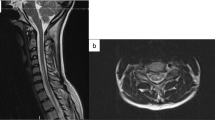
Abstract
Study design
Retrospective cohort study.
Objectives
To describe the demographics, clinical presentation, and functional outcomes of fibrocartilaginous embolic myelopathy (FCEM).
Setting
Academic inpatient rehabilitation unit in the midwestern United States.
Methods
We retrospectively searched our database to identify patients admitted between January 1, 1995 and March 31, 2016, with a high probability of FCEM. Demographic, clinical, and functional outcome measures, including Functional Independence Measure (FIM) information was obtained by chart review.
Results
We identified 31 patients with findings suggestive of FCEM (52% male), which was 2% of the nontraumatic spinal cord injury population admitted to inpatient rehabilitation. The age distribution was bimodal, with peaks in the second and sixth-to-seventh decades. The most common clinical presentation was acute pain and rapid progression of neurologic deficits consistent with a vascular myelopathy. Only three patients (10%) had FCEM documented as a diagnostic possibility. Most patients had paraplegia and neurologically incomplete injuries and were discharged to home. Nearly half of the patients required no assistive device for bladder management at discharge, but most were discharged with medications for bowel management. Median FIM walking locomotion score for all patients was 5, but most patients were discharged using a wheelchair for primary mobility. Median motor FIM subscale score was 36 at admission and 69 at discharge, with a median motor efficiency of 1.41.
Conclusions
FCEM may be underdiagnosed and should be considered in those with the appropriate clinical presentation, because their functional outcomes may be more favorable than those with other causes of spinal cord infarction.
Similar content being viewed by others
Introduction
Fibrocartilaginous embolic myelopathy (FCEM) is a type of ischemic myelopathy that occurs when a portion of the fibrocartilaginous nucleus pulposus from the intervertebral disk enters the nearby vascular system, which causes occlusion of the spinal cord vasculature. This phenomenon is well documented in the veterinary medicine literature as a cause of acute-onset myelopathy in animals and is thought to be the most common cause of ischemic myelopathy in dogs [1, 2]. FCEM in humans, although clearly established as a potential cause of myelopathy, is a less-studied and most likely underdiagnosed condition [3, 4].
There are several proposed mechanisms for the development of FCEM, with theories about how a portion of the nucleus pulposus gains spinal cord-specific vascular access. A portion of nucleus pulposus is thought to separate from the disc after a sudden increase in intradiscal pressure that may occur in instances of a sudden axial spinal load (e.g., heavy lifting, minor fall) or increased internal pressure through a Valsalva-type maneuver (e.g., straining, cough) [3,4,5,6]. The separated nucleus pulposus fragment is hypothesized to then embolize into the general spinal vasculature via adjacent vertebral sinusoids and vasculature in the case of Schmorl nodes, abnormal persistence of neonatal disc vasculature that may occur in children or young adults, or neovascularization of the disc from degenerative age-related changes [3,4,5,6,7,8]. Once inside the general vertebral arterial supply, the fibrocartilaginous embolus travels retrograde through a radicular artery, with subsequent anterograde flow into the anterior or posterior spinal arteries that serve the spinal cord. This theory is supported by research showing that intradiscal pressure can easily exceed arterial pressure during activities such as lifting. Alternatively, spinal cord venous system emboli may occur. After a fibrocartilaginous embolus enters the vertebral venous system, anterograde flow into the caval venous drainage system leads into the valveless Batson plexus where the embolus travels retrograde into the parenchyma of the spinal cord. This theory is supported by research showing that retrograde flow is possible in the setting of increased intrathoracic and intraabdominal pressure [3,4,5,6, 8].
