Abstract
Study design
Randomized controlled trial.
Objectives
To determine the effect of zoledronic acid on bone loss in people with acute spinal cord injury (SCI)
Settings
Sawai Man Singh Medical College, India.
Methods
Sixty patients with acute SCI were randomized to receive either standard treatment alone or standard treatment with zoledronic acid within 3 months after injury. Areal bone mineral density (aBMD) was measured at the hip using dual-energy X-ray absorptiometry (DXA) at baseline 3, 6, and 12 months.
Results
Significant differences in aBMD were found between the standard treatment alone and standard treatment plus zoledronic acid group at the femoral neck (−0.13; 95% CI, −0.18 to −0.09, p < 0.0001), and total hip (−0.16; 95% CI, −0.19 to −0.12, p < 0.0001), respectively, at 1 year and bone loss was reduced in the zoledronic acid treated group as compared to the standard treatment group. Significant differences in aBMD between the groups at 6 months post infusion was also observed at these sites. [Femoral neck −0.08; 95% CI, −0.12 to −0.03; p = 0.002 and total hip −0.12; 95% CI, −0.15 to −0.08; p < 0.0001]
Conclusion
A zoledronic acid 5 mg infusion given within 3 month significantly reduces bone loss at the hip after 6 months post infusion in patients with acute SCI.
Similar content being viewed by others
Introduction
Osteoporosis following spinal cord injury (SCI) is well known and is indicated by low bone mass with deterioration of skeletal micro-architecture [1]. Disuse is considered the underlying cause of osteoporosis subsequent to acute SCI [2]. Unloading, neural lesion and hormonal changes after SCI result in severe bone loss [3]. Following SCI and immobilization, bone loss occurs rapidly in the pelvis and lower extremities in patients with SCI due to a marked increase in osteoclastic bone resorption and a decrease in osteoblastic bone formation [4]. The loss of mechanical stimuli in the form of muscle contraction and the lack of weight bearing due to SCI commonly results in sub-lesional osteoporosis [5, 6]. This consequently leads to increased fragility fracture risk commonly at the proximal tibia and distal femur [7,8,9] and resulting in fractures after trivial trauma or even spontaneously [10, 11]. Increased bone loss following SCI is associated with fragility fractures, morbidity and mortality, and substantial cost to the health care systems [1].
Bone loss is inhibited by bisphosphonates because they reduce bone resorption [4, 12]. Patients with acute SCI are treated in supine position for initial care and it is advised that oral bisphosphonates should not be taken by persons who are not able to stand or sit upright for at least 30 min. This is not possible in the initial SCI situation. Intravenous bisphosphonates therefore have an advantage over oral compounds like Alendronate [12]. Zoledronic acid is a 3rd generation bisphosphonate with more potent suppression of osteoclast mediated bone resorption than other congeners and can be given by once yearly intravenous infusion [13,14,15]. Therefore the purpose of this study was to evaluate the effect of early administration of zoledronic acid infusion on reduction of bone loss in patients with acute SCI.
Methods
Patients with SCI and neurological deficits (ASIA Impairment Scale A, B, C) were invited to take part in this prospective randomized interventional study if they sustained their injury within 3 months, were aged over 18 years, and were admitted to the department of physical medicine and rehabilitation between February 2013 and January 2015. Institute ethics committee approval was taken from Sawai Man Singh Medical College and Hospitals ethics committee, Jaipur, Rajasthan, India.
Patients were excluded if they had hypocalcaemia (serum calcium < 8.5 mg/dl), vitamin-D deficiency (serum 25(OH) vitamin-D < 25 nmol/L), significant renal impairment (creatinine clearance < 30 ml/min) or any history of adverse reaction to bisphosphonates, iritis, uveitis, psychiatric illness. Female participants who were pregnant, lactating, or planning to conceive were also excluded.
Sample size was calculated at 80% study power and alpha error of 0.05 assuming a standard deviation of 9% in aBMD in 12 months for a minimum detectable change of percent change in aBMD score of 9. Thirty patients in each group were included in the study. Informed written consent was taken to participate in the study. A blocked randomization schedule was generated by computer. Participants randomized to the control group received standard nursing and medical treatment. Participants randomized to the intervention group received the same standard nursing and medical treatment but also received an intravenous zoledronic acid (5 mg/100 ml) infusion. Neither participants nor investigators were blinded to group allocation but the assessor was blinded. The allocation sequence was concealed from the investigators responsible for screening and consenting participants.
aBMD was measured at the hip (femoral neck and total hip) at baseline once patients were medically stable and at 3, 6, and 12 months from date of injury by Hologic QDR-Delphi dual X-ray absorptiometry (DXA) machine in the Department of Radio-diagnosis, Sawai Man Singh Hospital, Jaipur.
aBMD variables obtained by DXA scans were normally distributed. But, mean derived from within the group differences and between group differences were not normally distributed. Boot strapping techniques were used to overcome this problem. Statistical analysis was performed on SPSS software version 16.0. The data were presented as mean ± standard deviation. Continuous variables were compared by paired t-test. Paired t-tests were used for actual/observed aBMD values within group and Independent t-test was used for absolute change for intergroup analysis. P-values <0.05 were deemed significant.
