Abstract
Study design
Retrospective analysis of treated inpatients compared to expected neurorecovery from a propensity score-matched national database cohort.
Objective
Evaluate the effectiveness of buspirone on clinical neurorecovery following traumatic SCI when started during acute inpatient rehabilitation.
Setting
University-based hospital in Boston, USA.
Methods
Chart review yielded thirty-one individuals with acute, traumatic SCI treated with buspirone during inpatient rehabilitation from 2011–2017. Propensity score matching to a cohort of individuals from the spinal cord injury model systems (SCIMS) national database was completed. Changes in upper extremity motor score (UEMS), lower extremity motor score (LEMS), American Spinal Injury Association Impairment Scale (AIS), neurological level of injury (NLI), and functional impairment measure (FIM) from admission to discharge and discharge to 1 year were computed and compared between matched pairs (buspirone and mean national SCIMs cohort). A local control cohort not treated with buspirone was similarly compared to a matched mean national SCIMs group to identify location-specific effects.
Results
From admission to discharge from inpatient rehabilitation, 95% confidence intervals of changes in UEMS (−2.43 to +2.78), LEMS (−1.02 to +6.02), AIS (−0.04 to +0.35), NLI (−0.42 to +1.08), and FIM (−4.42 to +6.40) were not significantly different between those individuals who received buspirone and their propensity-matched SCIMS cohort. Similarly, changes in these metrics were not significantly different at 1-year follow up. Buspirone group individuals with initial clinically complete SCI demonstrated a higher 1-year conversion rate to incomplete injury (6 out of 14; 42.9%) compared to the matched national SCIMS cohort (14 out of 70; 21.2%, p = 0.047) though this was not significantly different from non-buspirone local controls (p = 0.25).
Conclusions
Retrospective analysis shows no statistically significant difference in gross markers of neurorecovery following acute traumatic SCI when buspirone is initiated indiscriminately during acute inpatient rehabilitation. In individuals with clinically complete SCI, findings suggest possible increased rates of 1-year conversion to incomplete injury.
Similar content being viewed by others
Introduction
Following spinal cord injury (SCI), loss of voluntary motor control is due to both damaged motorneurons and disruption of descending tracts from the brainstem that regulate locomotion via neurotransmitters including serotonin (5-HT) [1]. Animal studies have shown 5-HT release within the ventral horn of the spinal cord plays a critical role in locomotor system activation, modulation, and functional recovery, with descending axons requiring these neurotransmitters to prime them for movement generation [1, 2]. Following SCI, an acute relative deficit of 5-HT occurs caudal to the injury, often rendering motorneurons initially unexcitable, even though these neurons are often not directly injured [2, 3]. Functional recovery of locomotion has been elicited with activation of 5-HT receptors in different spinalized animal models [4,5,6]. While this evidence exists in animal models of SCI, clinically implementable interventions to capitalize on 5-HT activation in humans have been limited.
One such protentional medication is buspirone, a 5-HT receptor 1A agonist commonly used to manage anxiety. This United States Food and Drug Administration (FDA)-approved medication has been shown to facilitate reflex stepping, increase steps taken, and improve limb coordination in spinal cord injured mice [7]. In translation to humans, lab-based investigations involving patients with both complete and incomplete SCI have demonstrated increased muscle strength and enhanced locomotor-like activity when oral buspirone was combined with spinal cord electrical stimulation and physical therapy [8, 9]. In addition to potential neurofaciliatory effects, buspirone is an attractive therapeutic option because it is generally well tolerated with limited side effects, even in individuals with SCI [10, 11].
Based on this literature evidence and minimal risk for adverse drug reactions, several physicians at our inpatient rehabilitation hospital have begun prescribing buspirone to potentially aid in neurorecovery after SCI. This study aimed to evaluate the effectiveness of buspirone on motor recovery following SCI when given in the acute inpatient rehabilitation setting.
