Abstract
Study design
Within-subject, randomised cross-over trial.
Objectives
To determine whether a commercially available 3D head-mounted (HMD) virtual reality (VR) device results in significant reductions in neuropathic pain compared to using a 2D screen device in people with spinal cord injury (SCI).
Setting
Greenwich Hospital, Sydney, Australia.
Methods
Sixteen men with established SCI and chronic neuropathic pain participated in a single-session randomised cross-over trial. We compared the effects of 3D HMD VR and a 2D screen application on SCI neuropathic pain intensity and levels of perceived presence.
Results
Participants reported significantly lower pain intensity after 3D HMD VR compared to 2D screen application (1.9 ± SD 1.8 versus 3.4 ± SD 1.6, mean 95% CI: 1.5, P < 0.0001). Participants reported significantly higher perceived levels of presence with the 3D HMD VR compared to 2D screen of (49.6 ± SD 8.9 versus 32.8 ± SD 11.1, mean 95% CI: 16.6, P < 0.0001). Increased perceived presence was associated with significantly lower pain intensity regardless of randomised sequencing of the two conditions (mean 95% CI: 0.06, P = 0.005). Effect size for pain reduction using 3D HMD VR was 0.80.
Conclusions
We suggest that 3D HMD VR may provide neuropathic pain relief for people with SCI. Given the lack of cybersickness and ease of access, we propose that immersive VR could be a helpful adjunct to current pharmacotherapy. Further research is required to show that VR can be effective for more long-term reductions in SCI pain.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) causes debilitating loss of sensorimotor function and unrelenting neuropathic pain (NP) at and below the level of injury in over 50% of patients [1]. SCI-related NP is thought to be due to altered central neuronal activity with spontaneous firing and amplified responsiveness across the neuroaxis [2]. Sensory deafferentation during SCI produces neurophysiological changes in somatosensory spinal circuits thought to generate abnormal nociceptive impulses to the brain. Altered thalamic circuits are thought to amplify these nociceptive signals to the cortex, which eventually produce widespread and long-term reorganisation of central sensory circuits [3]. Not surprisingly, current pharmacological treatments are only partially beneficial. Difficulty in obtaining satisfactory relief is due mostly to functional and structural changes in the central nervous system, as well as psychological factors that also influence the experience of NP.
As such, alternative approaches such as virtual reality (VR) are being investigated for the treatment of several medical and psychological conditions. VR is a simulated construction of a 3D environment using computer technology that includes head-mounted devices (HMD) with 3D-enabled glasses, noise-cancelling headphones, body-tracking sensors, joysticks and data gloves. Together this forms a realistic multisensory experience that surrounds the user, currently described as a real or simulated environment where the perceiver experiences a sense of presence, defined as an illusion of ‘being there’ [4]. Investigations into the mechanisms of VR analgaesia in experimental settings show that the degree of analgaesic effect is dependent on the user’s sense of presence in the virtual environment. Moreover, recent meta-analysis shows no difference in effectiveness between specifically developed software and commercially available games using 3D environments [5], thus increasing access to affordable VR options for use in experimental and clinical pain settings.
Several feasibility studies using 3D HMD and 2D screen applications show reductions in SCI-related NP in over two-thirds of participants. For example using 3D VR movement exercises, Villiger et al. showed significant reductions in NP intensity and increases muscle strength in 14 people with incomplete SCI after a 4-week period [6]. These findings suggest that VR is an effective method of reducing pain in both the long and short term. However, no randomised studies comparing differences in short-term pain relief between 3D HMD and 2D screen applications in people with neuropathic SCI pain exist. Recent evidence suggests that compared to 2D screen, 3D VR technologies are more realistic [7] where 3D perception of a VR scene is considered to give people a greater sense of presence.
The aim of this study is to (a) determine whether using a 3D HMD VR device results in a significant reduction in NP compared to a 2D screen-based device running the same VR application and (b) determine whether the level of presence in the virtual environment during the two interventions predicts the degree of analgesic effect in people with NP following a SCI. We hypothesised that the level of presence in a 3D HMD VR environment would significantly reduce the intensity and negative perceptions of pain in people with SCI and NP, compared to interacting in the same scene on a 2D screen application.
