or weakened limbs to facilitate sit-to-stand, walking and, in some cases, climbing stairs. While these devices can be used by people with tetraplegia, most require a reasonable amount of trunk control to initiate starts and stops, and upper arm and hand function to operate either the controls or the assistive device being utilized. Furthermore, the cost of these powered exoskeletons is still quite prohibitive to the general population.
Much of the research attention on devices to improve locomotor ability has focused primarily on the capacity for functional gains and neural plasticity, and less on the potential for these devices to serve as an exercise stimulus that may be effective in improving fitness. While it appears that the added weight and passive resistance of powered exoskeletons typically requires users to expend more energy during walking at a given speed than what would normally be used for unassisted walking [10], it is not clear whether this “extra” energy requirement is of sufficient intensity to improve fitness and health in a user who is incapable of unassisted walking. Further, in the rehabilitation field, minimizing the metabolic energy cost of movement while wearing an exoskeleton has been considered to be advantageous, and a goal for new technology, as this would increase the potential for it to be used on a regular basis [11]. Of course, this improved metabolic efficiency in technological design would effectively decrease the potential utility of these devices to provide a sufficiently intense stimulus to qualify as exercise.
The following sections will aim to summarize locomotor training studies that have specifically evaluated aspects of health-related fitness in people with SCI.
Evidence supporting locomotor training as a means of improving cardiovascular fitness
It has been suggested that in order to improve aerobic capacity, a key metric of cardiovascular fitness, the exercise intensity must be equivalent to, at minimum, three times resting energy expenditure, or 3 METs [12]. Intensities between ~3 and 6 METs are considered to be “moderate” intensity, and those between 6 and 8.7 METs “vigorous” intensity [12]. Indeed, the PAGs for adults with SCI state the minimum requirement to increase cardiovascular fitness is 2, 20-min bouts of moderate-to-vigorous aerobic exercise per week [1]. With regard to locomotor training, it has been reported that walking on a BWS treadmill with manual assistance requires an energy demand approximately equivalent to 3 METs, or 50% of VO2 peak in persons with chronic incomplete SCI [13]. Walking on a treadmill with robotic assistance (e.g. Lokomat™) has been shown to require either less, the same, or even greater energy expenditure compared with manual assistance; the difference in energy cost seems to depend on the degree of “active contribution” on the part of the user, and whether or not the exoskeleton is providing 100% guidance support [13, 14].
In the past 5 years, a number of studies have evaluated the metabolic cost of walking overground with a robotic exoskeleton in people with SCI. Despite significantly slower walking speeds and virtually 100% guidance support by the robot, ambulating with the assistance of a powered exoskeleton has been shown to demand either a moderate [15, 16] or low–moderate [17, 18] level of intensity as indicated by either oxygen consumption or rating of perceived exertion (RPE). Although all of these studies were performed in people with motor-complete SCI, thus unable to voluntarily activate lower extremity muscles, it has been suggested that the increased activity of the upper extremity and trunk muscles during walking with the exoskeleton, necessary for both step initiation and utilization of canes or walker, contributes to the increased energy demand [15, 16]. In people with motor-incomplete SCI, the energy demand associated with walking with an exoskeleton has been shown to be quite variable, and very much dependent on the amount of assistance provided by the exoskeleton [19]. The presence/absence of muscle spasticity, as well as the possible anxiety associated with using the exoskeleton, have also been suggested to contribute to the energy demand associated with walking [19].
Appropriately measuring exercise intensity in the SCI population is often quite challenging. Exercise intensity is usually defined in the general population by a physiological measure such has heart rate (HR), but in people with SCI with injuries above T5, the HR response to exercise may be impaired due to dysfunction of sympathetic innervation of the heart [20]. Thus, the gauging of exercise intensity in the SCI population can be difficult, and very often needs to rely on subjective ratings of exertion as opposed to the direct measurement of HR.