FCEM was first identified in humans in a 1961 case report detailing an autopsy-proven fibrocartilaginous embolic infarct in the spinal cord of a 15-year-old boy who died suddenly after the development of acute-onset tetraplegia after a seemingly trivial fall [7]. Since then, 41 histopathologically confirmed cases of fibrocartilaginous embolism in humans have been reported [3]. In 2011, Mateen et al. [4] proposed criteria for antemortem diagnosis of FCEM. These criteria were later refined into an algorithmic approach for clinical diagnosis of FCEM by AbdelRazek et al. [3] in 2016. For a diagnosis of FCEM, this algorithm requires (1) a clinical syndrome of acute myelopathy; (2) no evidence of traumatic or compressive myelopathy on the basis of history and spinal imaging; (3) fulfillment of either one major criterion or two minor criteria for spinal cord infarction; (4) absence of a more common cause of spinal cord infarction (e.g., aortic pathologic process); and (5) presence of at least one of the three following criteria that established a high likelihood of FCEM: temporal relation to an event that can cause increased intradiscal or intraabdominal pressure, degenerative disc disease near the infarction level, or up to one vascular risk factor (defined as hypertension, diabetes mellitus, active smoking, peripheral arterial disease, age > 60 years, or prior stroke).
The literature to date on FCEM in humans primarily describes the clinical presentation of FCEM, with limited detail on the functional outcomes in this population. Most documented cases have been autopsy confirmed; in these incidences, FCEM was fatal, which suggests overall poor outcomes in this condition. Mateen et al. [4] found patients with FCEM to have moderate to severe disability and marked functional dependence using the Barthel index and modified Rankin Scale. No known studies have evaluated Functional Independence Measure (FIM) [9] scores in the FCEM population.
The purpose of this study was to (1) use the clinical diagnostic algorithm established by AbdelRazek et al. [3] to retrospectively identify patients with a high likelihood of FCEM; (2) add to the current literature about the clinical presentation of FCEM; and (3) more completely describe functional outcomes in this population.
Methods
The Mayo Clinic Institutional Review Board approved this study. We used the Advanced Cohort Explorer (ACE) program to identify potential cases for chart review. ACE is a searchable database maintained by the Unified Data Platform for research purposes. It contains information on all patients seen at multiple clinical and hospital source systems within Mayo Clinic, Rochester, Minnesota. Persons who decline chart review for research purposes are excluded from the ACE database. Using ACE, we searched for the records of all patients admitted to our inpatient rehabilitation unit between January 1, 1995 and March 31, 2016, with a diagnosis of spinal cord injury (SCI), myelopathy, tetraplegia, or paraplegia by using the International Classification of Diseases, Ninth Revision codes. Patients were excluded if they had a coexisting diagnosis of spinal surgery, spinal fracture, skull fracture, intracerebral or intraspinal hemorrhage, intraspinal abscess, intraspinal tumor, critical illness myopathy/polyneuropathy, aortic aneurysm/dissection, or aortic surgery within 90 days before or after the diagnosis of myelopathy or SCI (Supplemental Figure). Among patients initially identified with potential FCEM, we applied a diagnostic algorithm to identify patients that had a high probability of FCEM (described below). Potential cases of FCEM were additionally identified in a similar manner from the Mayo Clinic SCI database, initiated in 1972. Two physical medicine and rehabilitation resident physicians (BJM, AMB) performed all chart reviews.
To identify cases that fit the clinical diagnosis of FCEM, we used the schematic approach to diagnosing FCEM outlined by AbdelRazek et al. in 2016 [3]. For the purposes of this study, the algorithm was modified to identify cases with a high probability of FCEM (Fig. 1). The modification allowed patients to have up to one nonspecific inflammatory finding at initial evaluation (i.e., minimally increased cerebrospinal fluid (CSF) pleocytosis, increased IgG index, or gadolinium enhancement on magnetic resonance imaging (MRI)) if all other criteria were fulfilled. This modification was used because a substantial percentage of those with histopathologically confirmed FCEM had abnormal CSF findings (21%) or gadolinium enhancement (18%) when CSF or MRI findings were reported [3].