Methods
Study design
Using a within subject, randomised cross-over pilot trial, two sequential interventions were compared, one with 3D HMD VR and one with 2D screen applications using the same virtual environment in a convenience sample of 16 people with SCI and NP. Random allocation for the first administered intervention was performed where participants chose an enclosed text reading either ‘2D’ or ‘3D’ in separate using separate opaque sealed envelopes. The generation of random allocation sequencing, enrolment and intervention assignment were performed by PA.
Due to obvious differences in the appearance of the visual applications between the two interventions, participant and researcher blinding was not possible, however, because it was important for researchers (PA/PS) to show parity in describing both interventions, a script using neutral language was prepared. This study was an investigator-initiated feasibility trial funded by the Australian and New Zealand College of Anaesthetists, reference 19/002 and was registered by the Australia New Zealand Clinical Trials Registry, number ACTRN12618000959279 in May 2018. The Northern Sydney Local Health District Research Ethics Committee approved this single-site feasibility trial in September 2018, reference RESP/18/133.
Participants
We enroled participants with an established SCI and a diagnosis of chronic NP recruited through clinical contact and an SCI participant database. Inclusion criteria included males aged 18 years and older with complete or incomplete SCI of longer than 12 months duration in whom the lesion was at C6 level or below, a diagnosis of NP (>6 months), reported NP over the previous week and stable pharmacological or no pharmacological treatment for at least 4 weeks. Male participants were enroled as they account for ~80% of new SCI cases and because of significant differences in pain reporting and drug use between men and women [8]. Exclusion criteria included the presence of other types of pain more prominent at the time of the study, a SCI higher than C5 level, brain injury or other neurological diagnosis that would confound results. Level and extent of SCI was classified using the International Standards of Neurological Classification of SCI [9], while a diagnosis of NP was obtained using the PROMIS Measure of Neuropathic Pain Quality [10].
Study schedule
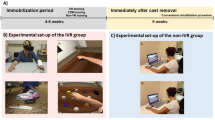
All participants visited on one occasion at the same time of day (11.00 a.m.) to account for circadian influences on wakefulness in people with SCI. Baseline NP measures were taken with an 11-point numerical pain rating scale (NPRS) for average, worst and least NP intensities over the previous week, in addition to current NP intensity. Importantly, participants were instructed only to rate their NP. We compared differences in effect between 3D HMD and 2D screen devices using the same VR software on SCI NP where the duration for each intervention was 15 min. NP scores reported immediately after each intervention were used for analysis. However, lowest and average NP scores during the intervention were also reported. To eliminate any carryover effect from the first intervention, a washout period separated the two treatment periods (see Fig. 1). Here, participants were free to move around in and out of the laboratory. Given that washout periods be at least five times the half-life of a given treatment [11], we chose 60 min, based on data showing that pain is significantly reduced immediately after VR exposures and that no difference exists between pain intensity scores at baseline and at 10 min after VR exposure [12]. The cross-over was counterbalanced to control for exposure to both interventions and the exposure sequence. Participants completed all self-report measures and VR applications in a temperature-controlled room maintained at 25 °C at a bench modified for wheelchair access. Participants were also required to report any headset discomfort and cybersickness (includes symptoms of nausea, vomiting, headache, vertigo and fatigue) prior to, during or after using the 3D HMD VR device.
VR interventions
3D HMD VR application
The Oculus Rift® headset is commercially available, widely used, inexpensive and utilised for VR studies in medical research [13]. For this study, participants viewed a 3D VR experience called Nature Trek®. Prior to use, participants were instructed by the researcher (PA) on the use of a handheld joystick, to move around a scenic meadow environment and make full use of the 360° scene. Given this was a single intervention study, sessions were only 15 min in length and to avoid cybersickness, the audio-visual experience was non interactive [14]. To avoid frustration for participants trying to master an interactive application in this short-time period, the application was standardised across the group. The VR headset was calibrated for participants’ eyesight in addition to advice on motion sickness prevention during VR such as reducing speed of their character and/or reducing head movement.
