Abstract
Study design
Retrospective chart study.
Objectives
The aim was to examine continuation of treatment of individuals with spinal cord injury including myelomeningocele and neurogenic detrusor overactivity, treated with repeated intra-detrusor Botulinum toxin A injections, and to investigate factors associated with discontinuation of treatment.
Setting
Rigshospitalet, Denmark
Methods
This study included 128 individuals with spinal cord injury and neurogenic detrusor overactivity, who were offered repeated Botulinum toxin A injections between 2001 and 2018. Continuation rates of the treatment were estimated using Kaplan Meier analysis. A Cox proportional hazard analysis was used to investigate factors predictive of discontinuation.
Results
A total of 1156 treatments were performed. The median number of treatments was six (IQR 9, range 1−51), and median follow-up was 10.6 years (IQR 8.5, range 0−16.9). All urodynamic parameters changed significantly after the first treatment (p < 0.001). The continuation group had significantly higher mean maximum bladder capacity after the first injections compared with the discontinuation group, with a mean difference between the groups of 84.5 mL (95% CI 4.7–164.2) (p = 0.038). The probability of continuing treatments after 5 years was 59% (95% CI 50.0–67.8) and 50% (95% CI 40.1–59.3) after 10 years. Individuals aged 31–50 years were more likely to continue treatment compared with those aged >50 years (95% CI 0.21‒0.79) (p = 0.008). No other factors predicted discontinuation.
Conclusions
This long-term follow-up study showed that 50% of people with spinal cord injury starting intra-detrusor Botulinum toxin A for neurogenic detrusor overactivity are still receiving injections after 10 years.
Similar content being viewed by others
Introduction
Individuals with spinal cord injury (SCI) including myelomeningocele (MMC) suffer a significant risk of developing long-term urinary tract complications and ultimately renal failure due to neurogenic detrusor overactivity (NDO) [1, 2]. Furthermore, NDO frequently results in urinary incontinence (UI), which has shown to impair quality of life (Qol) [3]. The aim of the neuro-urological care of these individuals is to protect the upper urinary tract by maintaining a low bladder-pressure, achieve urinary continence and improve Qol [4]. The use of Botulinum toxin A (Btx-A) was first reported in 2000 as a minimally invasive treatment for NDO [5]. It has since become a well-known and recommended second line treatment of NDO, in cases where antimuscarinic therapy is ineffective or not well tolerated [4]. The mean effect of Btx-A injections is ~9–11 months, however shorter durations of effect are found in the literature [6]. The treatment is primarily carried out under local anesthesia, but for some individuals it is necessary to undergo general anesthesia. Since its introduction by Schurch [5], it has been established in several studies, that Btx-A improves both the urodynamic parameters and the Qol. However, the number of studies investigating the long-term compliance with Btx-A is limited. Since 2001, the Urological department, Rigshospitalet, Copenhagen, Denmark has offered Btx-A injections as second line treatment for people diagnosed with SCI and NDO, free of charge through the Danish universal health care coverage [7]. After the initial treatment, the individuals are instructed to contact the clinic once symptoms of UI recurred, after which they receive a new treatment without further evaluation. The aim of this study was to evaluate continuation with treatment of NDO with repeated intra-detrusor Btx-A injections, and to investigate factors predictive of discontinuation of the treatment.
Methods
This study is a retrospective chart review. Data regarding the date of the first Btx-A treatment, number of treatments, time interval between treatments, cause of SCI, level and completeness of SCI including MMC according to International Standards for Neurological Classification of SCI, including the American Spinal Injury Association impairment scale (AIS) [8] or Frankel scale [9] were obtained from medical records. In cases where an AIS or Frankel assessment was not available, the degree of injury was assessed from medical records and if possible classified by a SCI expert. Individuals were grouped according to level and severity of the SCI, as recommended by Biering-Sørensen et al. [10].
Study population
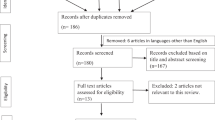
Individuals who had received their first intra-detrusor Btx-A injection at Rigshospitalet in the period 2001–2016 and were identified with SCI including MMC through the Clinic for Spinal Cord Injuries, Rigshospitalet were included in this study. Individuals were injected with Onabotulinum Toxin A, Allergan. Patients were only included if they had at least one urodynamic investigation (UDI) prior to the first Btx-A injection and had NDO on the pre-treatment UDI. Patients were either treated for the indication of UI due to NDO or high bladder pressure due to NDO. Individuals with bladder cancer, bladder stone or aged <15 years at first Btx-A injection were excluded. The selection of individuals for the study is shown in Fig. 1, including the subpopulation with a pre- and post-treatment UDI available. All patients were routinely treated with anti-cholinergics as first-line treatment, before starting Btx-A injections.