For patients deemed to have a high probability of FCEM, further detailed chart review was performed to abstract demographic, clinical, and functional outcome data. Demographic information included patient age, sex, and body mass index (BMI) at the time of initial hospitalization. Clinical outcome measures included vascular risk factors and clinical features at initial evaluation, physical examination findings, CSF findings, MRI findings, and documented discharge diagnosis. Patients were considered to have a vascular pattern on physical examination if there was documentation of spared vibration or proprioception. Vascular patterns on imaging were those with MRI T2 hyperintensity in an anterior spinal artery or posterior spinal artery distribution. Functional outcome measures included neurologic level of injury, neurologic completeness of injury, inpatient rehabilitation length of stay (LOS), discharge location, bladder management at discharge, bowel management at discharge, and mobility status at discharge. FIM scores at admission and discharge were obtained from corresponding insurance-reported information during hospitalization for inpatient rehabilitation. FIM motor efficiency was calculated by dividing motor FIM change by LOS in days. Functional outcome data from our SCI database were also obtained for patients with a documented vascular cause of myelopathy, including LOS and discharge location.
Data for the patient group were summarized by using basic statistical analyses and reported as counts (percentages) or median (range or interquartile range (IQR)).
Results
Among 689 patients initially identified from January 1995 through March 2016, 31 patients fulfilled the criteria for high probability of FCEM as the underlying cause of their acute myelopathy. During the same time frame, 2254 patients with SCI were admitted to the inpatient rehabilitation unit, with 1644 of those SCIs being nontraumatic. Thus, patients with a high probability of FCEM made up 1.9% of the nontraumatic SCI population and 1.4% of the total SCI population admitted for inpatient rehabilitation.
Demographic findings
The median age at injury was 53 years (range, 9–83 years) (Table 1). A bimodal age distribution was present, with higher incidence in the second and sixth to seventh decades. Sex distribution was essentially equal, with 52% of patients being male. Median BMI was 29.2 kg/m2.
Clinical and diagnostic findings
Most patients (68%) had 0 or 1 vascular risk factor, with the most common being age and premorbid diagnosis of hypertension. The most common presentations of FCEM were acute pain (77%), rapid progression of neurologic deficits (71%), and physical examination consistent with vascular-distribution myelopathy (75%) (Table 1). Initial work-up most commonly showed nonspecific increased CSF protein levels (74%), spinal MRI with vascular distribution abnormality (90%), and nearby degenerative spine disease (80%). In patients with increased CSF protein level (reference range either <35 or <45 mg/dL, per lab specifications at date of collection), the median (range) level was 60 (38–143) mg/dL. Most patients (65%) had no nonspecific inflammatory findings (CSF pleocytosis, increased IgG index, or enhancement on MRI). Some degree of spinal cord gadolinium enhancement was seen on MRI in 30% of patients imaged, with corresponding radiologic reports describing enhancement as “minimal,” “patchy,” or “consistent with evolving infarct.” One patient had an increased IgG index (2.26) without increased CSF protein, CSF pleocytosis, or MRI gadolinium enhancement. One patient had CSF pleocytosis (16 cells/mcL without lymphocytosis), with nonspecifically increased CSF protein (73 mg/dL) and no MRI gadolinium enhancement.
Discharge diagnosis was “spinal infarct—not otherwise specified” in 52% and “transverse myelitis” or “idiopathic transverse myelitis” in 26% of patients. Only 10% of patients (n = 3) had FCEM documented as a diagnostic possibility. The pediatric patients (n = 5) had increased CSF protein level (20%) or nearby degenerative spine disease (40%) less commonly than adult patients.