2D screen application
The same application was run on a 17.3-inch Alienware® laptop screen with the participant seated in the same position. This allowed for a reliable comparison between the effects of 3D VR and 2D screen experiences.
Primary outcome
The numerical pain rating scale. To investigate the effects of 3D HMD VR and 2D screen applications on SCI NP, participants first completed the 11-point NPRS after each intervention where participants not only reported levels of pain intensity immediately after intervention, but additionally reported their average pain intensity during each intervention, and lowest pain intensity during each intervention. The 11-point NPRS is a reliable and valid where meta-analysis shows use across many pain populations [15].
Secondary outcomes
The Depression Anxiety Stress Scale (DASS-21). To investigate the effects of 3D HMD VR on mood, participants completed the DASS-21 after each intervention. The DASS-21 is a valid and reliable set of three seven-item self-report scales designed to assess emotional states of depressive mood, anxiety and stress, where it has also been validated in people with NP, including those with SCI [16]. Participants rate the levels of their emotional states on a four-point Likert scale ranging from 0 (did not apply to me at all) to 3 (applied to me very much or most of the time).
iGroup Presence Questionnaire (IPQ). To investigate levels of presence during each VR session, participants completed the IPQ. The IPQ is a valid and reliable seven-point Likert scale for measuring spatial awareness, levels of involvement and experienced realism in a virtual environment ranging from 0 (not at all) to 6 (very much) [17].
Data analysis
Data analyses were performed using SPSS Statistics 24 [18]. Using a prevalidated case report form, descriptive statistics (means and standard deviations for continuous variables, frequencies for categorical variables) were drawn from demographic and clinical characteristics of the sample including age, years since injury, level of SCI lesion, SCI aetiology, SCI classification and prescribed pain medication in addition to average, least, worst NP over the previous week.
We used linear-mixed models analysis for repeated measures with post 3D HMD VR and 2D screen pain and presence scores as dependent variables for post 3D HMD VR and 2D screen time points. These regressions included a factor for the condition (3D HMD VR and 2D screen), the sequence (randomised sequence of conditions between subjects) and time (randomised sequence of conditions within subjects), where sequence was modelled as a random effect. The analysis controlled for baseline (pre-randomisation) pain intensity in models where post-intervention pain intensity was the dependent variable [19]. We based the sample size calculation on an effect size from a meta-analysis of previous studies using VR for chronic pain [20], which showed a mean effect size of 0.71. Using power of 0.8 and a type 1 error rate of 0.05, we calculated the required sample size was 16 [21]. We reported effect size using Hedge’s g.
Results
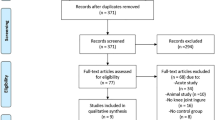
Seventeen men with SCI NP pain were enroled into the study, where one participant was excluded due to them reporting no pain over the previous week (Fig. 2). Table 1 shows the participants’ demographic characteristics including age, duration in years since SCI, level and extent of SCI, pain consistency and prescribed pain medication. Nine of the 16 eligible participants were randomly assigned to receive the 3D HMD VR application first. Table 2 shows the mean pain intensity scores over the week prior to their attendance, during and after VR interventions and levels of presence during VR interventions.
