Abstract
Study design
Cohort study.
Objectives
It is widely accepted that the prediction of long-term neurologic outcome after traumatic spinal cord injury (SCI) can be done more accurately with neurological examinations conducted days to weeks post injury. However, modern clinical trials of neuroprotective interventions often require patients be examined and enrolled within hours. Our objective was to determine whether variability in timing of neurological examinations within 48 h after SCI is associated with differences in observations of follow-up neurologic recovery.
Setting
Level I trauma hospital.
Methods
An observational analysis testing for differences in AIS conversion rates and changes in total motor scores by neurological examination timing, controlling for potential confounders with multivariate stepwise regression.
Results
We included 85 patients, whose mean times from injury to baseline and follow-up examinations were 11.8 h (SD 9.8) and 208.2 days (SD 75.2), respectively. AIS conversion by 1+ grade was significantly more likely in patients examined at ≤4 h in comparison with later examination (78% versus 47%, RR = 1.66, p = 0.04), even after controlling for timing of surgery, age, and sex (OR 5.0, 95% CI 1.1–10, p = 0.04). We failed to identify any statistically significant associations for total motor score recovery in unadjusted or adjusted analyses.
Conclusions
AIS grade conversion was significantly more likely in those examined ≤4 h of injury; the effect of timing on motor scores remains uncertain. Variability in neurological examination timing within hours after acute traumatic SCI may influence observations of long-term neurological recovery, which could introduce bias or lead to errors in interpretation of studies of therapeutic interventions.
Similar content being viewed by others
Introduction
Acute traumatic spinal cord injuries (SCI) are life-changing events that can substantially impair patients’ physical and psychosocial function, and patients with acute SCI often pose important questions about their expected extent of long-term neurological recovery. Clinicians might respond with some general guidance according to various clinical and radiological parameters, but accurate prediction of long-term outcomes in the acute setting is known to be highly uncertain. For example, consider a patient diagnosed with an American Spinal Injury Association (ASIA) Impairment Scale (AIS) A complete cervical SCI at 4 months, 4 days, or 4 h post injury. At 4 months post injury, many clinicians would confidently predict that this patient is likely to remain AIS A long-term. However, their confidence in doing so would be lower at 4 days, and much lower still at just 4 h. Patients who are evaluated very early after SCI are thought to have greater potential for spontaneous neurological recovery and it is intuitive that their prognosis could vary in comparison with patients evaluated much later [1].
While it may seem unreasonably ambitious or even unnecessary to predict long-term outcomes within hours after SCI, this is actually an important problem for modern clinical trials of neuroprotective interventions that are administered very early. Recent studies of minocycline, riluzole, and magnesium chloride required acute SCI patients to be resuscitated, evaluated, imaged, assigned a baseline AIS grade, and then consented and randomized with enough time to contact a hospital pharmacy and have study drugs delivered to the bedside within 12 h of injury [2,3,4]. Variability in spontaneous neurological recovery related simply to differences in neurological examination timing within hours after SCI could influence prognosis, introduce bias, and lead to errors in interpretation of results.
Although the issue of neurological examination timing in patients with SCI has been considered elsewhere in the literature, prior reports have only considered timing on the order of days or weeks [1]. The influence of varying neurological examination timing within just hours after SCI has not been investigated and remains a critical knowledge gap that is particularly relevant to the study of neuroprotective interventions for acute SCI. In this study, our objective was to determine whether variability in the timing of neurological examinations within the first 48 h of injury is associated with differences in observations of AIS conversion and motor score recovery at final follow-up. We hypothesized that earlier neurological examinations would be associated with improved neurological outcomes, due to greater spontaneous neurological recovery.
Methods
Study design
We performed an observational study with a retrospective analysis of data that were prospectively collected at the Vancouver General Hospital as part of the Rick Hansen Spinal Cord Injury Registry (RHSCIR) in Canada. Our methods were similar to those reported previously for other studies from this dataset [5,6,7,8,9,10]. RHSCIR is an ongoing prospective multicenter study of patients with acute traumatic spinal cord injuries, and the Vancouver General Hospital is a major academic quaternary care referral center in the RHSCIR network. We obtained local Research Ethics Board approval prior to enrolling patients, collecting data, and performing this study. Further descriptions of the RHSCIR data elements, procedures, governance structure, and privacy and confidentiality framework have been described elsewhere [11].