Flow diagram showing exclusion of patients. 1st exclusion: Misdiagnosed with SCI (n = 3) Records not found (n = 3) < 15 years old (n = 3) Received Btx injections in the urethral sphincter, not the detrusor (n = 1) Received first Btx injection at another hospital (n = 1) Multiple Sclerosis patient at injection time, patient suffered SCI later (n = 1) 2nd exclusion: Not found pretreatment urodynamics (n = 10).
Urodynamic assessment and intra-detrusor Btx-A injections
Patients were routinely assessed by UDI before and after the first Btx-A injection. NDO was defined as any detrusor overactivity during the storage phase [11]. In both the pre- and post-treatment UDI the following key parameters were obtained: (1) maximum detrusor pressure during filling, (2) cystometric capacity, (3) compliance, and (4) reflex volume, defined as the bladder volume, at which the first observed detrusor contraction occurred during the filling cystometry. If they did not have any DO on their posttreatment UDI, the reflex volume was defined as the cystometric capacity. Btx-A injections were performed sparing the trigone. The standard dose used was 300 U until 2012. Thereafter, the dose was changed to 200 U according to new phase 3 study-recommendations [12, 13]. In special cases, an alternate dose was used (50 U–400 U). In individuals with a preserved bladder sensibility, general or local anesthesia was required. In individuals with decreased or absent bladder sensibility, no anesthesia was used. At the beginning of the study period patients routinely received 500 mg Ciprofloxacin before Btx-A injections and twice after the injections. In 2011 the routine changed to 500 mg Ciprofloxacin administered once before the treatment.
Follow-up
The follow-up period was defined as the time interval between the first Btx-A treatment and October 2018, meaning everyone were followed for at least 2 years from their first Btx-A treatment. Individuals who died or were unable to continue with treatment due to pregnancy, bladder cancer, or change of geographical location during the study period were censored from the analysis at this time. The study population were divided into two groups, “continuation” and “discontinuation”, according to the time interval between their last Btx-A treatment and the follow-up date. Individuals with ≤2 years since last treatment were categorized as continuation group, while individuals with >2 years since last treatment were categorized as discontinuation group. Based on information obtained from medical records, reasons for discontinuing treatment in the discontinuation group were described.
Statistical analysis
Differences in urodynamic parameters before and after the first treatment were investigated with paired t test for normally distributed data and Wilcoxon signed rank test for non-normally distributed data. Differences in urodynamic changes between the continuation and discontinuation group were examined with Student’s t test for normally distributed data and Wilcoxon rank sum test for non-normally distributed data. Kaplan Meier analyses were used to examine the probability of continuation with treatment over time and according to number of treatments. The effect of the different covariates (gender, level and completeness of injury, etiology of injury, bladder emptying method, UI at baseline, or anesthesia at first treatment) on the risk of discontinuation with treatment were examined with Cox proportional hazards ratio analyses. The assumptions for using proportional hazards were analyzed with Wald test with a time-dependent covariate and the Schoenfeld residual plots. All statistical analyses were performed in SAS version 7.1 (SAS Institute, Cary, NC, USA), and P < 0.05 was considered statistically significant.
Results
Study population
Table 1 lists the characteristics of the 128 individuals included in the study. A total of 1156 Btx-A injections were performed in the 128 included individuals at Rigshospitalet during the follow-up period. A total of 115 people received more than one Btx-A injection and 13 individuals received a single injection. The median number of treatments was 6 (IQR 9, range 1–51), the median time-interval between treatments was 226 days and the median follow-up was 10.6 years (IQR 8.5, range 0–16.9). The median age at first Btx-A injection was 38 years (IQR 25.7, range 15–81). Of the 15 people with normal voiding before Btx-A injections, five continued to void normally, eight changed to a clean intermittent catheterization (CIC) regime, one changed to indwelling catheter and one voided by Valsalva after the first Btx-A injection. Of the ten people with indwelling catheter, six continued to use indwelling catheter and two changed to a CIC regime. Two individuals had unknown voiding after the first injection. No significant differences were found between the two groups with regards to baseline characteristics.