Methods
Study design and population
This study drew retrospective data from a local hospital model systems database and the National Spinal Cord Injury Model Systems (SCIMS) database [12]. The National SCIMS database is the largest SCI database in the world, currently including de-identified data on more than 32,000 individuals with acute, traumatic SCI through September 2016 [13]. These data examine changes in neurological function, functional independence, employment, and quality of life, with up to 40 years of follow-up for each individual.
All patients with a diagnosis of traumatic SCI admitted to our inpatient rehabilitation hospital between 2011–2017 were eligible for this study. Inclusion criteria for this study were: (1) adult patients over 18 years old at time of SCI; (2) enrolled as part of the National SCIMS database; and (3) had completed data for admission, discharge, and 1-year follow up assessments. Admission and discharge medication lists were reviewed for all eligible patients to identify all who had been prescribed buspirone during their inpatient rehabilitation hospitalization. If buspirone appeared as a new medication on their discharge medication list, regardless of treatment dose, the patient was stratified into the buspirone group. All others were included in a non-buspirone group that served as local controls. Per review with prescribing inpatient physicians, there were no clear criteria for starting patients on this medication. In addition, discharge medications were reviewed for other primary serotonergic medications, namely selective-serotonin reuptake inhibitors (SSRIs) and serotonin/norepinephrine reuptake inhibitors (SNRIs). To assess for implicit prescribing bias, presenting characteristics between individuals who were and were not prescribed buspirone were compared. Exclusion criteria included treatment with buspirone prior to admission to inpatient rehabilitation. To quantify differences in recovery outside of what was expected, a national cohort of individuals with similar injuries and completed 1-year follow up assessments from the SCIMS database was used (see detailed methods below).
This study was approved by the Partners Human Research Committee. In addition, The IRB approved the use of de-identified data obtained from the National Spinal cord Injury Statistical Center controlled access research database.
Primary outcome and study variables
The primary outcome measures were changes in upper extremity motor score (UEMS) and lower extremity motor score (LEMS) at discharge from the acute inpatient rehabilitation hospital and 1-year follow-up assessment relative to admission scores. Secondary outcome measures included changes in American Spinal Injury Association Impairment Scale (AIS) [14], neurological level of injury (NLI), and functional impairment measure (FIM) at discharge from acute inpatient rehabilitation hospital and 1-year follow-up assessment relative to admission values.
Statistical analysis
Baseline characteristics of our study population which influence neurorecovery were expectedly heterogeneous, as is common with this clinical population. Given this, propensity score matching to a cohort from the national SCIMS database was completed. Patients enrolled in the local hospital model systems were also enrolled in the National SCIMS database, and therefore were selectively removed from the matched national cohort prior to the analysis.
Each individual in the buspirone treatment group was first manually matched with up to 10 national SCIMS patients based on admission age (as quantified by the 15-year age range entered in standard SCIMS reporting), NLI, AIS, UEMS, and LEMS. These manual matches were completed to ensure residual motor scores below the NLI were as closely matched as possible. Since extended residual motor function is known clinically to indicate potential for improved recovery, manual matching served to reduce a potential confounder unable to be accounted for in our mathematical model. For example, for an individual in the buspirone treatment group with C6 AIS A SCI and with LEMS of 0, all of the national SCIMS matches also had LEMS of 0 (Table 1).
From these initial 1:10 pairings, propensity scores were calculated based upon logistic regressions incorporating age, NLI, AIS, UEMS, and LEMS using the statistical program R to generate a non-parsimonious model (MachIt software package, RStudio Inc. v1.1.463) [15]. Of note, if a matched individual had incomplete data, they were omitted from the model as any potential imputation was viewed as an avoidable source of possible confounding. Individuals in the buspirone group were then objectively matched to the SCIMS national cohort in a 1:5 ratio (matching the 5 individuals with the nearest propensity score). Furthermore, we used time from injury to inpatient rehabilitation admission as a proxy of other acute comorbidities which may have influenced neurorecovery, and ensured these values were similar between each individual in the treatment group and their matched SCIMS cohort using heteroscedastic t-tests.
