Abstract
Study design
Experimental animal study.
Objectives
Spastic hypertonia is originally believed to cause contractures from clinical observations. Botulinum toxin is effective for the treatment of spasticity and is widely used in patients who have joints with contractures. Using an established rat model with knee contractures after spinal cord injuries, we aimed to verify whether hypertonia contributes to contracture development, and the botulinum toxin improves structural changes in muscles and joint components responsible for contractures.
Setting
University laboratory in Japan.
Methods
To evaluate the effect of hypertonia on contracture development, the rats received botulinum toxin injections after spinal cord injuries. Knee extension motion was measured with a goniometer applying a standardized torque under anesthesia, and the contribution by muscle or non-muscle structures to contractures were calculated by measuring joint motion before and after the myotomies. We quantitatively measured the muscle atrophy, muscle fibrosis, and synovial intima length.
Results
Botulinum toxin injections significantly improved contractures, whereas did not completely prevent contracture development. Botulinum toxin was effective in improving the muscular factor, but little difference in the articular factor. Spinal cord injuries induced muscle atrophy, and botulinum toxin significantly accelerated muscle atrophy and fibrosis. The synovial intima length decreased significantly after spinal cord injuries, and botulinum toxin did not improve this shortening.
Conclusions
This animal study provides new evidence that hypertonia is not the sole cause rather is the partial contributor of contractures after spinal cord injuries. Furthermore, botulinum toxin has adverse effects in the muscle.
Similar content being viewed by others
Introduction
Joint contractures are major complications of spinal cord injuries (SCI) [1, 2], and are characterized by limitations in the passive range of motion (ROM) of the affected joints [3]. Decreased ROM limits activities of daily living for SCI patients, and predisposes them to other complications such as pressure sores [1]. Positioning, stretching, and physical therapy are advocated to prevent and treat contractures. The usefulness of passive joint motion exercises has been validated in many clinical studies that determined their therapeutic efficacy [2]. Nevertheless, we are often confronted with SCI patients who have contractures that limit limb function.
Based on clinical observations, spastic hypertonia is believed to cause contractures after central nervous system injuries for many years [3]. We have previously established SCI rat models with knee contractures [4] and proposed that both muscular and articular factors contribute almost equally to the overall progression of the contractures after 14 days of SCI [5]. Subsequently, we have shown that the intra-articular alterations after SCI exhibit the specific changes that differed from those observed in animal models with immobilized joints [6,7,8]. However, what causes the SCI-specific characteristics of joint contractures has not been previously investigated, and whether hypertonia plays a part in contracture development after SCI remains controversial.
Botulinum toxin (BTX) induces a reversible muscle relaxation by inhibiting acetylcholine release from the presynaptic terminals of the neuromuscular junction in the peripheral nervous system [9]. Clinically, BTX is considered safe and effective for the treatment of spasticity [9], and it has been widely used in patients with SCI [10], celebral palsy [11], and cerebrovascular disease [12]. The BTX injection also improves the limitations in ROM and the functional outcome [10], but it appears to be merely a consequence of muscle relaxation, not due to ameliorations of structural alterations. The effect of BTX injections on histopathological changes in muscles and periarticular structures have not been understood.
In this study, using an established SCI model with contractures, we aimed to verify whether (1) hypertonia contributes to contracture development and (2) the BTX injection improves structural changes in muscles and joint components responsible for contractures after SCI.
Methods
Experimental design
Total 20 male Wistar rats (SLC Japan Inc., Shizuoka, Japan), 10 weeks old, were used in this study. These rats were randomly divided into the following 3 groups: a healthy group that had no intervention (control group, n = 6 rats), an untreated group with SCI (SCI group, n = 6 rats), and a BTX injection group after SCI (BTX group, n = 8 rats). Knee flexion contractures develop in rats with SCI for first 14 days postinjury [4]. Therefore, to evaluate the effect of hypertonia on contracture development, the rats in the BTX group received injections at either the immediate or 14th postoperative day (n = each 4 rats). The rats at 14 and 28 days after the injections were evaluated and compared with the age-matched animals in the control and SCI groups (Fig. 1). The right and the left knee joint served as different samples. The sample sizes were calculated by a power analysis based on pilot results in order to detect a 10° difference in ROM 19 times out of 20 [5].