Patient sample
We included all consecutive patients enrolled from 2004 to 2017 that presented with complete AIS A acute traumatic cervical spinal cord injuries, were admitted and examined within 48 h of injury, and had complete baseline data with at least 2 months of follow-up. AIS A injuries are defined as complete injuries with no motor or sensory function preserved below the neurological level of injury and no sacral sparing, while AIS B have preserved sensory but not motor function, AIS C have some preservation of motor function in which more than half of key muscles below the neurological level have a muscle grade of less than 3, AIS D have some preservation of motor function in which at least half of key muscles below the neurological level have a muscle grade of 3 or more, and AIS E have normal motor and sensory function [12, 13]. We restricted our analysis to AIS A complete injuries because their prognosis is thought to be relatively homogeneous in comparison with incomplete injuries [1, 6].
Data sources
All data were collected by trained research personnel and entered into a standardized local RHSCIR database before being exported to the RHSCIR national office for centralized quality checks [5, 14]. We extracted age, sex, AIS, motor scores, timing of examinations, and timing of surgery. Neurological examinations were performed according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) by trained physicians, nurse practitioners, or physiotherapists [15]. ISNCSCI total motor scores can range from 0 (absent motor function) to 100 (intact motor function). ISNCSCI records were processed through a validated computerized algorithm that has been shown to maintain data consistency and high quality [14]. We considered baseline AIS grades and motor scores to be those obtained on admission to acute care, and final AIS grades and motor scores to be those obtained at the time of discharge to the community from acute care or inpatient rehabilitation.
Statistical analysis
We evaluated the influence of neurological examination timing within hours after SCI by testing for differences in rates of AIS conversion from A to B, C, D, or E and changes in ISNCSCI total motor scores among patients based on how early their neurologic examination was performed. We tested for both one- and two-grade AIS conversion because these approaches have been utilized in earlier literature [16], and we implemented a priori thresholds of 4, 8, 12, and 24 h post injury to define early versus late.
We report discrete variables as counts or proportions, normally distributed continuous variables as means with standard deviations (SD), and skewed continuous variables as medians with interquartile ranges. We used parametric tests for data with normal distributions and nonparametric tests for data without normal distributions. We tested univariate associations with the Pearson Correlation Coefficient, and we tested for differences in categorical variables using the Fisher exact test. We used the Fisher exact test as an alternative to the Pearson chi-square test because it is more conservative in most situations, valid for all sample sizes, and is the preferred approach when sample sizes are small or outcome events are uncommon [17]. We present unadjusted relative risk estimates as risk ratios (RR). We controlled for potential confounding due to timing of surgery using stepwise multiple logistic or linear regression, and we report coefficients as odds ratios (OR) with 95% confidence intervals (CIs).
Patients with missing data were excluded from each analysis and imputations were not performed. All tests of significance were two-tailed and p-values of less than 0.05 were considered significant. We performed our analyses with Microsoft Excel 2011 (Microsoft Corp., Redmond, WA), and IBM SPSS Version 23.0, 2015 (SPSS Inc., Chicago IL).
Results
Of 252 patients with AIS A cervical acute traumatic SCIs who were enrolled into RHSCIR at Vancouver General Hospital between July 2004 and August 2017, we excluded 31 because they had incomplete baseline data (e.g., missing time of injury or time of baseline exam), 77 because their baseline exam was performed more than 48 h post injury, 10 because they were missing follow-up AIS data, and 49 because they had less than 2 months of follow-up (Supplementary Fig. 1). In total, we included 85 cervical AIS A patients whose demographics are shown in Table 1. Mean age was 42.9 (SD 19.0) and 78% were male. The Injury Severity Score was at least 25 in 32%, and the leading mechanisms of injury were falls (39%), transport (32%), and sports (20%). Mean time from injury to baseline neurological examination was 11.8 h (SD 9.8) and from injury to discharge neurological examination was 208.2 days (SD 75.2) (Fig. 1). All patients underwent surgery except for two that were treated nonoperatively. A further 10 patients were subsequently excluded from our analyses of motor score outcomes because they had incomplete follow-up motor score data, yielding a sample of 75 for motor score outcomes.