Urodynamic findings in subpopulation
A total of 96 individuals had a pre- and post-treatment UDI. The remaining 32 had no post-treatment UDI due to either absence on the day of UDI or suspected urinary tract infection. In six of the 96 individuals with a post-treatment UDI, the post-treatment UDI was carried out after the second Btx-A injection. At the post-treatment UDI, a significant increase in the cystometric capacity and the reflex volume was found, as well as a significant decrease in maximum detrusor pressure during filling and the DO/cystometry ratio (Table 2). The continuation group had a 84.6 mL (CI 95% 4.7–164.2) increase in mean maximum bladder capacity after the first Btx-A injection compared with the discontinuation group (Table 3). In addition, the continuation group had a 72.1 mL (CI 95% (−3.9 to 148.2) larger increase in reflex volume than the discontinuation group, however this was not statistically significant.
Long term continuation
During the study period 12 individuals died, six changed geographical location and one became pregnant. An additional three individuals were lost to follow-up. The Kaplan Meier curves in Figs. 2 and 3 show the risk of discontinuation with continuous Btx-A injections according to time and number of treatments, respectively. The probability of receiving Btx-A treatments was 59.1% (95% CI 50.0–67.8) after 5 years and 50.1% (95% CI 40.1–59.3) after 10 years (Fig. 2). When looking at the number of treatments, 66.0% (95% CI 56.6–73.5) were still in continuous Btx-A treatment after five treatments, 53.2% (95% CI 43.3–62.0) after ten treatments and 49.6% (95% CI 39.2–59.1) after 15 treatments, with a stabilization of the continuation rate after eight treatments (Fig. 3). Individuals aged 31–50 years were more likely to continue treatment compared with individuals aged above 50 years (95% CI 0.21‒0.79) (p = 0.008). Compared with the group using CIC, individuals with indwelling catheter had an increased risk of discontinuation, however, this was not significant. No association was found between treatment discontinuation and gender, level and completeness of injury, etiology of injury (MMC vs. other etiologies to SCI), UI at baseline, and anesthesia during the first treatment (Table 4).
Reasons for discontinuing treatment
In all, 58 individuals discontinued treatment. Twenty individuals discontinued treatment due to patient-reported lack of effect. Seventeen individuals had previously reported a good effect of the Btx-A treatment but discontinued due to other reasons, including pain during procedure and patient reported side-effects. Thirteen individuals did not state a reason for discontinuation despite previously reporting good effect of the Btx-A treatment. An additional ten individuals discontinued due to unknown reasons. In addition, three people were lost to follow-up, five discontinued treatment due to concurrent medical conditions, and four reported to have secondary failure of Btx-A, with decreasing effect over time.
Discussion
This study presents data with a median follow up of 10.6 years of continuous intra-detrusor Btx-A injections in people with SCI. The study confirms that Btx-A injections are a highly effective method to lower the maximum detrusor pressure during filling and increase the cystometric capacity and reflex volume in individuals with SCI and NDO. Furthermore, we found a 59% continuation rate after 5 years and 50% continuation rate after 10 years with repeated Btx-A injections, suggesting a good long-term continuation with repeated Btx-A injections in people with SCI and NDO. The continuation rates in NDO patients have been reported between 36.2% and 71.1% [14,15,16]. The highest continuation rates were found by Joussain et al., who reported a 71.1% continuation rate after 5 years and by Leitner et al. who found that 60% of their study population continued treatment after 10–17 years follow-up [15]. Veeratterapillay et al. found a continuation rate of 75%, however this analysis only included individuals with two or more injections, excluding individuals who discontinued after a single treatment, thus making the continuation rate higher than other studies [17]. Conversely, Mohee et al. found lower continuation rates, where only 36.2 % was still in treatment after 5 years. This was however a population of both idiopathic detrusor overactivity (IDO) and NDO. Indeed, half of the individuals in the NDO group continued, while only a third from the IDO group continued, though this difference was not significant [14]. This is in concordance with other studies who reported better long-term success for NDO patients compared with IDO patients [18].