Borg’s RPE scale [21] is commonly used as an indicator of exercise intensity in this population, either in the 6–20 format or the Category Ratio 0–10 (CR10) format. While RPE has been shown to be both valid and reliable in both the assessment of peak exercise capacity [22] and guiding exercise prescription [23], the confidence in using RPE as a valid indicator of intensity is still up for debate in the literature [24].
The RPEs associated with walking with a powered exoskeleton (ReWalk™) in people with paraplegia (ASIA A and B) have been shown to vary between 7 and 13 (“very very light” to “somewhat hard”) on the Borg 6–20 scale, which is just marginally lower than the “moderate” level of intensity that is suggested to be needed to improve cardiovascular fitness [15, 17]. The perceived effort associated with locomotor training is undoubtedly related to the degree of active muscle involvement and effort exerted by the participant; for instance, RPEs of 1 (“very easy”) have been reported while walking with 100% guidance support by the Lokomat, yet when the same participants walked on the BWS treadmill with manual assistance or overground with a robotic overhead track (ZeroG™), the associated RPEs (CR10) were 4 (“somewhat hard”) and 5 (“hard”), respectively [13]. Interpreting RPE data in the context of walking with an exoskeleton should be done with caution, given the fact that both the novelty of the device and/or the anxiety associated with using the device might contribute to a higher RPE. Further, it has been demonstrated that both the RPE and the associated oxygen demand of walking with an exoskeleton is directly related to the level and severity of the SCI [25].
It is understood that in order to improve cardiovascular fitness, it is necessary to not only exercise at a minimum moderate-level intensity, but also to maintain that intensity for at least 20 min 2–3 times/week [1]. A 12-week training study (5 times/week) in people with incomplete SCI randomized to either Lokomat™, BWS treadmill, overground walking or transcutaneous electrical stimulation showed significantly improved VO2 and walking speed in all conditions except the Lokomat™ [26]. These results are in contrast to a later study that reported significant increases in VO2 peak after 12 weeks of 3 ×/week training on the Lokomat™ [27], however the discrepant results could have been due to the amount of effort expended and/or feedback provided by the Lokomat™ to the study participants. The model of the Lokomat™ used in the Kressler et al. [26] study did not permit the same degree of guidance control and visual feedback as in the Gorman et. al. [27] study, which may have resulted in participants not exerting as much effort during training. Training studies employing powered exoskeletons are scarce. While there are reports of increases in mean arterial pressure and total walking time after either five consecutive days of training [28], or once-weekly training for 10–15 weeks [29], both studies using the Ekso™, there is still insufficient evidence in the literature to determine if these locomotor devices are either feasible to use on a regular basis or challenging enough, with regard to intensity, to induce improvements in aerobic capacity. Table 1 summarizes the results from studies that have evaluated outcomes related to cardiovascular fitness after both single bouts and more long-term locomotor training.
It is noteworthy that a recent systematic review evaluating the effectiveness of training with overground robotic exoskeletons on indices of cardiovascular demand revealed that locomotor training with these devices is not sufficient to induce any significant or clinically important changes in either HR or BP [30]. It was noted, however, that the studies included in the review were highly variable in terms of level and severity of injury across participants, and the inclusion of participants with injuries above T5, with accompanying sympathetic dysfunction, may have contributed to the variability in responses in these two outcomes. It was also noted that studies that reported RPE values found that they were in the ranges associated with light-to-moderate exercise intensity, and that participants across the various studies were able to tolerate longer training sessions and walk greater distances at a lower RPE after training. The authors took this as possible evidence that regular use of these devices might impart some health benefits.
Evidence supporting locomotor training as a means of improving muscular fitness
Any training-induced increase in muscle size, strength or function can be interpreted as an increase in muscular fitness. After SCI, the musculature of the paralyzed limbs undergoes a pronounced atrophy together with a shift in fibre type distribution towards the fast twitch, type IIx, fibre types [31]. These changes, concomitant with a decrease in muscle oxidative capacity, contribute to an increase in muscle fatiguability, which presents a significant challenge to rehabilitation strategies. Fortunately, as long as there remains a viable nerve–muscle connection after SCI, the evidence suggests that paralyzed muscle retains the potential for both muscle hypertrophy and fibre type change in response to training.