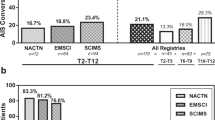
The overall rate of conversion from complete (AIS A) to incomplete SCI (AIS B, C, or D) in our cohort was 52%. Forty-one patients (48%) remained AIS A, 22 converted to B (26%), 15 to C (18%), and 7 to D (8%). Patients examined earlier postinjury experienced AIS grade conversion more frequently in comparison with those examined later (Table 2), and the timing of baseline neurological examination at 4 h or less post injury was associated with statistically significant increases in rates of AIS conversion in comparison with more than 4 h. Specifically, 78% of patients examined at 4 h or less post injury converted by at least one grade in comparison with 47% of patients examined at more than 4 h (RR = 1.66, p = 0.04, Fig. 2a). Similarly, 50% of patients examined at 4 h or less converted by at least two grades in comparison with 21% of patients examined at more than 4 h (RR = 2.4, p = 0.04). There were no significant differences in rates of AIS grade conversion at thresholds of 8, 12, or 24 h.
Timing of baseline neurological examination post injury was statistically significantly correlated with timing of surgery post injury (r = 0.44, p < 0.01). We controlled for this potential confounder, as well as age and sex, by performing adjusted analyses with stepwise multiple logistic regression. In the adjusted analysis, timing of baseline neurological examination at 4 h or less post injury was independently associated with increased rates of AIS conversion (OR 5.0, 95% CI 1.1–10, p = 0.04, Table 3).
Mean motor score recovery across the cohort was 13.0 points (SD 14.1). Patients examined earlier appeared to experience greater motor score improvement in comparison with than those examined later (Table 4), but comparisons of motor score recovery among patients examined before and after thresholds of 4 (Fig. 2b), 8, 12, or 24 h were not statistically significant. These findings were consistent in adjusted analyses that controlled for timing of surgery, age, and sex.
Discussion
We performed an observational study to evaluate the influence of neurological examination timing within hours after acute traumatic SCI on final neurological outcomes among patients with cervical complete SCI. We found that patients examined earlier converted by one and two or more AIS grades more frequently in comparison with those examined later, and that a timing threshold of 4 h or less post injury was statistically significant even after controlling for timing of surgery. Patients examined earlier also appeared to experience greater motor score improvement in comparison with those examined later, but a statistically significant association for this outcome was not established across multiple timing thresholds in unadjusted and adjusted analyses.
Limitation and strengths
We did not perform a sample size calculation when designing this study, which raises the possibility that our failure to identify an effect of neurological examination timing on total motor score recovery could represent a type II error [18]. Indeed, conventional sample size formulae suggest that we would have required ~500 patients to confidently identify our observed differences in motor scores [19]. Conventional sample size formulae also suggest that our study was more than adequately powered to detect our observed statistically significant differences in AIS conversion rates, but our p-values were modest and we did not correct for multiple comparisons. Further analyses from larger or combined datasets of other studies that prospectively enrolled patients early after SCI are warranted to clarify our findings.
Likewise, we explored a priori thresholds of 4, 8, 12, and 24 h post injury because of their potential relevance to the design and conduct of future clinical trials and perceived clinical importance, but it is plausible that the influence of neurological examination timing could be even more granular. The biological processes of neurological recovery after acute traumatic SCI are time dependent and nonlinear, and clinically important effects due to variable examination timing might exist within even the first hour post injury. At one extreme, it is typical for patients who suffer even the most “mild” traumatic SCI to experience a period of complete paralysis immediately after the injury. Examining such patients at the scene of the accident could certainly lead to the assessment of being classified as clinically “complete”. Examining patients within the first hour seems unfeasible, but longitudinal analyses of repeated prospective measurements that begin as early as possible could inform about the very early neurological trajectories of patients with SCI and the most optimal times to perform a baseline examination. Acknowledging this issue is particularly important when considering the possibility of conducting a clinical trial of a neuroprotective intervention that is deployed to the field to be administered by paramedics. If dependent on the baseline neurologic assessment at such an early time point, such an interventional trial would be wise to consider the effect of the timing of this evaluation in predicting the variability of spontaneous recovery in the control population.