A diagram of the experimental design is shown. The rats were randomly divided into the control, SCI, and BTX groups (n = 2 rats per timepoint). The rats of the BTX group received BTX injections into both sides of all knee flexors at 0, 14 or at 28 days after SCI, and were evaluated and compared with the age-matched animals in the control and SCI groups at 14, 28 or 42 days respectively after SCI. The right and the left knee joint from same animal served as independent samples (n = 4 knees from 2 rats per timepoint for all groups)
Surgical procedures and postoperative care conformed to those in our previous studies [4,5,6,7,8]. Rats in the SCI and BTX groups were anesthetized by an intraperitoneal administration of 40 mg/kg of sodium pentobarbital and subcutaneously injected with 0.02 mg/kg buprenorphine to give relief of pain. Then, their spinal cords were transected completely at the T8 level. This procedure led to the development of knee joint flexion contractures [4, 5]. Postoperative pain was controlled with 0.05 mg/kg of buprenorphine given subcutaneously every 8–12 h for the first 72 h. The rats were housed in sterilized cages with bedding (cedar shavings), and were maintained under artificial conditions at 22 ± 1 °C and a cycle of 12 h of light and 12 h of dark. The animals had free and easy access to food and tap water, and unlimited activity. The behavior of all animals was observed every day throughout the experimental period. Before surgery and at the end of each timepoint, withdrawal reactions to stimuli (extension, pain, and pressure) were evaluated.
BTX injections
The lyophilized clostridium botulinum toxin type A neurotoxin complex (BOTOX; Allergan, Coolock, Dublin, Ireland) was diluted with saline to a final concentration of 1 UI/ml. BTX solution was injected into both sides of all knee flexors (biceps femoris, semitendinosus, semimembranosus, gastrocnemius, and gracilis) of the rats in the BTX group (0.2 UI/ml was given in each muscle). This dose of BTX per muscle has been shown previously to produce muscle relaxant for 14 days [13, 14]. Therefore, the rats evaluated at 42 days after SCI were injected once every 14 days (Fig. 1).
ROM measurements
ROM measurements of knee extension were performed under anesthesia with isoflurane according to the previously described method [5, 15, 16]. Briefly, knee motion in extension was measured with a mechanical goniometer applying a standardized torque (0.06Nm) by intervals of 1°. The lateral femoral condyle was the pivot point while the femur was fixed, and extension moments were applied to the tibia. The degree of the limitation in ROM was assessed by measuring the femorotibial angle. Normal extension ROM of healthy rats is ~15° [4, 5]. The measurements were done blindly by 2 investigators and repeated them 5 times for each knee. Values were the mean of the 10 measurements, the combined measurements by both investigators.
The animals were killed by exsanguination. Myotomies of the trans-articular muscles were then performed, and ROM was measured again in extension. Measurements after myotomies were completed within 15 min of the animal’s deaths, in order to minimize the possibility of postmortem rigidity. The muscular factor that contributes to contractures was defined as limited ROM in the muscles including tendon and fascia, and the articular factor was defined as limited ROM in the articular components (bone, cartilage, synovium, capsules, and ligaments) [17]. According to our previous method [15, 16], the formulas assessed by measuring ROM before and after the myotomies allow isolation of the muscular and articular factors responsible for contractures and are as follows: muscular factors = ROM no myotomy–ROM after myotomy (within each group); articular factors = ROM after myotomy of each group–ROM after myotomy of the control group.
Sampling and histological preparation
The biceps femoris muscles, which are the biggest in rat knee flexors and therefore contribute to the development of knee flexion contractures, were harvested and the ratio of skeletal muscle wet weight to whole-body weight was calculated. Frozen sections 10μm thick were cut from muscle samples and were then stained with hematoxylin and eosin. Muscle fiber cross-sectional area were measured in over 100 muscle fibers from each muscle in each animal. After the knee joints and surrounding soft tissue were harvested, standardized 5μm sections were obtained at the medial midcondylar level in the sagittal plane following a previously published method of Kawamoto [18]. The sections of each knee were stained with hematoxylin and eosin.
Quantification of fibrosis in muscle tissue
We assessed muscular fibrosis leading to poor muscle extensibility in muscular contracture by slight modification of the method according to Hadi et al. [19]. The histologic sections from the biceps femoris muscles were stained with picrosirius red. Fibrosis was quantified for an average of 10 images obtained at ×10 magnification randomly chosen at the middle third of each section. The yellow color of the muscle cells and the red color of the connective tissue were identified on the sections. The area of each color was measured separately with Image Tool software (Image J 1.47 v; National Institutes of Health, Bethesda, MD, USA), and the percentage of connective tissue area was then calculated.