Effects of 3D HMD VR compared to 2D screen applications on pain intensity
Linear-mixed model analysis showed that 3D HMD VR was associated with significantly greater reductions in pain intensity from baseline in all participants compared to a 2D screen application (1.9 ± SD 1.8 versus 3.4 ± SD 1.6, 95% CI: 0.96–1.98, P < 0.0001) (Fig. 2). The standardised mean difference (Hedges’ g) for pain reduction using 3D HMD VR compared to 2D screen application at the post intervention time point was 0.80, which indicates a large effect. Participants reported an average decrease in pain of over 65% using 3D HMD VR compared to 35% using the 2D screen application. Here, there were moderate correlations between baseline and post 3D VR (r = −0.68, P = 0.003) and weak correlations between baseline and 2D screen NP scores (r = −0.42, P = 0.11). Concerning 3D VR, there were also significantly greater NP reductions in participants with NP below lesion level (n = 10, P > 0.0001) compared to those with NP at and below (n = 5, P = 0.03). Only one participant reported pain at lesion level. Additionally, those with complete spinal lesions reported significantly greater NP reductions (n = 11, P > 0.0001) compared to those with incomplete spinal lesions (n = 5, P = 0.005). Participants reporting SCI NP for <20 years, showed significantly greater reductions in post 3D VR pain (n = 7, P = 0.0001), compared to participants reporting up to 10 years (n = 5, P = 0.013) and between 11 and 20 years (n = 4, P = 0.011).
Importantly, the effects of randomised VR condition sequencing both between and within subjects on post-VR pain intensity were not significant (P = 0.34, P = 1.0 respectively) (Table 3).
Effects of 3D HMD VR and 2D screen applications on levels of presence
Linear-mixed model analysis also showed that 3D HMD VR was associated with significantly greater levels of presence compared to a 2D screen application (48.8 ± SD 8.8 versus 31.7 ± SD 11.1, mean 95% CI: 9.93–23.97, P < 0.0001) (Fig. 3). Furthermore, mixed-model analysis also showed that increases in levels of reported presence were associated with greater reductions in pain intensity, regardless of randomised sequencing of VR conditions (P = 0.002) (Table 3). However, although VR condition and presence predict pain separately, the effects of presence on pain intensity are non-significant (P = 0.60) when VR conditions are controlled for.
Effects of 3D HMD VR and 2D screen applications on mood
Mean DASS-21 subscale scores were within the normal clinical range at baseline (stress = mean 5.56 ± 2.92 SD, anxiety = 3.19 ± 2.79 SD, depressive mood = 2.69 ± 2.93 SD). Additionally, after each intervention, there were no significant differences between 3D HMD and 2D screen applications for changes in the DASS-21 subscale scores. Furthermore, linear-mixed model analysis confirmed that neither levels of presence nor the type of VR had significant effects on levels of mood.
Discussion
People with neuropathic SCI pain reported significant decreases in pain intensity during and immediately after taking part in both 3D HMD and 2D screen sessions. However, the reduction in pain intensity was significantly greater with 3D HMD VR when compared with the 2D screen application. Importantly, the effect size for 3D VR distraction was 0.8, which is typically considered representative of a large effect.
Participants also reported significantly greater levels of presence during 3D HMD VR compared to a 2D screen session where they reported that the 3D scene felt more real and were less aware of the real environment around them. It could be argued that the observed reductions in SCI NP may be due to placebo responses. Although, we cannot rule out such responses, recent meta-analyses show insignificant placebo responses in people with SCI NP suggesting that the responses observed in the present study are indeed significant [22]. Here, it is suggested that people with SCI have little expectation for pain relief, where in many cases; modes of previously attempted pain relief do not work [23]. Thus, our findings suggest that 3D HMD VR may offer an effective modality for the management of SCI NP. These findings are encouraging clinically as 3D HMD VR equipment is accessible and offers inexpensive and safe pain relief. Although the type of VR condition and levels of presence predict short-term decreases in pain intensity separately, further analysis revealed that the effects of levels of presence on changes in pain intensity are insignificant when VR conditions are controlled for. Thus, our findings suggest that while the level of presence is an important factor in obtaining analgesic relief, possibly through pain modulatory brain regions associated with distraction [10] using both 3D VR and 2D screens, the type of VR is the only independent factor associated with pain relief.