It was inherent to the RHSCIR study design that our final neurological examinations were obtained at the time of discharge to the community from acute care or inpatient rehabilitation, rather than at regular long-term follow-up at a minimum of 1 year of more. Whereas neurological recovery after SCI may continue for at least that long, albeit slowly and to a much lesser extent than occurs acutely, this limitation could have led to diminished final neurological outcomes in our study. However, our mean follow-up of ~7 months post injury likely captured the majority of neurological recovery in most patients, and longer follow-up would not likely have altered our main conclusions.
There exists some controversy about whether acute SCI patients without demonstrable sensory or motor function below their level of injury might be in a state of “spinal shock” that precludes an accurate diagnosis of AIS A complete SCI. Spinal shock has been described as a temporary state of apparent flaccid paralysis in which an injured cord is completely nonfunctional even though it may be structurally intact [20, 21]. This state may occur immediately and then persist for days or even month postinjury, classically until the bulbocavernosus reflex returns. In the strictest of terms, the assignment of an AIS grade is technically not possible prior to the resolution of spinal shock. However, if we strictly adhered to this guidance, we would simply never be able to actually conduct an acute interventional clinical trial, as it would be nearly impossible to actually assign a “valid” baseline to subjects prior to enrollment.
The Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) was a prospective evaluation of early versus delayed surgical decompression, and the patients who underwent “early surgery” underwent surgery by, on average, 14 h post injury [16]. The fact that some of these patients might have technically been in “spinal shock” when they were assessed in the early hours after their injury and assigned a baseline AIS grade does not negate the value of that study—it simply reflects clinical reality. In practical terms, the evaluation of neuroprotective interventions for acute SCI will require that we utilize baseline neurologic examinations conducted very early after injury when “the BCR has not yet returned”. Understanding how the timing of this examination influences outcome is therefore important for this aspect of translational research. To our knowledge, this study is the first to specifically examine the influence of neurological examination timing within hours post injury.
We limited this study to patients with AIS A injuries because their prognosis is thought to be relatively homogeneous in comparison with patients with incomplete injuries, but we demonstrated substantial variability in their rates of AIS conversion. Our findings deviate from those of Fawcett et al. [1], who reported based on data from multiple databases that ~80% of initial AIS A patients remain AIS at 1 year of follow-up in comparison with as little as 20% of initial AIS B or C patients.
Relation to previous literature
Fawcett et al. reanalyzed data from the Sygen (GM-1 ganglioside) study [22] database and found that the rate of change in motor grade at 1 year among patients evaluated at 3 days after SCI was much greater than that among patients evaluated at 8 weeks [1]. This result provided empiric evidence to support an observation that is largely intuitive to all clinicians: the prediction of neurologic outcomes becomes easier as time goes on because there is less spontaneous recovery. In contrast to our study, these analyses stopped with the earliest baseline assessments at 3 days post injury and did not examine time points relevant to modern acute SCI trials. In STASCIS, decompression prior to 24 h post injury was associated with significantly higher rates of AIS conversion by two or more grades. Recent prospective studies of minocycline, riluzole, and magnesium chloride have all involved drug administration within 12 h post injury [2,3,4].
To our knowledge, this study is the first to specifically examine the influence of neurological examination timing within hours post injury. Maynard et al. reviewed 123 patients in 1979, performed baseline examinations at 72 h post injury for administrative reasons, and advised that 72 h was preferable because delayed examinations were more likely to improve predictive accuracy [23]. Only 20% of patients with complete injuries in their series converted AIS grades by 1 year. Brown et al., and Herbison et al., later compared manual motor testing at 72 h versus less than 24 h and up to 2 weeks and concluded that 72 h examinations were most reliable for predicting outcomes at up to 6 months post injury [24, 25]. A more recent analysis of 1436 patients by Marino et al. in 2011 failed to identify an effect of neurological examination timing at earlier versus later than 72 h [26].
Implications
From a methodological perspective, our study suggests that the timing of neurologic examination is a parameter that warrants consideration when interpreting the findings from acute neuroprotective interventions. Our data support that differences in neurological examination timing within hours post injury may contribute to differences in observed neurological outcomes, which could be an important source of bias. This is particularly relevant when comparing rates of neurologic recovery with “historical controls” where the baseline neurologic examination may be done at later time points when there is less variability in spontaneous recovery. Studies that involve the administration of interventions very early post injury may be at particular risk, as may be studies that compare prospective data with early examination timing to historical data with delayed examination timing. In the future, it may be worthwhile considering the implementation of strategies to control for differences in neurological examination timing in the design, conduct, and interpretation of clinical trials.