Quantification of synovial intima length
To evaluate the shortening of the joint capsule responsible for articular contracture, we measured posterior synovial intima length as described by Ando et al. [20]. The synovial lining contour from the synovio–cartilage junction of the femur or tibia to the posterior horn of the meniscus was traced with Adobe Photoshop CS2 (Adobe Systems Inc, San Jose, CA, USA), and then its length was measured with Image Tool software. The length of the superior and inferior subdivisions of the synovial intima in the posterior joint capsule were summed to provide the total length of the synovial intima.
Statistical analyses
The results for ROM, muscle wet weight, muscle fiber cross-sectional area, muscle fibrosis, and synovial intima length were analyzed statistically with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [21]. The results were compared among all groups within timepoint using analysis of variance following Tukey’s honestly significant difference test. An alpha of <0.05 was chosen as the significance level for all statistical analyses.
Statistical analyses of muscular and articular factors in contractures were conducted with Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA). ROM data from each condition were averaged for each group in the muscular and articular factors. The standard deviations (SD) on muscular and articular factors estimates were derived according to a method of calculating SD for differences in group means [22]. We calculated the SD for the mean differences among the groups and 95% confidence intervals (CI) were estimated. When the length of the 95% CI of the between-group mean difference did not overlap zero, we identified muscular and articular factors contributed significantly to contracture development [5, 15,16,17].
Results
Functional outcome
The rats with SCI demonstrated complete flaccid paraplegia after injury, and thereafter showed kick movements, clonic and high-frequency flexion-extension movements, and hyperreflexia which were characteristic in spasticity [23]. Meanwhile, the rats after the BTX injection showed the reflex response to stimuli, but kick movements and hyperreflexia were not observed. This result indicated that the dose of BTX was adequate to suppress spasticity after SCI.
Limitations in ROM
The limitation in knee extension ROM developed significantly in rats with SCI (Fig. 2 and Supplementary Table 1). BTX injections significantly improved the limitation in ROM after SCI (Fig. 2 and Supplementary Table 1), whereas BTX did not completely prevent the development of limitations in ROM after SCI (Fig. 2 and Supplementary Table 1).
The graph shows knee motion measured for extension with a goniometer. Two approaches to BTX injections were evaluated: BTX injections was provided at the immediate after SCI (BTX 0) and BTX injections was given on the 14th day postoperatively (BTX 14). Data are presented as the mean ± SD. *Indicates a significant difference when compared to the age matched control. †Indicates a significant difference between the SCI and BTX groups. Four knees from each group were evaluated at each timepoint
Muscular and articular factors of contractures
BTX injections significantly improved the muscular factor at each timepoint, when compared to the SCI groups (Table 1). In the articular factor, no differences were found between the SCI and BTX groups, except for the timepoint of the BTX injection at day 0 and evaluation after day 28 of SCI (Table 1).
Changes in the muscle
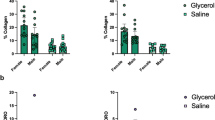
Microscopic findings showed that SCI induced muscle atrophy (Fig. 3a–j). Likewise, the muscle fiber cross-sectional area was decreased significantly after SCI compared to the control group, although no statistically significant differences were found between the SCI and BTX groups (data not shown). In addition, the muscle wet weight decreased significantly after SCI, and BTX group was more prominent (Fig. 3k and Supplementary Table 2). BTX injections significantly accelerated muscle fibrosis and increased muscle fibrosis at day 28 and 42 (Fig. 3l and Supplementary Table 3).