Functional outcomes
Median inpatient rehabilitation LOS for FCEM patients was 23 days (Table 2). Most patients had paraplegia (65%), had incomplete SCI (94%), and were discharged to home (84%). The pediatric subset, however, was slightly more likely to have tetraplegia (60%), with two of the five pediatric patients having high tetraplegia (C1−C4 neurologic level of injury). FIM scores were available for 29 of the 31 patients. Median motor FIM subscale was 36 at admission, which improved to 69 at discharge. Median motor FIM change was 28, and median LOS efficiency was 1.41 points/day. At discharge, nearly half the patients (48%) required no assistive device for bladder management. Median bladder FIM score at admission was 1, with improvement to 5 at discharge (IQR, 1–6), which indicated that they required assistance with setup or supervision, or had 1 episode of urinary incontinence per week.
The majority of patients were discharged with medications for bowel management (84%) (Table 2). Median bowel FIM score at admission was 1 with an improvement to 6 at discharge (IQR, 1–6). Nearly half of patients (45%) at discharge were ambulating independently or with supervision with or without a gait aid, and the rest (55%) were mobilizing primarily from a wheelchair base. The median walking locomotion FIM score, available for 26 patients, was 5 (IQR, 1–6), which indicates that either standby supervision was required or independent ambulation took more than a reasonable amount of time.
Median LOS for all patients with vascular causes of SCI at our institution was 16 days, compared with the estimated LOS of 23 days for the FCEM patients. Rate of discharge to home for all patients with vascular causes of SCI (81%) was similar to that for the FCEM patients.
Discussion
This study suggests that FCEM may affect a unique patient population compared with other causes of SCI. Table 3 compares the patients with highly probable FCEM in this study with published data for other categories of SCI. The equal sex distribution for highly probable FCEM is in contrast to the male predominance of traumatic SCI [10] and the modest male predominance seen in the spinal cord infarction population as a whole [11, 12]. The median age at presentation of highly probable FCEM is older than for both traumatic SCI [10] and transverse myelitis populations [13] and is younger than for spinal cord infarction [11]. In addition, our data suggest a possible bimodal age distribution for FCEM. This may reflect the different theorized pathophysiologic mechanisms in FCEM, with abnormal persistence of neonatal disc vasculature potentially affecting teenagers and younger adults, and neovascularization of discs with access through Schmorl nodes potentially affecting middle-age to older adults. The median BMI in this population was high (29.2 kg/m2), but this is believed to be a reflection of the local population.
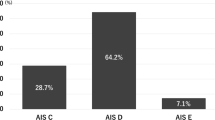
Differences were also seen when comparing the neurologic involvement and functional outcomes of patients with highly probable FCEM with those from published data for other causes of SCI. The percentage of people with tetraplegia in highly probable FCEM was lower than in traumatic SCI and higher than the incidence in published reports on spinal cord infarction [12, 14, 15]. This discrepancy most likely stems from most spinal cord infarctions being related to aortic pathologic processes, such as complications of thoracoabdominal aortic aneurysm repair, which are, thus, more likely to affect the thoracic spinal cord [15,16,17]. In contrast, FCEM theoretically can occur anywhere along the cord. The pediatric subset of highly probable FCEM was even more likely to have tetraplegia, with three of five pediatric patients having tetraplegia. Of interest, the only two patients in this study with high tetraplegia (C1−C4) were children. Although patients with highly probable FCEM had a higher neurologic level of injury, they were more likely to have an incomplete SCI than those with spinal cord infarction [12, 14, 15].
With regard to functional outcomes, FIM motor efficiency was superior in the highly probable FCEM patients than patients with spinal cord infarction [14, 15] but was similar to efficiency data for traumatic SCI [18]. These findings, taken together, suggest that although persons with FCEM may be more likely to have a higher neurologic level of injury than for all other causes of ischemic myelopathy, the myelopathy severity may be milder. This may be partly due to the increased likelihood of an incomplete SCI as well as the younger age of presentation in FCEM.
Compared with all vascular causes of SCI at our institution, patients with highly probable FCEM had longer overall inpatient rehabilitation unit LOS, with similar discharge rates to home. The longer LOS may have been related to the higher neurologic levels of injury or because they were demonstrating greater functional improvements, as evidenced by higher FIM efficiency scores.