From a clinical perspective, our study confirms that the final neurological outcomes of patients evaluated within hours post injury may vary substantially. Our data suggest that frontline clinicians who care for patients very early after SCI should recognize this initial uncertainty when counseling about prognosis for functional improvement, and should continue to perform serial neurological examinations over time. Patients with cervical AIS A injuries are often thought to experience relatively little neurological recovery, but more than half of the patients with cervical complete injuries in our study converted by at least one AIS grade, and some experienced marked improvement within a relatively short follow-up period. Clinicians caring for patients later in their course of recovery can be more confident when discussing and planning long-term rehabilitation. Our study also highlights the importance of performing serial neurological examinations early in patients’ clinical courses. Rather than attempting to counsel individual patients with generic numbers or percentage of AIS conversion rates, clinicians should tailor their discussions based on their serial neurological examinations over time
Conclusions
Variability in neurological examination timing within hours after acute traumatic SCI may influence observations of long-term neurological recovery. AIS grade conversion in AIS A complete SCI patients was significantly more likely in those whose neurologic assessment was performed within 4 h of injury; the effect of timing on motor scores remains uncertain. Further research is warranted to clarify our findings in larger datasets and investigate the influence of timing in patients with incomplete injuries. However, our results highlight the need to consider variations in the timing of baseline neurologic assessment when interpreting neurologic recovery in studies of therapeutic interventions.
Data availability
The raw data analyzed in this study are not publicly available due to stipulations about their use with the participating hospital facility and the participants themselves, but an application for data access can be made to the Rick Hansen Institute’s Data Steward, via the corresponding author, and data may be available on reasonable request with permission from the participating hospital facility’s principal investigator.
Change history
09 January 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41393-020-0413-y
References
Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205.
Grossman RG, Fehlings MG, Frankowski RF, Burau KD, Chow DSL, Tator C et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31:239–55.
Casha S, Zygun D, McGowan MD, Bains I, Yong VW, John Hurlbert R. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135:1224–36.
Refinement P, Kwon BK, Roy J, Lee JHT, Okon E, Zhang H, et al. Magnesium chloride in a polyethylene glycol formulation as a neuroprotective therapy for acute spinal cord injury. J Neurotrauma. 2009;26:1379–93.
Evaniew N, Noonan VK, Fallah N, Kwon BK, Rivers CS, Ahn H, et al. Methylprednisolone for the treatment of patients with acute spinal cord injuries: a propensity score-matched cohort study from a Canadian multi-center spinal cord injury registry. J Neurotrauma. 2015;32:1674–83.
Dvorak MF, Noonan VK, Fallah N, Fisher CG, Rivers CS, Ahn H, et al. Minimizing errors in acute traumatic spinal cord injury trials by acknowledging the heterogeneity of spinal cord anatomy and injury severity: an observational Canadian cohort analysis. J Neurotrauma. 2014;31:1540–7.
Ahn H, Bailey CS, Rivers CS, Noonan VK, Tsai EC, Fourney DR, et al. Effect of older age on treatment decisions and outcomes among patients with traumatic spinal cord injury. CMAJ. 2015;187:873–80.
Dvorak MF, Noonan VK, Fallah N, Fisher CG, Finkelstein J, Kwon BK, et al. The influence of time from injury to surgery on motor recovery and length of hospital stay in acute traumatic spinal cord injury: an observational Canadian cohort study. J Neurotrauma. 2015;32:645–54.
Paquet J, Rivers CS, Kurban D, Finkelstein J, Tee JW, Noonan VK, et al. The impact of spine stability on cervical spinal cord injury with respect to demographics, management, and outcome: a prospective cohort from a national spinal cord injury registry. Spine J. 2018;18:88–98.
Holtz KA, Szefer E, Noonan VK, Kwon BK, Mills PB. Treatment patterns of in-patient spasticity medication use after traumatic spinal cord injury: a prospective cohort study. Spinal Cord. https://doi.org/10.1038/s41393-018-0165-0.
Noonan V, Kwon B, Soril L, Fehlings M, Hurlbert R, Townson A, et al. The Rick Hansen spinal cord injury registry (RHSCIR): a national patient-registry. Spinal Cord. 2012;50:22–27.