Demographic information regarding ethnicity and medical co-morbidities were not included in the matching process. While health-care outcome discrepancies exist between ethnicities following SCI, no differences in self-care and mobility outcomes have been observed across racial and ethnic groups at 1-year follow-up [16]. Similarly, the presence of medical comorbidities likely impacts length of stay and return to acute care during rehabilitation, but impact on functional outcomes is not clear [17].
Mean values for NLI, AIS, UEMS, LEMS, and FIM were calculated for each of the matched SCIMS cohorts. FIM gains and FIM efficiency (FIM gains normalized to length of inpatient rehabilitation stay) were calculated at discharge from rehab hospitalization only. Changes in NLI, AIS, UEMS, and LEMS from admission to discharge and admission to 1 year were computed and compared between matched pairs (buspirone and mean national SCIMs cohort). With regard to UEMS change, this metric was included only for those with initial tetraplegia in an effort to not dilute the population with individuals with paraplegia without any expected upper extremity motor changes.
In order to account for any potential location-specific effects on neurorecovery from our local center, an additional 15 individuals in the non-buspirone group were selected. These individuals had similar 1:10 manual and 1:5 propensity score matching to individuals from the national SCIMS database. Changes in the same metrics were calculated, with differences between the buspirone treated local group versus national cohort compared to the difference between the local non-buspirone group versus national cohort servings as an estimate of any potential location-specific effects from our center. Since all dosages of buspirone were grouped together in the treatment group, post hoc analysis was performed to assess if dosage played a potential role, analyzing daily prescribed doses of <20 mg vs daily doses of 20–60 mg.
To assess potential effects on conversion from clinically complete (AIS A) SCI to incomplete injury, subgroup analysis of all local individuals with initial AIS A and their national SCIMS cohort were compared. Heteroscedastic t-tests and chi-squared tests were used to analyze between group differences with p-values of <0.05 treated as statistically significant. Standard deviations and 95% confidence intervals were further calculated to quantify estimates of effect size.
Results
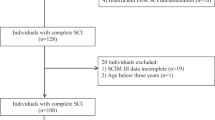
One hundred and twenty-four adults with traumatic SCI were admitted to our local rehab hospital during the study window and had full admission, discharge, and 1-year data recorded. Of these eligible patients, 31 were prescribed buspirone during inpatient rehabilitation hospitalization and were included in the buspirone treatment group for analysis (Fig. 1). Daily prescribed doses ranged from 5 mg to 60 mg in this group. Demographics for this buspirone treatment group and local non-buspirone group appear in Table 2. In assessing for implicit prescribing bias, the buspirone treatment group (n = 31) did not differ significantly from the non-buspirone local individuals (n = 93) with regards to age (p = 0.174), NLI (p = 0.649), AIS (p = 0.094), or time from injury to admission (p = 0.370).
Of the 32,159 records in the SCIMS database, only 983 had complete admission, discharge and 1-year data. Of these eligible 983 patients, a total of 460 were manually matched to the local cohort groups (Fig. 1). Propensity matching further distilled the SCIMS patients in final 1:5 ratio, yielding a total of 230 matched SCIMS participants used for direct statistical comparison. Sampling with replacement was used during this study and 6 of the 155 national SCIMS patients matched to the local buspirone group were used twice, a compromise we felt was acceptable given the 1:5 matching dilutes individual effects. Propensity score matching with both the buspirone treatment group (n = 31) versus matched SCIMS cohort (n = 155) and local non-buspirone control group (n = 15) versus matched SCIMS cohort (n = 75) demonstrated a strong match, with minimal standardized differences between groups (Table 3, Supplementary Fig. 1). Of note, age did differ by more than the typically accepted 10% standardized difference, though as ages were reported in SCIMS in 15-year increments, the 2.7 year mean difference between groups is of unclear clinical significance.