Only two previous studies have examined short-term alterations in pain intensity in people with SCI, the first using 3D HMD VR and the second, a 3D screen. In the first study, Pozeg and colleagues used a virtual leg illusion together with actual and virtual tactile stimuli to investigate changes in body ownership in 20 people with SCI NP. Here, they showed mild but significant reductions in NP (P = 0.04) and significantly stronger experiences of illusionary ownership of the virtual legs [24]. In the second, Jordan and Richardson investigated the effects of virtual walking (treatment) and virtual wheelchair wheeling (control) in 15 people with SCI NP. Their findings showed mild but significant decreases in SCI NP during virtual walking, compared to virtual wheeling (P = 0.03) [25].
Short-term pain relief after a single session of 3D HMD VR has also been investigated in people with chronic pain and phantom-limb pain. For example, Jones and colleagues reported significant decreases in pain intensity (P = 0.001) after a single session in 30 people with chronic pain [26], while similar levels of pain relief are shown in people with phantom-limb pain [27]. These and our findings suggest that uncontrolled attention to ʻbottom-up’ influences of pain may be modulated in a ʻtop-down’ manner by voluntary relaxation and/or goal-directed attention
Our VR protocols were designed for distraction, where participants were required only to move around and observe a nature scene. However, given that presence was not an independent factor for pain relief, it is feasible that concerns such as hardware mastery and the effects of neurological injury on hand movement and control may reduce immediate analgesic efficacy of VR. Thus, future studies should examine the effects of different types of VR application such as interactive gaming, interactive injury-specific or non-interactive nature and relaxation-based applications. Furthermore, combining VR with other techniques, such as mindfulness and hypnosis strategies, are shown to reduce levels of pain intensity in several pain conditions and lessen in negative emotions in psychological settings including SCI [28].
The duration of VR-analgesia is an important factor. Here, the actions of VR on pain mechanisms are divided into two types: distraction and neuroplasticity. Distraction refers to the short-term diversion of attention away from pain towards an alternative stimulus whereby, VR may act on pain by ‘hijacking’ attention, emotion and memory. These effects, depending on the level of immersion, may be due to excitability of neuron populations in brain regions associated with pain modulation. For example, healthy participants reporting decreases in pain intensity from painful thermal stimulation during distraction show decreases in thalamic, insular and anterior cingulate cortex activation using fMRI [10]. Alternatively, neuroplasticity refers to long-term functional and structural changes in neuronal pathways and synapses that may occur following long-term practice of skills, such as playing a musical instrument or VR use involving interactive real-time simulations of scenes or activities. In this study, we investigated only short-term analgesic effects that were assessed during and immediately following each intervention session. Although we can provide no evidence, the analgesic effect of the VR application for pain in this population, attention distraction is most likely the best explanation, especially concerning short-term or single session VR use. These immediate effects of VR are shown to be due to temporary activation of top-down pain modulatory pathways via the cingulo-frontal cortex and periaqueductal grey [29].
Despite current awareness of short-term benefits of VR in people with chronic pain conditions, long-term effects of VR on pain intensity, whilst promising, require further investigation. A recent meta-analysis investigating the effect of VR on pain perception shows that although frequency and time of exposure appear to be important aspects of VR interventions when managing chronic pain, current evidence is inconsistent. Encouragingly, however, studies investigating long-term VR exposure on SCI pain do show that exposures over longer periods do show greater reductions in pain intensity. Here, VR has been used alone, or in combination with other forms of treatment such as transcranial direct current stimulation and exoskeleton muscle training. VR (3D and 2D) exposure over multiple sessions show the greatest reductions in pain severity where in some cases, analgesic effects continued several weeks after treatment [30], suggesting long-term neuroplastic changes to central pain pathways. Given these findings, larger studies are required to determine the long-term analgesic effects of different types of VR application alone and whether VR such as the one used in this study are cumulative in the duration of pain relief over time.
Promisingly in this study, all participants completed both VR interventions with none reporting discomfort with the VR headset, or cybersickness during or after using the 3D HMD VR. To optimise user friendliness, we selected a VR application with no pitch or roll elements while participants used a handheld device that enabled them to stop, start or change direction when they chose.