Kirshblum S, Waring W. Updates for the International standards for neurological classification of spinal cord injury. Phys Med Rehabil Clin N Am. 2014;25:505–17.
Furlan JC, Fehlings MG, Tator CH, Davis AM. Motor and sensory assessment of patients in clinical trials for pharmacological therapy of acute spinal cord injury: psychometric properties of the ASIA Standards. J Neurotrauma. 2008;25:1273–301.
Walden K, Bélanger LM, Biering-Sørensen F, Burns SP, Echeverria E, Kirshblum S, et al. Development and validation of a computerized algorithm for International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI). Spinal Cord. 2016;54:197–203.
Kirshblum SC, Burns SP, Biering-Sørensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (Revised 2011). J Spinal Cord Med. 2011;34:535–46.
Fehlings MG, Vaccaro A, Wilson JR, Singh A, W Cadotte D, Harrop JS, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS ONE. 2012;7:e32037.
Evaniew N, Files C, Smith C, Bhandari M, Ghert M, Walsh M, et al. The fragility of statistically significant findings from randomized trials in spine surgery: a systematic survey. Spine J. 2015;15:2188–97.
Bailey CS, Fisher CG, Dvorak MF. Type II error in the spine surgical literature. Spine. 2004;29:1146–9.
Chow S-C. Sample size calculations for clinical trials. Wiley Interdiscip Rev Comput Stat. 2011;3:414–27.
Ko H-Y. Revisit spinal shock: pattern of reflex evolution during spinal shock. Korean J Neurotrauma. 2018;14:47–54.
Schouten R, Albert T, Kwon BK. The spine-injured patient: initial assessment and emergency treatment. J Am Acad Orthop Surg. 2012;20:336–46.
Geisler FH, Coleman WP, Grieco G, Poonian D. The Sygen multicenter acute spinal cord injury study. Spine. 2001;26:S87–S98.
Maynard FM, Reynolds GG, Fountain S, Wilmot C, Hamilton R. Neurological prognosis after traumatic quadriplegia. Three-year experience of California Regional Spinal Cord Injury Care System. J Neurosurg. 1979;50:611–6.
Brown PJ, Marino RJ, Herbison GJ, Ditunno JF. The 72-hour examination as a predictor of recovery in motor complete quadriplegia. Arch Phys Med Rehabil. 1991;72:546–8.
Herbison GJ, Zerby SA, Cohen ME, Marino RJ, Ditunno JF Jr. Motor power differences within the first two weeks post-SCI in cervical spinal cord-injured quadriplegic subjects. J Neurotrauma. 1992;9:373–80.
Marino RJ, Burns S, Graves DE, Leiby BE, Kirshblum S, Lammertse DP. Upper- and lower-extremity motor recovery after traumatic cervical spinal cord injury: an update from the national spinal cord injury database. Arch Phys Med Rehabil. 2011;92:369–75.
Acknowledgements
We would like to thank the Vancouver Spine Research Program team at Vancouver General Hospital for their help in data collection. BKK is the Canada Research Chair in Spinal Cord Injury and Dvorak Chair in Spine Trauma.
Funding
The Rick Hansen Spinal Cord Injury Registry and this work are supported by funding from the Rick Hansen Institute, Health Canada and Western Economic Diversification Canada.
Author information
Authors and Affiliations
Contributions
BKK, NE, NF, VKN, and BS designed the study. TA, RC-M, MD, ND, NE, CF, BKK, VKN, SP, BS, JS, and ZW assisted with the data acquisition. NE, NF, and BS performed the analysis of the data. NE led paper preparation and all authors contributed to the interpretation of the data and helped to write, review and edit the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All local research ethics board approvals were obtained prior to recruitment of participants. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Evaniew, N., Sharifi, B., Waheed, Z. et al. The influence of neurological examination timing within hours after acute traumatic spinal cord injuries: an observational study. Spinal Cord 58, 247–254 (2020). https://doi.org/10.1038/s41393-019-0359-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-019-0359-0
This article is cited by
-
Improving Diagnostic Workup Following Traumatic Spinal Cord Injury: Advances in Biomarkers
Current Neurology and Neuroscience Reports (2021)