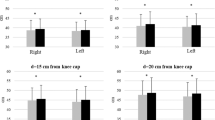
With regards to muscle size at the whole muscle level, increases in lean mass or muscle cross-sectional area have been reported after manual BWS treadmill training in both chronic [32, 33] and acute [34] SCI. Participants in those studies had motor-incomplete SCI (American Spinal Injury Association Impairment Scale (AIS) C) and training protocols lasted between 9 and 36 weeks. Less is known about the effects of training on robotic exoskeletons to improve muscle size, but one recent study showed significant increases in leg lean mass and calf cross-sectional area after 6 weeks of thrice-weekly training with the Ekso™ in people with complete SCI [35].
Only two studies have utilized the muscle biopsy technique to assess fibre size and fibre type distribution in the vastus lateralis after locomotor training in people with SCI. These studies offer important information on the plasticity of the physiological state of the muscle, as any decrease in the ratio of type II to type I fibre types after training would make the muscle less fatiguable. Significant increases in type I and type IIa fibre cross-sectional area were reported after 6 months of thrice-weekly manual BWS training in people with chronic, motor-incomplete SCI [36]. Further, these authors reported increases in the percent distribution of type IIa fibres, together with decreases in the distribution of type IIx fibres after training, supporting a transition to a more fatigue-resistant phenotype. The participants in that study (n = 9) all had motor-incomplete SCI (AIS C and D) so were capable of some voluntary activation of their lower limbs. It does not appear, however, that this voluntary activation is an absolute requirement for muscle fibre type adaptations to training. A case study of a gentleman with a C4 motor-complete SCI showed increases in mean muscle fibre area of the vastus lateralis, together with a change in fibre type distribution favouring increased representation of type I fibres and decreases in both type IIa and IIx fibres, after 4 months of thrice-weekly manual BWS treadmill training [37].
As muscle strength is directly related to muscle cross-sectional area, one would expect to find a training-induced increase in strength to go along with the increases in muscle size reported above. Unfortunately, few studies have incorporated a measure of leg muscle strength in their outcomes. A significant increase in plantar flexor and knee extensor torque was reported after 9 weeks of locomotor training on a BWS treadmill [33] in people with motor-incomplete SCI, and an increase of 5.4 points in the lower extremity motor score has been reported after 20 weeks of Lokomat™ training [38], also in people with motor-incomplete SCI. Evidence for improvement in strength-dependent functional tasks after locomotor training is equivocal, both improvements [29, 36] and lack of change [38] in walking speed/performance have been reported.
Evidence supporting locomotor training as a means of improving metabolic health
Improvements in physical fitness parameters such as aerobic capacity and muscle strength/function after training are typically associated with positive changes in metabolic profile. Improvements in blood lipid profile and/or glucose regulation can decrease risk for secondary co-morbidities such as cardiovascular disease and type II diabetes, both of which are common health complications in people with SCI [4, 5]. A recent systematic review supported the efficacy of aerobic and resistance training to improve cardiometabolic health in adults with chronic SCI [39], but no direct mention was made of locomotor training as an effective stimulus to improve this outcome.