Currently, FCEM is considered an uncommon cause of spinal cord infarction in humans, but it may be an underrecognized diagnosis [3]. Our data support this perspective, because FCEM was mentioned as a diagnostic possibility for only 10% of the identified patients. After spinal cord infarct—not otherwise specified, idiopathic transverse myelitis was the most commonly encountered discharge diagnosis in these patients. This may reflect a decreased awareness of FCEM compared with more familiar diagnoses such as transverse myelitis. Unfortunately, misdiagnosis of FCEM may result in ineffective treatments that have potential risk, such as immunosuppressant medications or plasma exchange.
The incidence of FCEM is currently unknown. FCEM has been reported to account for 5.5% of all cases of acute spinal cord infarction [4]. Those data, however, most likely underestimate the frequency, because they did not account for potential misdiagnoses of nonvascular causes of myelopathy, such as idiopathic transverse myelitis. Vascular myelopathies are estimated to represent 5−8% of acute myelopathies [16]. The current study findings, with FCEM capable of representing 2% of all nontraumatic spinal cord injuries in patients admitted to the inpatient rehabilitation unit, suggest that FCEM is more common than previously thought.
This study has some limitations. It is a retrospective chart review that is limited to the information available in the medical record. In addition, the most definitive way to diagnose FCEM is by autopsy. To improve the reliability of patient classification, a diagnostic algorithm described by AbdelRazek et al. [3] was used to identify cases of FCEM. Although imperfect, the algorithm is the best available tool to diagnose FCEM in living patients. The sample size is also relatively small, and the follow-up duration is relatively short. This study, however, is one of the largest published on FCEM and provides an alternative perspective regarding outcome potential. Finally, because the target population focused on persons who were admitted to inpatient rehabilitation, the results may not be generalizable to patients with FCEM overall.
Conclusion
FCEM may be an underrecognized cause of spinal cord infarction and should be considered in cases of acute-onset myelopathy without other identifiable causes, especially in patients without corresponding inflammatory markers. FCEM appears different from other causes of nontraumatic myelopathy in that it has a bimodal age distribution and equal sex distribution. Patients we identified as having a high likelihood of FCEM also have favorable functional outcomes at the time of discharge from inpatient rehabilitation, which contradicts the suggested notion of poor outcomes.
Disclaimer
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
De Risio L. A review of fibrocartilaginous embolic myelopathy and different types of peracute non-compressive intervertebral disk extrusions in dogs and cats. Front Vet Sci. 2015;2:24.
Cauzinille L, Kornegay JN. Fibrocartilaginous embolism of the spinal cord in dogs: review of 36 histologically confirmed cases and retrospective study of 26 suspected cases. J Vet Intern Med. 1996;10:241–5.
AbdelRazek MA, Mowla A, Farooq S, Silvestri N, Sawyer R, Wolfe G. Fibrocartilaginous embolism: a comprehensive review of an under-studied cause of spinal cord infarction and proposed diagnostic criteria. J Spinal Cord Med. 2016;39:146–54.
Mateen FJ, Monrad PA, Hunderfund AN, Robertson CE, Sorenson EJ. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218–25.
Thone J, Hohaus A, Bickel A, Erbguth F. Severe spinal cord ischemia subsequent to fibrocartilaginous embolism. J Neurol Sci. 2007;263:211–3.
Han JJ, Massagli TL, Jaffe KM. Fibrocartilaginous embolism—an uncommon cause of spinal cord infarction: a case report and review of the literature. Arch Phys Med Rehabil. 2004;85:153–7.
Naiman JL, Donohue WL, Prichard JS. Fatal nucleus pulposus embolism of spinal cord after trauma. Neurology. 1961;11:83–87.