Results
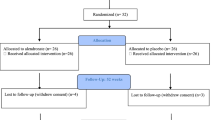
A total of 212 patients with SCI admitted between February 2013 and January 2015 were screened for eligibility. A total of 152 were excluded because they did not meet inclusion criteria or declined to participate. Sixty eligible participants were assigned either to control group or to intervention group according to blocked randomization schedule (Fig. 1). Both the groups were comparable at baseline (Table 1). As a standard treatment, all acute SCI patients were treated with intravenous methylprednisolone acetate for 72 h and IM enoxaparin for 6 weeks following injury.
Consort flow chart 212 SCI patients were admitted to the department of physical medicine and rehabilitation. All were screened for inclusion. Sixty patients were eligible for enrollment. They were assigned to either control group or intervention group based on the predefined computer generated random numbers. Baseline DXA scans were done once the patients were medically stabilized. Standard medical and nursing treatments were given to both the groups along with zoledronic acid infusion to the intervention group. Then follow-up scans were done at 3, 6, and 12 months. Two participants in the control group and one participant in the intervention group were lost in follow-up
No significant differences in aBMD between groups were observed at 3 months at any of the sites of the hip. Similarly there was no significant differences at the femoral neck at 6 months (0.787 ± 0.095 vs. 0.806 ± 0.106, p = 0.473). But there was a significant difference at the total hip (0.785 ± 0.085 vs. 0.859 ± 0.129, p = 0.014) (Fig. 2).
Significant differences in aBMD were observed between the groups at 12 months at the femoral neck (0.729 ± 0.085 vs. 0.806 ± 0.102, p = 0.003) and total hip (0.734 ± 0.074 vs. 0.845 ± 0.125, p < 0.001) (Fig. 2).
On intergroup analysis for absolute change in aBMD, significant differences were observed from 3 to 12 months. But there was non-significant difference at the femoral neck at 3 months (−0.01; 95% CI, −0.05–0.03; p = 0.65) (Table 2).
Zoledronic acid infusion was tolerated well and no adverse effects were documented. No participants had documented symptomatic hypocalcemia or renal function deterioration.
Discussion
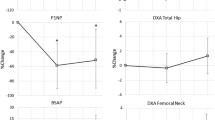
Our study reaffirms that aBMD at hip decreases early and rapidly following acute SCI. Considerable reduction of aBMD at the femoral neck (19.7%) and total hip (21.1%) over 12 months was observed (Fig. 3; Supplementary file).
Similar to our study, Gilchrist et al. [16] also reported a randomized controlled trial on oral aledronate (70 mg/week) in 31 patients after acute SCI to demonstrate preservation of aBMD at all hip sites. The aBMD decline in their control group was similar to ours (total hip—20.9 ± 1.9%, trochanter—26.3 ± 2.4%, femoral neck—16.4 ± 2%). The treatment effect of aledronate on aBMD in this study was similar to the effect of zoledronic acid in our study.
Shapiro et al. [4] performed a double-blind, randomized, placebo-controlled trial in 17 patients with SCI which received zoledronic acid 4 or 5 mg once or placebo. They concluded that a single administration of zoledronic acid will ameliorate bone loss and maintain parameters of bone strength at the three proximal femur sites for 6 months and at the femur intertrochanteric and shaft sites for 12 months. However, Bubbear et al. [12] reported a randomized, open-label study of 14 patients with acute SCI randomized to receive 4 mg IV zoledronic acid or standard treatment. The reduction of aBMD loss was significant for total hip and trochanter but not for the femoral neck, probably due to the small patient numbers. Other studies also found reduced bone loss with oral alendronate and intravenous pamidronate [17, 18].Similarly Schnitzer et al. found zoledronic acid 5 mg infusion to effectively slow down the bone loss in the lower extremity (i.e., hip more than knee) compared to placebo [19].
Zoledronic acid given once within 3 months after SCI reduced bone loss at the hip and was well tolerated. IV zoledronic acid avoids potential adherence problems seen with oral bisphosphonates [20].
Limitations
Participants who regained ability to walk with aids were not considered separately, and the possible anti-resorptive effects of rehabilitation program (time of commencement and intensity) on preservation of aBMD were not considered.
Conclusion
Early treatment with zoledronic acid is an effective treatment for the reduction of bone loss at the hip for 12 months following SCI. But bone resorption continues due to off-loading of bone and further treatment may be needed. Also larger studies with a longer follow-up to assess the magnitude of bone preservation and further treatment with zoledronic acid are required.