From admission to discharge from inpatient rehabilitation, changes in NLI, AIS, UEMS, LEMS, FIM, and FIM efficiency were not significantly different between those individuals who received buspirone and their propensity-matched SCIMS cohort (Fig. 2). Further, when considering location-specific effects by analyzing the 15 individuals who did not receive buspirone from our institution compared to their own propensity-matched SCIM cohort, there was overlap in all 95% confidence intervals with the estimates of buspirone treatment effect. Similarly, at one-year follow-up, there were no significant differences in changes in NLI, AIS, UEMS, LEMS, or FIM following buspirone administration compared to the matched cohort. With overlapping 95% CI, there were no statistically significant differences in effects between the buspirone treatment group and the local non-buspirone cohort.
 compared to SCI model systems propensity-matched peers (1:5), 95% CI.
compared to SCI model systems propensity-matched peers (1:5), 95% CI.When comparing patients within the local buspirone group who received high-dose buspirone (≥20 mg per day) (n = 10) versus low-dose buspirone (<20 mg per day) (n = 21), there was no statistically significant difference between any outcome measure (Supplementary Table 1, p = 0.22–0.97). A total of 55 patients included in this cohort had an SSRI or SNRI on their discharge medication list. Out of the buspirone treatment group, 24 patients were also prescribed SSRI or SNRI. In the control group, 31 patients were prescribed SSRI/SNRI at discharge (p < 0.0001).
Within individuals with clinically complete SCI (AIS A) who received buspirone (n = 15) 2 out of 15 (13.3%) converted from complete to incomplete at discharge versus 9 out of 75 (11.6%) in this subgroup’s propensity-matched national SCIMS cohort (p = 0.86). From admission to 1-year post injury (n = 14), 6 out of 14 (42.9%) of the buspirone group had converted from clinically complete to incomplete SCI versus 14 out of 70 (21.2%) in the propensity-matched cohort (p = 0.047). In assessing for location-specific effects, only eight individuals had clinically complete SCI in local non-buspirone cohort. There was no statistically significant difference between conversion rates of buspirone treated and local non-buspirone groups (p = 0.25), though given these low sample sizes, conclusions from this post-hoc analysis are limited.
Discussion
To our knowledge, this is the first study examining the effects of buspirone on SCI neurorecovery in a clinical, acute rehabilitation hospital setting. In this retrospective analysis, our results did not show a statistically significant difference in the changes of outcome measures at discharge from inpatient rehabilitation hospital or at 1-year follow-up. One reason for this may be that these clinical measures are not sensitive enough to detect slight improvements. Both the NLI and AIS further take into account sensory deficits. While serotonergic neurons do project to the dorsal horns, where sensory information is processed, 5-HT is relevant for transmission, processing, and control of nociceptive signals [18, 19]. 5-HT can act to both diminish and potentiate nociceptive pain, but the exact mechanisms remain unclear. As such, it is currently unknown if supplementation of 5-HT has any role in recovery of diminished sensation, which could in turn impact the clinical measures of AIS and NLI.
Numerous studies have evaluated the effects of serotonin agonists on lower extremity neurorecovery, though few studies exist using buspirone or any other serotonin agonist as a treatment for tetraplegia. Freyvert et al. showed that transcutaneous epidural stimulation applied to cervical spinal circuits could strengthen hand grip in individuals with chronic tetraplegia, and the addition of buspirone further enhanced grip strength [11]. These results suggest that stimulation and buspirone interventions increase the level of excitability of pre-motor spinal circuitries that mediate hand function. While the mechanism is not fully understood, Freyvert et al. hypothesized that buspirone may serve as a priming agent to help facilitate neurorecovery. If this priming effect is a key mechanism, the amount of focused therapy may play a role in maximizing gains. This was not accounted for in our study and may contribute to why the buspirone group did not demonstrate any significant effects beyond expected recovery on our clinical metrics.
Structural and functional changes in 5-HT receptors on motorneurons below the injury aide in residual locomotor function. Following SCI, the depletion of descending 5-HT caudal to the injury leads to a compensatory overexpression of receptors, hypersensitivity to residual 5-HT input, and constitutive activity (receptor activity in the absence of 5HT) [20, 21]. In animal models, these changes occur as early as day 1 after injury [22]. This upregulation provides motorneurons an enhanced response to residual descending 5-HT input and is a prime target for exogeneous 5-HT. While these adaptive mechanisms are thought to be responsible for residual motor output, excessive upregulation may produce poorly regulated motoneuron excitability and uncoordinated muscle spasms [20, 23]. Further investigation on potential spasticity effects is thus warranted.