Representative photomicrographs show the biceps femoris muscles stained with picrosirius red in the control a–c, SCI d–f, and BTX g–h groups at each timepoint. Two approaches to BTX injections were evaluated: BTX injections was provided at the immediate after SCI (BTX 0) and BTX injections was given on the 14th day postoperatively (BTX 14). Scale bars = 100 µm. The graphs show the muscle wet weight g and the muscle fibrosis h in each group. Data are presented as the mean ± SD. * Indicates a significant difference when compared to the age matched control. † Indicates a significant difference between the SCI and BTX groups. Four knees from each group were evaluated at each timepoint
Changes in the joint capsule
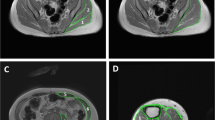
Microscopic findings showed that adhesions between synovial fold and the synovial membrane of the posterior joint capsule were observed after SCI, although there were no differences between the SCI and BTX groups (Fig. 4a–j). In line with these observations, the synovial intima length decreased significantly after SCI, and BTX injections did not improve this shortening (Fig. 4k and Supplementary Table 4).
Representative photomicrographs show the synovial membrane in the posterior capsule around the femur stained with hematoxylin and eosin in the control a–c, SCI d–f, and BTX g–h groups at each timepoint. Two approaches to BTX injections were evaluated: BTX injections was provided at the immediate after SCI (BTX 0) and BTX injections was given on the 14th day postoperatively (BTX 14). Scale bars = 1 mm. The graph shows the posterior synovial intima’s length in each group k. Data are presented as the mean ± SD. *Indicates a significant difference when compared to the age matched control. Four knees from each group were evaluated at each timepoint
Discussion
Our study had 2 objectives: to verify whether hypertonia contributes to contracture development after SCI and to examine the effects of BTX on contracture-associated alterations of muscles and joint components. The widely held belief that spastic hypertonia causes joint contracture was not always true. The results presented here suggest that hypertonia is the partial contributor rather than the sole cause of contractures after SCI. BTX injections significantly improved contractures, but did not completely prevent contracture development. Moreover, the findings indicated that BTX injections significantly improved the muscular factor responsible for contractures, whereas BTX induced marked muscle atrophy and accelerated fibrosis. In contrast, BTX had no effect on articular contractures.
Spastic hypertonia is originally believed to cause contractures following paralysis from clinical observations [2, 3]. In an attempt to elucidate experimentally this clinical observation, BTX was injected into all the knee flexors immediately after SCI and then suppressed hypertonia. Consequently, BTX did not prevent contracture development after SCI. On the other hand, we have reported previously that knee flexion contractures developed in rats with SCI for first 14 days postinjury [5], and therefore we investigated the effect of treatment with BTX by injections at the 14th postoperative day. Thus, BTX had treatment effects on joint ROM limitations. We also found little differences between the results of ROM at the immediate and 14th postoperative day. This would indicate that the improvement in ROM limitations by BTX injections may be due to treatment effects, but not preventive effects. Overall, our findings cast doubt whether only hypertonia causes joint contracture.
The muscle fibrosis is closely related to contracture development after joint immobilization [24], and therefore we evaluated the fibrosis. Although there was not a significant increase in muscle fibrosis after SCI, BTX significantly induced the fibrosis with longer duration after the injections. BTX induces the time- and dose-dependent increases in type I and III collagens, IGF-1, and TGF-β expression associated with fibrosis [25] and vimentin staining, a marker for fibrosis, in rabbit muscles [26]. Similarly, in human extraocular muscles, BTX induces muscle fibrosis [27]. These earlier reports support the present results. Besides, our results demonstrated that muscle atrophy occurred in animals with SCI and was more marked by BTX injections. Muscle atrophy is a well-known phenomenon observed in SCI patients [28], and BTX injections commonly induce human muscle atrophy [29]. Our findings in the rat model with SCI closely reflect these outcomes in humans. Additionally, the passive stiffness decreases in atrophied muscle by BTX injections [30]. Taken together, improved muscular contractures by BTX injections in this study may not result from improvements in structural changes of muscles.
The shortening of the synovial intima length proved an excellent marker for the incidence and severity of knee flexion contractures after SCI [8, 16] or immobilization [20]. This led us to conclude that BTX injections into muscles had no effect on articular contractures. BTX is an agent inhibiting acetylcholine release from the neuromuscular junction in the peripheral nervous system [9]; thus this explanation may be quite plausible. Previously, we reported that joint movement (ie, mechanical stimuli) are crucial for improvements in articular alterations causing contractures after SCI [15, 16]. The present findings are also in line with our previous results. Both muscular and articular factors contribute almost equally to the overall progression of the contractures after 14 days of SCI [5]. Indeed, BTX improved muscular factors, even apparent improvements; however, ROM in the BTX group did not recover to the same range as that in the control group, indicating that articular factors are critically involved in the contracture development.