This study had several limitations. First, our sample size was relatively small with a low number of people with SCI and NP responding to the study invitation, who were residing in the same city and willing to travel to the hospital for testing. Second, the group included people on various types of prescribed pain medications. However, our sample was relatively homogeneous in relation to having predominantly neuropathic SCI pain. Third, our study focused on short-term outcomes and it is not possible to draw conclusions about long-term or cumulative effects. Although any effective treatment option with a short-term effect is arguably beneficial in this sample, it is important to determine whether this can be extended by repeated use or different protocols. Nevertheless, our findings highlight the efficacy of accessible, inexpensive 3D HMD VR applications for the short-term relief of neuropathic SCI pain.
Conclusion
We suggest that 3D HMD VR may provide NP relief for people with SCI. Given the lack of cybersickness and increasing ease of access, we also suggest 3D HMD VR applications could be a helpful adjunct to centrally-acting pain medications to control symptoms of this long-term problem. Further research is required to show that VR can be effective for more long-term reductions in neuropathic SCI pain.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Burke D, Fullen BM, Stokes D, Lennon O. Neuropathic pain prevalence following spinal cord injury: a systematic review and meta-analysis. Eur J Pain. 2017;21:29–44.
Hadjipavlou G, Cortese AM, Ramaswamy B. Spinal cord injury and chronic pain. BJA Educ. 2016;16:264–8.
Gerke MB, Duggan AW, Xu L, Siddall PJ. Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience. 2003;117:715–22.
Weech S, Kenny S, Barnett-Cowan M. Presence and cybersickness in virtual reality are negatively related: a review. Front Psychol. 2019;10:1–19.
Kenny MP, Milling LS. The effectiveness of virtual reality distraction for reducing pain: a meta-analysis. Psychol Conscious Theory Res Pract. 2016;3:199–210.
Villiger M, Bohli D, Kiper D, Pyk P, Spillmann J, Meilick B, et al. Virtual reality-augmented neurorehabilitation improves motor function and reduces neuropathic pain in patients with incomplete spinal cord injury. Neurorehabil Neural Repair. 2013;27:675–83.
Rooney B, Hennessy E. Actually in the cinema: a field study comparing real 3D and 2D movie patrons’ attention, emotion, and film satisfaction. Media Psychol. 2013;16:441–60.
Norrbrink Budh C, Lund I, Hultling C, Levi R, Werhagen L, Ertzgaard P, et al. Gender related differences in pain in spinal cord injured individuals. Spinal Cord. 2003;41:122–8.
The International Spinal Cord Society. International standards for neurological classification of spinal cord injury. Aylesbury, UK: International Spinal Cord Society; 2019. https://www.iscos.org.uk/international-standards-for-neurological-classification-of-spinal-cord-injury-isncsci.
Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–9.
Evans SR. Clinical trial structures. J Exp Stroke Transl Med. 2010;3:8–18.
Jin W, Choo A, Gromala D, Shaw C, Squire P. A virtual reality game for chronic pain management: a randomized, controlled clinical study. Stud Health Technol Inform. 2016;220:154–60.
Fowler CA, Ballistrea LM, Mazzone KE, Martin AM, Kaplan H, Kip KE, et al. A virtual reality intervention for fear of movement for Veterans with chronic pain: protocol for a feasibility study. Pilot Feasibility Stud. 2019;5:146.
LaViola JJ. A discussion of cybersickness in virtual environments. SIGCHI Bull. 2000;32:47–56.
Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58.
Mitchell MC, Burns NR, Dorstyn DS. Screening for depression and anxiety in spinal cord injury with DASS-21. Spinal Cord. 2008;46:547–51.
Schwind V, Knierim P, Haas N, Henze N. Using presence questionnaires in virtual reality. Proceedings of the 2019 CHI conference on human factors in computing systems; Glasgow, Scotland Uk: Association for Computing Machinery; 2019. pp. 360.
IBM C. IBM SPSS statistics for Windows, version 25.0. Armonk, NY: IBM Corp; 2018.