McLean JM, Palagallo GL, Henderson JP, Kimm JA. Myelopathy associated with fibrocartilaginous emboli (FE): review and two suspected cases. Surg Neurol. 1995;44:228–34. discussion 234−5
Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18.
National Spinal Cord Injury Statistical Center. Annual Statistical Report for the Spinal Cord Injury Model Systems: Public Version. University of Alabama at Birmingham. 2016. https://www.nscisc.452uab.edu/
Robertson CE, Brown RD Jr., Wijdicks EF, Rabinstein AA. Recovery after spinal cord infarcts: long-term outcome in 115 patients. Neurology. 2012;78:114–21.
Cheshire WP, Santos CC, Massey EW, Howard JF Jr.. Spinal cord infarction: etiology and outcome. Neurology. 1996;47:321–30.
Ho CH, Wuermser LA, Priebe MM, Chiodo AE, Scelza WM, Kirshblum SC. Spinal cord injury medicine. 1. Epidemiology and classification. Arch Phys Med Rehabil. 2007;88:S49–S54.
New PW, McFarlane CL. Retrospective case series of outcomes following spinal cord infarction. Eur J Neurol. 2012;19:1207–12.
McKinley W, Sinha A, Ketchum J, Deng X. Comparison of rehabilitation outcomes following vascular-related and traumatic spinal cord injury. J Spinal Cord Med. 2011;34:410–5.
Rubin MN, Rabinstein AA. Vascular diseases of the spinal cord. Neurol Clin. 2013;31:153–81.
Augoustides JG, Stone ME, Drenger B. Novel approaches to spinal cord protection during thoracoabdominal aortic interventions. Curr Opin Anaesthesiol. 2014;27:98–105.
Granger CV, Karmarkar AM, Graham JE, Deutsch A, Niewczyk P, Divita MA, et al. The uniform data system for medical rehabilitation: report of patients with traumatic spinal cord injury discharged from rehabilitation programs in 2002−2010. Am J Phys Med Rehabil. 2012;91:289–99.
National Spinal Cord Injury Statistical Center. Facts and Figures at a Glance. University of Alabama at Birmingham. 2017. https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%20-%202017.pdf.
Cobo Calvo A, Mane Martinez MA, Alentorn-Palau A, Bruna Escuer J, Romero Pinel L, Martinez-Yelamos S. Idiopathic acute transverse myelitis: outcome and conversion to multiple sclerosis in a large series. BMC Neurol. 2013;13:135.
Gupta A, Kumar SN, Taly AB. Neurological and functional recovery in acute transverse myelitis patients with inpatient rehabilitation and magnetic resonance imaging correlates. Spinal Cord. 2016;54:804–8.
Acknowledgements
Funding
This publication was supported by Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS).
Author contributions
AMB was responsible for designing the study protocol, writing the protocol, performing chart review of potential eligible study subjects, extracting and analyzing data of eligible study subjects, interpreting results, drafting and revising the manuscript, and creating Table 2 and the supplemental figure. BJM was responsible for designing the study protocol, writing the protocol, performing chart review of potential eligible study subjects, extracting and analyzing data of eligible study subjects, interpreting results, drafting and revising the manuscript, creating Table 1, and updating the reference lists. MTL was responsible for identifying potential eligible study subjects. RKR was responsible for designing the study protocol, interpreting results, and manuscript revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Moore, B.J., Batterson, A.M., Luetmer, M.T. et al. Fibrocartilaginous embolic myelopathy: demographics, clinical presentation, and functional outcomes. Spinal Cord 56, 1144–1150 (2018). https://doi.org/10.1038/s41393-018-0159-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-018-0159-y
This article is cited by
-
The trends in sports-related spinal cord injury in China
Spinal Cord (2023)
-
Fibrocartilaginous embolism of the posterior spinal artery: A case report regarding the responsible intervertebral disc on magnetic resonance imaging
Spinal Cord Series and Cases (2022)