Our study has several limitations. First, we used the right and the left knee joint served as different samples from same rats. The use of both joints has the advantages of minimizing the number of experimental animals needed for ethical reasons and providing equivalency of sample size for statistical purposes. However, its use cannot preclude chance findings attributable to intra-animal and inter-animal variation. That is, outcomes in one knee is likely to be very similar to outcomes from the other knee because both knees belong to the same rat. The lack of accounting for the correlation between knees belonging to the same rat produces confidence intervals that are narrower than what they should be. The second limitation is that we assessed only histologic changes responsible for contractures and did not analyze other factors (eg, mRNA and protein levels) involved in structural changes in muscles and joint components. Finally, and most importantly, we did not examine spasticity using electromyography more specifically. Thus, the question remains as to whether spasticity directly contributes to contracture development. There are possible other factors may cause contracture or mediate the effect of spasticity on contracture. In addition, to clarify the direct effect of BTX, it may be necessary to administer BTX to healthy rats without contractures, although the effect of BTX on normal muscle is well documented.
In conclusion, here we provide new evidence that hypertonia is not the sole cause rather is the partial contributor of contractures. Furthermore, in the treatment aimed to improve spasticity and/or contracture after SCI, BTX can suppress spasticity but has adverse effects of muscle atrophy and fibrosis; thus may be less predictably true effective against contractures.
Data archiving
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Dalyan M, Sherman A, Cardenas DD. Factors associated with contractures in acute spinal cord injury. Spinal Cord. 1998;36:405–8.
Harvey LA, Herbert RD. Muscle stretching for treatment and prevention of contracture in people with spinal cord injury. Spinal Cord. 2002;40:1–9.
Botte MJ, Nickel VL, Akeson WH. Spasticity and contracture. Physiologic aspects of formation. Clin Orthop Relat Res. 1988;233:7–18.
Moriyama H, Yoshimura O, Sunahori H, Nitta H, Imakita H, Saka Y, et al. Progression and direction of contractures of knee joints following spinal cord injury in the rat. Tohoku J Exp Med. 2004;204:37–44.
Moriyama H, Yoshimura O, Sunahori H, Tobimatsu Y. Comparison of muscular and articular factors in the progression of contractures after spinal cord injury in rats. Spinal Cord. 2006;44:174–81.
Moriyama H, Nishihara K, Hosoda M, Saka Y, Kanemura N, Takayanagi K, et al. Contrasting alteration patterns of different cartilage plates in knee articular cartilage after spinal cord injury in rats. Spinal Cord. 2009;47:218–24.
Moriyama H, Yoshimura O, Kawamata S, Takayanagi K, Kurose T, Kubota A, et al. Alteration in articular cartilage of rat knee joints after spinal cord injury. Osteoarthr Cartil. 2008;16:392–8.
Moriyama H, Yoshimura O, Kawamata S, Takemoto H, Saka Y, Tobimatsu Y. Alteration of knee joint connective tissues during contracture formation in spastic rats after an experimentally induced spinal cord injury. Connect Tissue Res. 2007;48:180–7.
Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43:249–59.
Catz A, Barkol H, Steinberg F, Ronen J, Bluvshtein V, Keren O. Repeated botulinum toxin injections can improve mobility in patients with spinal cord lesions. Eura Med. 2007;43:319–25.
Lidman G, Nachemson A, Peny-Dahlstrand M, Himmelmann K. Botulinum toxin A injections and occupational therapy in children with unilateral spastic cerebral palsy: a randomized controlled trial. Dev Med Child Neurol. 2015;57:754–61.
Pittock SJ, Moore AP, Hardiman O, Ehler E, Kovac M, Bojakowski J, et al. A double-blind randomised placebo-controlled evaluation of three doses of botulinum toxin type A (Dysport) in the treatment of spastic equinovarus deformity after stroke. Cereb Dis. 2003;15:289–300.
Pickett A, O’Keeffe R, Judge A, Dodd S. The in vivo rat muscle force model is a reliable and clinically relevant test of consistency among botulinum toxin preparations. Toxicon. 2008;52:455–64.
Sakitani N, Iwasawa H, Nomura M, Miura Y, Kuroki H, Ozawa J, et al. Mechanical stress by spasticity accelerates fracture healing after spinal cord injury. Calcif Tissue Int. 2017;101:384–95.