Kenward MG, Roger JH. The use of baseline covariates in crossover studies. Biostatistics. 2010;11:1–17.
Mallari B, Spaeth EK, Goh H, Boyd BS. Virtual reality as an analgesic for acute and chronic pain in adults: a systematic review and meta-analysis. J Pain Res. 2019;12:2053–85.
Rosner B. Hypothesis testing: two-sample inference. fundamentals of biostatistics. 7th ed. CENGAGE Learning Inc; Boston USA, 1995. p. 271.
Jutzeler CR, Warner FM, Cragg JJ, Haefeli J, Richards JS, Andresen SR, et al. Placebo response in neuropathic pain after spinal cord injury: a meta-analysis of individual participant data. J Pain Res. 2018;11:901–12.
Eippert F, Finsterbusch J, Bingel U, Büchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326:404.
Pozeg P, Palluel E, Ronchi R, Solcà M, Al-Khodairy AW, Jordan X, et al. Virtual reality improves embodiment and neuropathic pain caused by spinal cord injury. Neurology. 2017;89:1894–903.
Jordan M, Richardson EJ. Effects of virtual walking treatment on spinal cord injury-related neuropathic pain: pilot results and trends related to location of pain and at-level neuronal hypersensitivity. Am J Phys Med Rehabil. 2016;95:390–6.
Jones T, Moore T, Choo J. The impact of virtual reality on chronic pain. PloS ONE. 2016;11:e0167523.
Wake N, Sano Y, Oya R, Sumitani M, Kumagaya S, Kuniyoshi Y. Multimodal virtual reality platform for the rehabilitation of phantom limb pain. In: 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, 2015, pp. 787–90.
Flores A, Linehan MM, Todd SR, Hoffman HG. The use of virtual reality to facilitate mindfulness skills training in dialectical behavioral therapy for spinal cord injury: a case study. Front Psychol. 2018;9:531.
Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain-an fMRI analysis. Pain. 2004;109:399–408.
Donati ARC, Shokur S, Morya E, Campos DSF, Moioli RC, Gitti CM, et al. Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci Rep. 2016;6:30383.
Funding
The postdoctoral researcher (PA) was funded by Australian and New Zealand College of Anaesthetists project grant (19/002) for this study.
Author information
Authors and Affiliations
Contributions
PA—principle investigator. Contributed to the design of study protocols, screening and recruitment of subjects, administering questionnaires, organising clinic visits, performance of VR, collection and analysis of data and drafting presentations, reports and the current article. AC—contributed to the design of study protocols, recruitment of subjects. He also contributed to the writing while also provided feedback on the article. JM—contributed to the design of study protocols, location and recruitment of subjects. He also contributed to the writing while also provided feedback on the article. YT—contributed to the design of study protocols, development of the study. She also contributed to the writing while also provided feedback on the article. DC—designed and conducted statistical analysis. He also contributed to the writing of the current study article. PW—contributed to the design of study protocols, screening and recruitment of subjects. He also contributed to the writing of the current study article. PS—chief investigator. Initiated and contributed to the design of study protocols, screening and recruitment of subjects. He also contributed to the writing while also provided feedback on the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteer were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Austin, P.D., Craig, A., Middleton, J.W. et al. The short-term effects of head-mounted virtual-reality on neuropathic pain intensity in people with spinal cord injury pain: a randomised cross-over pilot study. Spinal Cord 59, 738–746 (2021). https://doi.org/10.1038/s41393-020-00569-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-020-00569-2
This article is cited by
-
Virtual Reality in Acute and Chronic Pain Medicine: An Updated Review
Current Pain and Headache Reports (2024)
-
Machine Learning in Clinical Trials: A Primer with Applications to Neurology
Neurotherapeutics (2023)
-
Digital therapeutics in neurology
Journal of Neurology (2022)
-
Feasibility and acceptability of virtual reality for cancer pain in people receiving palliative care: a randomised cross-over study
Supportive Care in Cancer (2022)