By duplicating our methods on a subset of non-buspirone individuals with SCI at our institution, we were able to estimate location-specific effects in the assessed metrics. These effects overlapped 95% confidence intervals with all buspirone treatment group metrics, indicating no statistically significant relative changes with this medication. Further, increased dosage of this medication was not found to clinically alter expected recovery to a significant degree. Buspirone is applied as a psychotropic drug at mean daily dose of 20–30 mg [24, 25]. Recent findings suggest that moderate release of 5-HT facilitates locomotion and promotes the excitability of motoneurons, while stronger release inhibits rhythmic activity and motoneuron firing [26, 27]. Our results show no difference in high-dose or low-dose buspirone. So, while buspirone is generally well tolerated, its effects on neurorecovery and preferred treatment doses remain to be elucidated.
In addition, the confounding effects of concomitant use of other serotonergic medications remains to be seen. While a large percentage of patients in the treatment group received both buspirone as well as an SSRI/SNRI, it is reasonable to postulate that additional serotonergic pharmacology would potentially be helpful. In pre-clinical SCI animal studies, fluoxetine was been shown to be neuroprotective as well as a facilitator of functional recovery [28, 29]. Furthermore, in humans with chronic motor-incomplete SCI, one dose of the SSRI escitalopram acutely increased muscle activity seen on EMG, despite no improvement in locomotor function [30]. Similar to buspirone, preferred dosing of SSRIs/SNRIs to augment functional neurorecovery following SCI has yet to be determined.
In the buspirone group, the rate of conversion from clinically complete (AIS A) to incomplete injury at 1-year post injury was significantly higher than that of the matched SCIMS cohort. This may be due to the higher degree of 5-HT receptor alterations seen in complete injuries compared to incomplete injuries. While exogenous 5-HT agonists improve motorneuron excitability in acute spinal transection animal models, the same may not be as effective for more clinically-relevant, incomplete SCI paradigms such as spinal cord contusion. Hayashi et al demonstrated that neither direct nor indirect 5-HT agonists improved motor function after incomplete contusion lesions in an animal model, despite 5HT receptor upregulation [6]. With increased spared supraspinal serotonergic projections, incomplete SCI may not display a sufficient degree of receptor changes to affect motor function when exposed to 5-HT agonists alone. Given our small sample size for this sub-analysis (n = 14) and the insignificant between our local, non-buspirone clinically complete cohort (n = 8), these results point to the need for further robust studies specifically assessing this clinically important metric with greater scrutiny. Additional studies on 1-year conversion to incomplete injury, taking into account buspirone dosage and continuity of medication throughout the year post injury are further needed.
Initially, the difference in SSRI/SNRI exposure and potential effects on neurorecovery were considered. The specific medication as well as dosage for both classes of serotonergic medications were recorded from chart review of the discharge medication list for the local buspirone group. Ultimately, there were too many variables including variable use of specific drug, new versus chronic drug exposure, and dosage. While not analyzed in this study, this is clinical data that can inform the future studies in analyzing serotonergic pharmacology for neurorecovery following SCI.
Limitations
The limitations of this study should be considered when interpreting the clinical significance of our results. As a retrospective study, we were unable to decipher precise drug exposure. The start date of buspirone treatment during inpatient rehabilitation and timing of drug discontinuation was also unclear and warrant additional investigation. Furthermore, it was not known if patients continued to take buspirone throughout the year after injury or whether it was self-discontinued or discontinued by another provider. Additionally, our use of propensity score matching for the control group assumes that individuals from the national SCIMS database were not newly prescribed buspirone during their acute inpatient rehabilitation stays. While not commonly prescribed long-term in the rehabilitation setting for neurorecovery, we are unable to say with certainty whether or not buspirone was given as a chronic medication during the rehabilitation stay or prescribed as a new medication on discharge for these SCIMS patients from other institutions as discharge medication lists were not available. As noted above, our 1:5 matching does dilute individual effects such as this.