A single bout of locomotor training is probably not a strong enough stimulus to induce any significant change in metabolic parameters, although trends for higher levels of fat oxidation and improved glucose uptake have been reported [18, 19]. To the author’s knowledge, only four studies have explored indices of metabolic health after locomotor training of 12–24 weeks, all in individuals with AIS C or D motor-incomplete SCI. Kressler et al. [26] evaluated substrate utilization at different self-selected walking speeds in a large sample of people with incomplete SCI undergoing 12 weeks of different types of locomotor training. By evaluating respiratory exchange ratios before and after training they concluded that there was evidence for a shift towards greater fat oxidation after training. In two papers from the same laboratory, improvements in blood lipid profile, glucose homoeostasis and muscle oxidative capacity were reported after 24 weeks of thrice-weekly training on a BWS treadmill with manual assistance [36, 40]. More specifically, significant decreases in total cholesterol, LDL cholesterol and the ratio of total cholesterol to HDL cholesterol were found after training [36]. Further, the areas under the curve for both glucose and insulin during a modified oral glucose tolerance test were significantly reduced after training [40]. Biopsy results from the vastus lateralis indicated a significant increase in the content of the GLUT-4 transporter protein together with increases in two key enzymes involved in oxidative metabolism, citrate synthase and 3-hydroxyacyl-CoA-dehydrogenase activity [36, 40]. In contrast to these promising results, no change in blood lipids or calculated Quantitative Insulin Sensitivity Check were found in one report after a similar period of training [41], however this study used a combination of progressive resistance training, FES cycling and locomotor training. Although no data were provided, it was noted that the majority of participants began their training programme with a “healthy” lipid profile, which may have contributed to the lack of any significant training effect.
Table 2 summarizes the studies that included muscle function and/or metabolic health as outcomes after locomotor training. While promising, it is clear that more research is needed to definitively determine if locomotor training provides sufficient muscle activation to induce metabolic changes that would translate into improved cardiometabolic health in people with SCI.
Summary and concluding statements
The last two decades have seen tremendous advances in the technology behind locomotor training for people with SCI. While the early BWS treadmills (with or without robotic exoskeleton support) were primarily developed for retraining gait in the clinic, the industry has now moved towards developing sophisticated powered exoskeletons that can potentially be used within the community. Collectively, these devices all carry the same common goal, to improve walking function and walking performance. Being upright, however, and supporting part or all of one’s bodyweight offers unique physiological challenges to both the cardiovascular and muscular system, so it is reasonable to conceive that regular access to locomotor training might also serve as an exercise stimulus if it is performed at a sufficient intensity and duration.
Based on the still rather limited research in the field, it can be cautiously concluded that locomotor training can meet the minimum “moderate intensity” threshold considered to be necessary for cardiovascular fitness benefits. However, only a handful of studies have used a sufficient training duration to impart measureable changes in cardiovascular benefits. Perhaps more compelling is the evidence showing the positive effects of locomotor training on the paralyzed muscle in terms of improving muscle size, muscle strength and muscle oxidative capacity. These adaptations may have the potential to not only improve metabolic health, but will also make the muscle less fatiguable and thus better able to withstand increased daily activity. One of the biggest challenges in interpreting results from the published literature on locomotor training paradigms in people with SCI is that many of the studies have been powered to evaluate changes in walking function, not physical fitness. More studies that identify fitness (or cardiometabolic health) as a primary outcome are needed.
There is no denying that robotics technology is an extremely fast-moving field, and powered exoskeletons are likely going to continue being refined to the point that they become easier to use and more readily accessible to people with SCI living in the community. If users can be encouraged to utilise these devices according to the minimum thresholds recommended by the PAGs, there is the possibility for not only improvements in walking function and performance, but also in health-related fitness.
References
Martin Ginis K, van der Scheer J, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P, et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 2018;56:308–21.
Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–31.
Martin Ginis KA, Arbour-Nicitopoulos KP, Latimer AE, Buchholz AC, Bray SR, Craven BC, et al. Leisure time physical activity in a population-based sample of people with spinal cord injury part II: activity types, intensities, and durations. Arch Phys Med Rehabil. 2010;91:729–33.
Lai YJ, Lin CL, Chang YJ, et al. Spinal cord injury increases the risk of type 2 diabetes: a population-based cohort study. Spine J. 2014;14:1957–64.
Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86:142–52.
Buchholz AC, Martin Ginis KA, Bray SR, Craven BC, Hicks AL, Hayes KC, et al. Greater daily leisure time physical activity is associated with lower chronic disease risk in adults with spinal cord injury. Appl Physiol Nutr Metab. 2009;34:640–7.