Iwasawa H, Nomura M, Sakitani N, Watanabe K, Watanabe D, Moriyama H. Stretching after heat but not after cold decreases contractures after spinal cord injury in rats. Clin Orthop Relat Res. 2016;474:2692–701.
Moriyama H, Tobimatsu Y, Ozawa J, Kito N, Tanaka R. Amount of torque and duration of stretching affects correction of knee contracture in a rat model of spinal cord injury. Clin Orthop Relat Res. 2013;471:3626–36.
Trudel G, Uhthoff HK. Contractures secondary to immobility: is the restriction articular or muscular? An experimental longitudinal study in the rat knee. Arch Phys Med Rehabil. 2000;81:6–13.
Kawamoto T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch Histol Cytol. 2003;66:123–43.
Hadi AM, Mouchaers KT, Schalij I, Grunberg K, Meijer GA, Vonk-Noordegraaf A, et al. Rapid quantification of myocardial fibrosis: a new macro-based automated analysis. Cell Oncol (Dordr). 2011;34:343–54.
Ando A, Hagiwara Y, Onoda Y, Hatori K, Suda H, Chimoto E, et al. Distribution of type A and B synoviocytes in the adhesive and shortened synovial membrane during immobilization of the knee joint in rats. Tohoku J Exp Med. 2010;221:161–8.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Hedges LV, Olkin I Statistical methods for meta-analysis. (Academic Press, Orlando, 1985).
van de Meent H, Hamers FP, Lankhorst AJ, Buise MP, Joosten EA, Gispen WH. New assessment techniques for evaluation of posttraumatic spinal cord function in the rat. J Neurotrauma. 1996;13:741–54.
Honda Y, Sakamoto J, Nakano J, Kataoka H, Sasabe R, Goto K, et al. Upregulation of interleukin-1beta/transforming growth factor-beta1 and hypoxia relate to molecular mechanisms underlying immobilization-induced muscle contracture. Muscle Nerve. 2015;52:419–27.
Fortuna R, Vaz MA, Sawatsky A, Hart DA, Herzog W. A clinically relevant BTX-A injection protocol leads to persistent weakness, contractile material loss, and an altered mRNA expression phenotype in rabbit quadriceps muscles. J Biomech. 2015;48:1700–6.
Olabisi R, Chamberlain CS, Petr S, Steiner S, Consigny D, Best TM, et al. The effects of botulinum toxin A on muscle histology during distraction osteogenesis. J Orthop Res. 2009;27:310–7.
Li J, Allende A, Martin F, Fraser CL. Histopathological changes of fibrosis in human extra-ocular muscle caused by botulinum toxin A. J Aapos. 2016;20:544–6.
Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–7.
Choi WH, Song CW, Kim YB, Ha CS, Yang GH, Woo HD, et al. Skeletal muscle atrophy induced by intramuscular repeated dose of botulinum toxin type A in rats. Drug Chem Toxicol. 2007;30:217–27.
Thacker BE, Tomiya A, Hulst JB, Suzuki KP, Bremner SN, Gastwirt RF, et al. Passive mechanical properties and related proteins change with botulinum neurotoxin A injection of normal skeletal muscle. J Orthop Res. 2012;30:497–502.
Acknowledgements
We thank Naoyoshi Sakitani, Shin Ogasawara, Ryota Suzuki, Eriko Mizuno, and Masato Nomura for their skilled technical assistance.
Funding
This work was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant no. 17K19908.
Author information
Authors and Affiliations
Contributions
HM was responsible for designing and directing the protocol, interpreting results, and writing the manuscript. JO was responsible for designing and directing the protocol, interpreting results, and revising the manuscript. TY, SI, and TW was responsible for conducting the experiment, extracting and analyzing data, and revising the manuscript. NK, YS, and TA was responsible for designing the protocol, interpreting results, and revising the manuscript.
Corresponding author
Ethics declarations
Ethics
This study was approved by the Institutional Animal Care and Use Committee (Permission number: P130408) and carried out according to the Kobe University Animal Experimentation Regulations.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Moriyama, H., Ozawa, J., Yakuwa, T. et al. Effects of hypertonia on contracture development in rat spinal cord injury. Spinal Cord 57, 850–857 (2019). https://doi.org/10.1038/s41393-019-0312-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-019-0312-2