The outcomes used in the study (UEMS, LEMS, AIS, NLI, and FIM) are routinely documented outcomes during inpatient rehabilitation and provide insight into gross functional assessment after injury. This study is unable to assess potential nuanced functional improvements and future studies may consider relevant outcome assessments involving force contraction measurements, EMG, handgrip strength testing, etc.
Conclusion
Retrospective analysis comparing local individuals treated with oral buspirone during inpatient rehabilitation compared to a propensity-matched SCIMS database cohort did not show a statistically significant difference in gross measurements of neurorecovery following acute traumatic SCI. In individuals with clinically complete SCI, findings suggest possible increased rates of 1-year conversion to incomplete injury, though further well-powered studies are needed to examine these findings.
Data availability
The datasets generated during and/or analyzed during the current study are available in the National Spinal Cord Injury Model Systems (SCIMS) Database and can be requested per their internal policy upon approval [https://www.nscisc.uab.edu/Research/PublicDataRegister].
References
Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–91.
Jacobs BL. Martı́n-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Rev. 2002;40:45–52.
Carlsson A, Magnusson T, Rosengren E. 5-Hydroxytryptamine of the spinal cord normally and after transection. Experientia. 1963;19:359–359.
Cazalets JR, Sqalli‐Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol. 1992;455:187–204.
Madriaga MA, McPhee LC, Chersa T, Christie KJ, Whelan PJ. Modulation of locomotor activity by multiple 5-HT and dopaminergic receptor subtypes in the neonatal mouse spinal cord. J Neurophysiol. 2004;92:1566–76.
Hayashi Y, Jacob-Vadakot S, Dugan EA, McBride S, Olexa R, Simansky K, et al. 5-HT precursor loading, but not 5-HT receptor agonists, increases motor function after spinal cord contusion in adult rats. Exp Neurol. 2010;221:68–78.
Jeffrey-Gauthier R, Josset N, Bretzner F, Leblond H. Facilitation of locomotor spinal networks activity by buspirone after a complete spinal cord lesion in mice. J Neurotrauma. 2018;35:2208–21.
Gerasimenko YP, Lu DC, Modaber M, Zdunowski S, Gad P, Sayenko DG, et al. Noninvasive reactivation of motor descending control after paralysis. J Neurotrauma. 2015;32:1968–80.
Moshonkina TR, Shapkova EY, Sukhotina IA, Emeljannikov DV, Gerasimenko YP. Effect of combination of non-invasive spinal cord electrical stimulation and serotonin receptor activation in patients with chronic spinal cord lesion. Bull Exp Biol Med. 2016;161:749.
Guertin PA, Brochu C. Preliminary evidence of safety following administration of L-DOPA and buspirone in an incomplete monoplegic patient. Spinal Cord. 2009;47:91–2.
Freyvert Y, Yong NA, Morikawa E, Zdunowski S, Sarino ME, Gerasimenko Y, et al. Engaging cervical spinal circuitry with non-invasive spinal stimulation and buspirone to restore hand function in chronic motor complete patients. Sci Rep. 2018;8:1–10.
Chen Y, DeVivo MJ, Richards JS, SanAgustin TB. Spinal cord injury model systems: review of program and national database from 1970 to 2015. Arch Phys Med Rehabil. 2016;97:1797–804.
Chen Y. National spinal cord injury model systems database. Version 2016ARPublic. Birmingham, AL: National Spinal Cord Injury Statistical Center [distributor]. 2016. https://doi.org/10.17605/OSF.IO/NP24C.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34:535–46.
Sekhon JS. Multivariate and propensity score matching software with automated balance optimization: the matching package for R. J Stat Softw. 2011;42:1–52.
Fyffe DC, Deutsch A, Botticello AL, Kirshblum S, Ottenbacher KJ. Racial and ethnic disparities in functioning at discharge and follow-up among patients with motor complete spinal cord injury. Arch Phys Med Rehab. 2014;95:2140–51.