Ditunno J, Scivoletto G. Clinical relevance of gait research applied to clinical trials in spinal cord injury. Brain Res Bull. 2009;78:35–42.
Dobkin BH, Duncan PW. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil Neural Repair. 2012;26:308–17.
Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil. 2013;94:2297–308.
Chang SR, Kobetic R, Triolo RJ. Effect of exoskeletal joint constraint and passive resistance on metabolic energy expenditure: Implications for walking in paraplegia. PLoS ONE. 2017;12:e0183125. https://doi.org/10.1371/journal.pone.0183125.
Ferris DP, Sawicki GS, Daley MA. A physiologist’s perspective on robotic exoskeletons for human locomotion. Int J HR. 2007;4:507–28.
Garber C, Blissmer B, Deschenes M, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59.
Fenuta AM, Hicks AL. Metabolic demand and muscle activation during different forms of bodyweight supported locomotion in men with incomplete SCI. Biomed Res Int. 2014;2014:632765. https://doi.org/10.1155/2014/632765.
Israel JF, Campbell DD, Kahn JH, Hornby GT. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Phys Ther. 2006;86:1466–78.
Escalona MJ, Brosseau R, Vermette M, Comtois AS, Duclos C, Aubertin-Leheudre M, et al. Cardiorespiratory demand and rate of perceived exertion during overground walking with a robotic exoskeleton in long-term manual wheelchair users with chronic spinal cord injury: a cross-sectional study. Ann Phys Rehabil Med. 2018;61:215–23.
Evans N, Hartigan C, Kandilakis C, Pharo E, Clesson I. Responses during exoskeleton-assisted walking overground among persons with chronic spinal cord injury. Top Spinal Cord Inj Rehabil. 2015;21:122–32.
Asselin P, Knezevic S, Kornfeld S, Cirnigliaro C, Agronaova-Breyter PT, Bauman WA, et al. Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. JRRD. 2015;52:147–58.
Maher JL, Baunsgaard CB, van Gerven J, Palermo AE, Biering-Sorensen F, Mendez A, et al. Differences in acute metabolic responses to bionic and nonbionic ambulation in spinal cord injured humans and controls. Arch Phys Med Rehabil. 2020;101:121–9.
Kressler J, Wymer T, Domingo A. Respiratory, cardiovascular and metabolic responses during different modes of overground bionic ambulation in persons with motor-incomplete spinal cord injury: a case series. J Rehabil Med. 2018;50:173–80.
Currie KD, West CR, Hubli M, Gee CM, Krassioukov AV. Peak heart rates and sympathetic function in tetraplegic nonathletes and athletes. Med Sci Sports Exerc. 2015;47:1259–64.
Borg G. A category scale with ratio properties for intermodal and interindividual comparisons. Psychophysical Judgement and the process of perception. In: Geissler G, Petzol P, editors. Proceedings of the 22nd International Congress of Psychology. Amsterdam: North Holland; 1980. p. 25–34.
Gossey-Tolfrey VL, Paulson TA, Tolfrey K, Easton RG. Prediction of peak oxygen uptake from differentiated ratings of perceived exertion during wheelchair propulstion in trained wheelchair sportspersons. Eur J Appl Physiol. 2014;114:1251–8.
Pelletier CA, Totosy de Zepetnek J, MacDonald M, Hicks A. A 16-week randomized controlled trial evaluating the physical activity guidelines for adults with spinal cord injury. Spinal Cord. 2015;53:363–7.
van der Scheer JW, Hutchinson M, Paulson T, Martin Ginis KA, Goosey-Tolfrey VL. Reliability and validity of subjective measures of aerobic intensity in adults with spinal cord injury: a systematic review. PMR. 2018;10:194–207.
Kawashima N, Taguchi D, Nakazawa K, Akai M. Effect of lesion level on the orthotic gait performance in individuals with complete paraplegia. Spinal Cord. 2006;44:487–94.