Our study is unique in only including people with SCI including MMC as opposed to a mixed NDO population consisting of both SCI and multiple sclerosis (MS). Even though both SCI and MS patients suffer from NDO, the two populations may vary in adherence to Btx-A injection. Individuals with SCI are neurologically stable where MS patients’ neurological symptoms vary over time, which may make for example CIC an increasing challenge over time. Leitner et al., who found that 60% of their population of mixed NDO patients continued treatment, observed that 67.5% of individuals with SCI continued treatment, as opposed to only 14% of the people with MS [15]. Joussain et al. also had a population of mixed NDO, where 84.6% of the population suffered from traumatic SCI and only 10.6% suffered from MS. Yet, the MS group accounted for 54.4% of all CIC-related withdrawals from Btx-A treatment, which was the main reason for discontinuation along with personal convenience.
In our population the main reasons for discontinuing treatment was reported lack of effect (20 individuals) and the individuals’ wished to stop treatment, despite having reported a good effect of Btx-A earlier (17 individuals). These findings are similar to the observations of Leitner et al., who found that 50% of the people who discontinued treatment with Btx-A had no effect of the treatment. The continuation rates of this study are slightly lower than the ones found by Joussain et al. and Leitner et al. [15, 16]. This might be due to our population being less selected than the populations of Leitner et al. and Joussan et el., who only included individuals with more than two Btx-A treatments, and individuals who performed CIC before Btx-A, respectively. Furthermore, we found a stabilization of the discontinuation rate after eight treatments (Fig. 3) suggesting a good long-term continuation for individuals who have received more than eight treatments, which was the case for 52 of the patients.
We found that individuals aged 31–50 years were significantly more likely to continue Btx-A treatment compared with people above 50 years. Interestingly Mohee and Joussain found older age was predictive of long-term continuation [14, 16]. In contrast to this study as well as Leitner’s and Mohee’s studies, Joussain et al. found multiple parameters associated with discontinuation. People with SCI face several different challenges in their everyday life, which may vary greatly from individual to individual. Perhaps the decision to continue or discontinue Btx-A treatment has more to do with the current life circumstances of the individual than any one parameter alone. Even though it is not possible to predict who will continue and who will not in this study, the discontinuation rate is still valuable information to an individual starting Btx-A injections.
Strengths
This study was carried out in a single center, providing a standardized treatment and follow-up for all individuals. In addition, the study had a long follow-up of up to 17 years and the people in the present study were selected based on SCI diagnosis and urodynamically confirmed NDO, thus, increasing the generalizability of the results to a general clinical setting. Furthermore, the treatment was offered free of charge to all individuals through the Danish health care system, eliminating discontinuation due to financial reasons and including all people regardless of income status.
Limitations
A total of 23 out of the 58 individuals who discontinued did not state a reason for discontinuing the treatment. Therefore, there is a risk that this study has underestimated the continuation rate, since some of these individuals may have moved, become pregnant or had other concurrent medical conditions without reporting it to either the urological department or the SCI clinic.
Discontinuation was defined as absence from Btx-A treatment for more than 2 years in this study. According to the literature, the mean duration of Btx-A is 9–11 months, hence it could be argued a 2-year cutoff may overestimate the continuation rate, however, too short a cutoff may overestimate discontinuation. As several individuals from our population reported effect duration for more than one year and to account for a delay in time from contacting the clinic, to the next treatment is carried out, the authors found that a 2 year cutoff was appropriate.
The retrospective nature of this study has some limitations. Data regarding UI was not routinely recorded by physicians, and therefore, this study relies on the urodynamic investigation instead of the symptoms of the patients. However, improvement of the urodynamic parameters may not always result in continence of the individual, and therefore information regarding incontinence before and after the first treatment could possibly allow for a more complete analysis of discontinuation of the treatment. In addition, multivariate statistical analyses would have been preferable in investigating factors predictive of discontinuation, however, this was not possible due to the limited study population.
Conclusions
In conclusion, the present study confirms the beneficial effect of intra-detrusor Btx-A injections on urodynamic parameters, as it significantly reduced the maximum detrusor pressure during filling and increased both the cystometric capacity and reflex volume. In the long-term, 50% of people with SCI are still receiving Btx-A injections after 10 years with a stabilization of the discontinuation rate after eight treatments, suggesting a good long-term continuation with Btx-A injections.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Nseyo U, Santiago-lastra Y. Long-term complications of the neurogenic bladder. Urol Clin North Am. 2017;44:355–66.
Gormley EA. Urologic complications of the neurogenic bladder. Urol Clin North Am. 2010;37:601–7.