Horn SD, Smout RJ, DeJong G, Dijkers MP, Hsieh CH, Lammertse D, et al. Association of various comorbidity measures with spinal cord injury rehabilitation outcomes. Arch Phys Med Rehab. 2013;94:S75–86.
Eide PK, Hole K. The role of 5‐hydroxytryptamine (5‐HT) receptor subtypes and plasticity in the 5‐HT systems in the regulation of nociceptive sensitivity. Cephalalgia. 1993;13:75–85.
Kayser V, Bourgoin S, Viguier F, Michot B, Hamon M. Toward deciphering the respective roles of multiple 5-HT receptors in the complex serotonin-mediated control of pain. Pharmacol Pain. 2010:185–207.
Murray KC, Nakae A, Stephens MJ, Rank M, D'amico J, Harvey PJ, et al. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT 2C receptors. Nat Med. 2010;16:694.
Fouad K, Rank MM, Vavrek R, Murray KC, Sanelli L, Bennett DJ. Locomotion after spinal cord injury depends on constitutive activity in serotonin receptors. J Neurophysiol. 2010;104:2975–84.
Kong XY, Wienecke J, Hultborn H, Zhang M. Robust upregulation of serotonin 2A receptors after chronic spinal transection of rats: an immunohistochemical study. Brain Res. 2010;1320:60–8.
Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–58.
Loane C, Politis M. Buspirone: what is it all about? Brain Res. 2012;1461:111–8.
Sramek JJ, Hong WW, Hamid S, Nape B, Cutler NR. Meta‐analysis of the safety and tolerability of two dose regimens of buspirone in patients with persistent anxiety. Depression Anxiety. 1999;9:131–4.
Perrin FE, Gerber YN, Teigell M, Lonjon N, Boniface G, Bauchet L, et al. Anatomical study of serotonergic innervation and 5-HT 1A receptor in the human spinal cord. Cell Death Dis. 2011;2:e218–e218.
Dunbar MJ, Tran MA, Whelan PJ. Endogenous extracellular serotonin modulates the spinal locomotor network of the neonatal mouse. J Physiol. 2010;588:139–56.
Lee JY, Kang SR, Yune TY. Fluoxetine prevents oligodendrocyte cell death by inhibiting microglia activation after spinal cord injury. J Neurotrauma. 2015;32:633–44.
Scali M, Begenisic T, Mainardi M, Milanese M, Bonifacino T, Bonanno G, et al. Fluoxetine treatment promotes functional recovery in a rat model of cervical spinal cord injury. Sci Rep. 2013;3:2217.
Leech KA, Kinnaird CR, Hornby TG. Effects of serotonergic medications on locomotor performance in humans with incomplete spinal cord injury. J Neurotrauma. 2014;31:1334–42.
Acknowledgements
The authors would like to specifically acknowledge the collective work of the National Spinal Cord Injury Statistical Center and Model Systems contributors, without whom this study would not have been possible.
Author information
Authors and Affiliations
Contributions
JWM was responsible for conducting literature review, designing the study protocol, extracting and analyzing data, interpreting results, and writing the report. RS was responsible for designing the study protocol, extracting and analyzing data, creating tables and figures, interpreting results, and writing the report.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This study has been reviewed and approved by the Partners Human Research Committee (Protocol #: 2018P000667/PHS). In addition, The IRB has reviewed and approved to use research data obtained from Protocol 2011P002173 and HIPAA de-identified data obtained from the National Spinal cord Injury Statistical Center controlled access research database. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Morgan, J.W., Solinsky, R. Buspirone for functional improvement after acute traumatic spinal cord injury: a propensity score-matched cohort study. Spinal Cord 59, 563–570 (2021). https://doi.org/10.1038/s41393-020-00606-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-020-00606-0


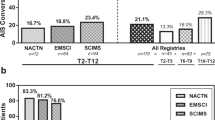


 Location effects
Location effects  specific to our institution (15 individuals who did not receive buspirone compared to similarly matched model systems peers, 1:5).
specific to our institution (15 individuals who did not receive buspirone compared to similarly matched model systems peers, 1:5).