References
Sheng-Dan J, Li-Yang D, Lei-Sheng J. Osteoporosis after spinal cord injury. Osteoporos Int. 2006;17:180–92.
Kiratli BJ, Smith AE, Nauenberg T, Kallfelz CF, Perkash I. Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. J Rehabil Res Dev. 2000;37:225–33.
Sheng-Dan J, Lei-Sheng J, Li-Yang D. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol. 2006;65:555–65.
Shapiro J, Smith B, Beck T, Ballard P, Dapthary M, BrintzenhofeSzoc K, et al. Treatment with zoledronic acid ameliorates negative geometric changes in the proximal femur following acute spinal cord injury. Calcified Tissue Int. 2007; 80:316–22.
Slade JM, Bickel CS, Modlesky CM, Majumdar S, Dudley GA. Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free postmenopausal women. Osteoporos Int. 2005;16:263–72.
Modlesky CM, Majumdar S, Narasimhan A, Dudley GA. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J Bone Miner Res. 2004;19:48–55.
Nottage W. A review of long bone fractures in patients with spinal cord injuries. Clin Orthop Relat Res. 1981;155:65–70.
Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36:790–6.
Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe J. Supralesional and sublesional bone mineral density in spinal cord injured patients. Bone. 2000;27:305–9.
Übelhart D, Demiaux-Domenech B, Roth M, Chantraine A. Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilisation. A review. Paraplegia. 1995;33:669–73.
Sabo D, Blaich S, Wenz W, Hohmann M, Loew M, Gerner HJ. Osteoporosis in patients with paralysis after spinal cord injury: a cross sectional study in 46 male patients with dual-energy X-ray absorptiometry. Arch Orthop Trauma Surg. 2001;121:75–8.
Bubbear JS, Gall A, Middleton FR, Ferguson-Pell M, Swaminathan R, Keen RW. Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporos Int. 2011;22:271–9.
Green JR, Muller K, Jaeggi KA. Preclinical pharmacology of CGP 42′446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res. 1994;9:745–51.
Jonathan RG, Michael JR. Pharmacologic profile of zoledronic acid: a highly potent inhibitor of bone resorption. Drug Dev Res. 2002;55:210–24.
Widler L, Jaeggi K, Glatt M, Müller K, Bachmann R, Bisping M, et al. Highly potent geminal bisphosphonates from pamidronate disodium (Aredia) to zoledronic acid (Zometa). J Med Chem. 2002;45:3721–38.
Gilchrist NL, Frampton CM, Acland RH, Nicholls MG, March RL, Maguire P, et al. Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007;92:1385–90.
Zehnder Y, Risi S, Michel D, Knecht H, Perrelet R, Kraenzlin M, et al. Prevention of bone loss in paraplegics over 2 years with alendronate. J Bone Miner Res. 2004;19:1067–74.
Nance P, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil. 1999;80:243–51.
Schnitzer TJ, Kim K, Marks J, Yeasted R, Simonian N, Chen D. Zoledronic acid treatment after acute spinal cord injury: results of a randomized, placebo controlled pilot trial. PM R. 2016;8:833–43.
Cranney A, Wells G, Willan A, Griffith L, Zytaruk N, Robinson V, Black D, Adachi J, Shea B, Tugwell P, Guyatt G. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev. 2002;23:508–16.
Acknowledgements
We are grateful to Dr. Prof. Kusum Lata Gaur, Department of Preventive and Social Medicine, SMS Medical College, Jaipur for giving me valuable suggestions and for her help with the statistical analyses. We would like to acknowledge Mr. Shrikant Sharma, Senior radiographer, Department of Radio-diagnosis for his assistance with the DXA scans.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Goenka, S., Sethi, S., Pandey, N. et al. Effect of early treatment with zoledronic acid on prevention of bone loss in patients with acute spinal cord injury: a randomized controlled trial. Spinal Cord 56, 1207–1211 (2018). https://doi.org/10.1038/s41393-018-0195-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-018-0195-7
This article is cited by
-
Zoledronic acid after spinal cord injury mitigates losses in proximal femoral strength independent of ambulation ability
Osteoporosis International (2023)
-
Loss of lower extremity bone mineral density 1 year after denosumab is discontinued in persons with subacute spinal cord injury
Osteoporosis International (2023)
-
Preventive treatment with alendronate of loss of bone mineral density in acute traumatic spinal cord injury. Randomized controlled clinical trial
Spinal Cord (2022)
-
The efficacy and safety of bisphosphonate analogs for treatment of osteoporosis after spinal cord injury: a systematic review and meta-analysis of randomized controlled trials
Osteoporosis International (2021)
-
The effect of zoledronic acid on attenuation of bone loss at the hip and knee following acute traumatic spinal cord injury: a randomized-controlled study
Spinal Cord (2020)