Hicken BL, Putzke JD, Richards JS. Bladder management and quality of life after spinal cord injury. Am J Phys Med Rehabil. 2001;80:916–22.
Blok B, Pannek J, Castro-Diaz D, Del Popolo G, Groen J, Hamid R et al. EAU Guidelines. Edn. presented at the EAU Annual Congress Copenhagen 2018.
Schurch B, Stöhrer G, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum toxin-A for treating detrusor hyperreflexia in spinal cord injured patientpt 1s: a new alternative to anticholinergic drugs? Preliminary results. J Urol 2000;164(3 Pt 1):692–7.
Goessaert A, Everaert K. Onabotulinum toxin A for the treatment of neurogenic detrusor overactivity due to spinal cord injury or multiple sclerosis. Exp Rev Neurother. 2012;12:763–75.
Bagi P, Biering-Sørensen F. Botulinum toxin A for treatment of neurogenic detrusor overactivity and incontinence in patients with spinal cord lesions. Scand J Urol Nephrol. 2004;38:495–8.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. Reference for the 2011 revision of the international standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2011;34:547–54.
Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia 1969;7:179–92.
Biering-Sørensen F, DeVivo MJ, Charlifue S, Chen Y, New PW, Noonan V, et al. International Spinal Cord Injury Core Data Set (version 2.0)—including standardization of reporting. Spinal Cord 2017;55:759–64.
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003;61:37–49.
Cruz F, Herschorn S, Aliotta P, Brin M, Thompson C, Lam W, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol 2011;60:742–50.
Ginsberg D, Gousse A, Keppenne V, Sievert KD, Thompson C, Lam W, et al. Phase 3 efficacy and tolerability study of onabotulinumtoxin A for urinary incontinence from neurogenic detrusor overactivity. J Urol 2012;187:2131–9.
Mohee A, Khan A, Harris N, Eardley I. Long-term outcome of the use of intravesical botulinum toxin for the treatment of overactive bladder (OAB). BJU Int 2013;111:106–13.
Leitner L, Guggenbühl-Roy S, Knüpfer SC, Walter M, Schneider MP, Tornic J, et al. More than 15 years of experience with intradetrusor onabotulinumtoxina injections for treating refractory neurogenic detrusor overactivity: lessons to be learned. Eur Urol 2016;70:522–8.
Joussain C, Popoff M, Phé V, Evan A, Bosset PO, Pottier S, et al. Long-term outcomes and risks factors for failure of intradetrusor onabotulinumtoxin A injections for the treatment of refractory neurogenic detrusor overactivity. Neurourol Urodyn 2018;37:799–806.
Veeratterapillay R, Harding C, Teo L, Vesdev N, Abroaf A, Dorkin TJ, et al. Discontinuation rates and inter-injection interval for repeated intravesical botulinum toxin type A injections for detrusor overactivity. Int J Urol. 2014;21:175–8.
Rahnama’i MS, Marcelissen TAT, Brierley B, Schurch B, de Vries P. Long-term compliance and results of intravesical botulinum toxin A injections in male patients. Neurourol Urodyn 2017;36:1855–9.
Biering-Sørensen F, Craggs M, Kennelly M, Schick E, Wyndaele JJ. International lower urinary tract function basic spinal cord injury data set. Spinal Cord 2008;46:325–30.
Author information
Authors and Affiliations
Contributions
KPH was responsible for collecting data, analyzing and interpreting data, and writing the report. NK was responsible for designing the protocol, contributed to analyzing and interpreting data, and revised the article critically. PB and FBS was responsible for designing the protocol, interpreting data, and revised the article critically. ME was responsible for designing and writing the protocol, interpreted and analyzed the data, conducted the Cox proportional Hazard analysis, and revised the article critically.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Danish data protection agency and the Danish patient safety authority. We certify that all applicable institutional and governmental regulations concerning the ethical use of data originally collected for clinical purposes were followed during the course of this research.
Conflict of interest
NK and KPH have received travel grants from Contura. ME has received honoraria and travel grants from Contura. However, Contura had no involvement in the collection, analysis, interpretation, or write up of this paper. PB and FBS have no disclosures to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hebert, K.P., Klarskov, N., Bagi, P. et al. Long term continuation with repeated Botulinum toxin A injections in people with neurogenic detrusor overactivity after spinal cord injury. Spinal Cord 58, 675–681 (2020). https://doi.org/10.1038/s41393-019-0411-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-019-0411-0