Kressler J, Nash MS, Burns PA, Field-Fote EC. Metabolic responses to 4 different body weight-supported locomotor training approaches in persons with incomplete spinal cord injury. Arch Phys Med Rehabil. 2013;94:1436–42.
Gorman PH, Scott W, York H, Theyagaraj M, Price-Miller N, McQuaid J, et al. Robotically assisted treadmill exercise training for improving peak fitness in chronic motor incomplete spinal cord injury: a randomized controlled trial. JSCM. 2016;39:32–44.
Faulkner J, Martinelli L, Cook K, Stoner L, Ryan-Stewart H, Paine E, et al. Effects of robotic-assisted gait training on the central vascular health of individuals with spinal cord injury: a pilot study. J Spinal Cord Med. 2019. https://doi.org/10.1080/10790268.2019.1656849.
Gorgey AS, Wade R, Sumrell R, Villadelgado L, Khalil RE, Lavis T. Exoskeleton training may improve level of physical activity after spinal cord injury: a case series. Top Spinal Cord Inj Rehabil. 2017;23:245–55.
Shackleton C, Evans R, Shamley D, West S, Albertus Y. Effectiveness of over-ground robotic locomotor training in improving walking performance, cardiovascular demands, secondary complications and user satisfaction in individuals with spinal cord injuries: a systematic review. J Rehabil Med. 2019. https://doi.org/10.2340/16501977-2601.
Castro MJ, Apple DF Jr, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999;86:350–8.
Giangregorio LM, Webber CE, Phillips SM, Hicks AL, Craven BC, Bugaresti JM, et al. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nut Metab. 2006;31:283–91.
Jayaraman A, Shah P, Gregory C, Bowden M, Stevens J, Bishop M, et al. Locomotor training and muscle function after incomplete spinal cord injury: case series. J Spinal Cord Med. 2008;31:185–93.
Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, et al. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord. 2005;43:649–57.
Karelis AD, Carvalho LP, Escalona MJ, Gagnon DH, Aubertin-Leheudre M. Effect on body composition and bone mineral density of walking with a robotic exoskeleton in adults with chronic spinal cord injury. J Rehabil Med. 2017;49:84–7.
Stewart BG, Tarnopolsky MA, Hicks AL, McCartney N, Mahoney DJ, Staron RS, et al. Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve. 2004;30:61–8.
Adams MM, Ditor DS, Tarnopolsky MA, Phillips SM, McCartney N, Hicks AL. The effect of body weight-supported treadmill training on muscle morphology in an individual with chronic, motor-complete spinal cord injury: a case study. J Spinal Cord Med. 2006;29:167–71.
Piira A, Lannem AM, Sorensen M, Glott T, Knutsen R, Jorgensen L, et al. Robot-assisted locomotor training did not improve walking function in patients with chronic incomplete spinal cord injury: a randomized clinical trial. J Rehabil Med. 2019;51:385–9.
van der Scheer JW, Martin Ginis KA, Ditor DS, Goosey-Tolfrey VL, Hicks AL, West CR, et al. Effects of exercise on fitness and health of adults with spinal cord injury. A systematic review. Neurology. 2017;89:736–45.
Phillips SM, Stewart BG, Mahoney DJ, Hicks AL, McCartney N, Tang JE, et al. Body-weight-support treadmill training improves blood glucose regulation in persons with incomplete spinal cord injury. J Appl Phsiol. 2004;97:716–24.
Jones ML, Evans N, Tefertiller C, Backus D, Sweatman M, Tansey K, et al. Activity-based therapy for recovery of walking in individuals with chronic spinal cord injury: results from a randomized clinical trial. Arch Phys Med Rehabil. 2014;95:2239–46.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hicks, A.L. Locomotor training in people with spinal cord injury: is this exercise?. Spinal Cord 59, 9–16 (2021). https://doi.org/10.1038/s41393-020-0502-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-020-0502-y
This article is cited by
-
Wearable Robotic Exoskeletons for Overground Walking in Rehabilitation: Specific Contexts of Use and Improvement Opportunities
Current Robotics Reports (2020)