Abstract
Study design
Psychometric study using retrospectively collected data.
Objectives
We investigated the comparability of quantitative computed tomography (qCT) in assessing bone mineral density (BMD) with dual energy X-ray absorptiometry (DXA). We evaluated how well previously suggested normal values for spinal Hounsfield units (HU) correlated with routine DXA results in patients with chronic spinal cord injury (SCI). Furthermore, we investigated inter/intra-observer reliability of measuring HU in the spine.
Setting
Academic medical center in Tehran, Iran.
Methods
Spinal CT scans of 44 male participants with chronic SCI who had undergone DXA studies on the same day were selected. The main outcome measures were sensitivity, specificity, and area under curve (AUC) of HU at each spinal region against DXA results of areal BMD. The secondary outcome was inter/intra-observer reliability of measuring HU in the spinal column.
Results
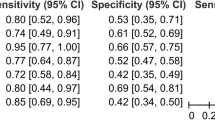
We found no significant difference between qCT and DXA results (p-value = 0.237, R = 0.188). However, the two methods showed overall unfavorable comparability, with a sensitivity of 0%, 0%, and 80%, specificity of 50%, 90%, and 85%, and area under curve (AUC) of 0.27, 0.53, and 0.83 for cervical, thoracic, and lumbar spine, respectively. The best comparability was achieved at the lumbar region although not statistically significant (p-value = 0.072). Measuring HU was reliable (inter/intra-observer reliability >98%).
Conclusions
This study demonstrates that currently proposed normal values result in unfavorable comparability in the cervical and thoracic regions; however, as the agreement improved at the lumbar spine, it is possible that qCT could become an indicator of bone strength with further research.
Similar content being viewed by others
Introduction
Disuse osteoporosis is one of the most common complications of spinal cord injury (SCI) which causes significant morbidity in patients and increased toll for health-care systems [1, 2]. The process of bone loss is rapid and severe and starts as early as in the first year after SCI [3,4,5,6]. Different mechanisms contribute to this process such as physical unloading and loss of innervation [2,3,4]. Bone loss after SCI causes increased risk of fractures due to little or no trauma. It has been estimated that as many as 50% of the patients with SCI experience fractures at some point in their lifetime and the duration of injury is the most important predictor for the occurence of fractures [5,6,7,8]. Fractures may cause various complications such as non-union fractures, delayed healing, and pressure ulcers resulting from bracing, and it has been shown that such events plague more than 50% of fracture-related hospitalizations [9,10,11,12,13]. Dual energy X-ray absorptiometry (DXA) is the gold-standard method widely used to assess areal bone mineral density (aBMD); however, some believe that quantitative computed tomography (qCT) may provide better insight into the boney structure specifically in patients with SCI [2, 3]. New developments in the computed tomography (CT) technology, such as automatic exposure control and thin sectioning, subject the spinal column to a more homogenous X-ray beam. As a result, Hounsfield unit (HU) measurements are closely dependent on the composition of the targeted tissue which theoretically eliminates the necessity of using calibrating phantoms in assessing BMD. [9] It is noteworthy that many patients with SCI undergo CT studies for various reasons throughout the years after injury. The reasons include investigational purposes to determine sources of chronic pain or to evaluate skeletal abnormalities like mal-alignments. In this context, it seems beneficial to incorporate HU-based BMD assessment in routine radiologic reports as it is a measure readily available. The goal of the present study is to determine whether the results obtained using DXA are comparable with HU-based results of the whole spine or regionally in the ability to detect low BMD.
Methods
A database containing imaging studies of war veterans with chronic SCI was retrospectively reviewed. The data were later compared to the medical records of patients such as their ambulatory status, their neurological-deficit status based on American Spinal Injury Association (ASIA) Impairment Scale, and the duration of injury.
Participants who had both DXA and spinal CT investigations at the site of their injury within the same screening visit were included. Those who had instrumental fixations, previous spinal interventions such as vertebroplasty or fusion, were excluded. Using these criteria, 44 male war veterans were included in this study. The measurement of HU was done according to the method described by Schreiber et al. [9].
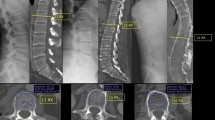
All DXA scans were obtained using a DMS Stratos device (France, 2015). Measurements were carried out from the first to the fourth lumbar vertebrae and were reported in g/cm2, and the respective average Z- and T-scores were calculated. qCT was performed using 16 channel helical CT scanner SOMATOM Emotion 6 (Siemens, Erlangen, Germany, 1994) in all patients. CT parameters included: slice thickness of 1.25 mm, with 1.0 s interval, tube voltage 130 kV, effective tube current 150 mAs (obtained using CARE dose 4D), and boney reconstruction in three planes and no phantoms were used. Picture Archiving and Communications System (PACS) used was INFINITT software (version 3.0.11.3, South Korea). To measure the average HU in axial planes, an elliptical area was drawn in the vertebral bodies in frames immediately inferior to superior end plate, mid vertebral, and immediately superior to inferior end plate below the site of SCI. This elliptical region did not contain cortical boney tissue (as shown in Fig. 1). HU measurements were done by two different observers (HH and NM) according to the method described by Schreiber et al., and the observers were blinded to DXA result.
Categorizing HU into different groups of BMD status was done according to the suggested values by Schreiber et al. for measurements done in lumbar vertebrae and Marinova et al. [14] for measurements done in the thoracic region. For the cervical region, limited amount of evidence existed; however, we referenced work by Weishauptet al. [1] for 25 male participants and an average of 250 was chosen which was in compliance with other work done by Yoganandan et al. [15] in female participants. DXA reported T-scores were categorized into groups of T-scores higher than −2.5 standard deviations (SD) and −2.5 SD or below. The suggestions of the International Society for Clinical Densitometry, the American College of Radiology, and the American College of Physicians for diagnosing osteoporosis in men who are at least 50-years old and have additional risk factors, and other studies [1,2,3,4,5,6,7, 9,10,11,12,13,14,15,16], were taken into consideration for choosing this cutoff.
Statistical analysis
Normal distribution of quantitative variables was assessed using Shapiro–Wilk test and visual inspection of histograms. Inter-observer and intra-observer reliability were assessed using interclass correlation coefficient reported as a score between 0 representing no agreement and 1 representing perfect agreement. Scores higher than 0.8 are considered as an excellent agreement. Further analysis to compare subgroups in aspects of age and time since injury was done using one-way ANOVA, and using Fisher’s exact test for ambulation status and partial or complete motor injury. Measures of diagnostic accuracy were calculated using receiver operating characteristic (ROC) analysis. Comparison between the two methods was carried out using Fisher’s exact test.
Results
Reliability
Measuring HU according to the method proposed by Schreiber et al. was reliable with inter-observer reliability of 0.988 and intra-observer reliability of 0.992, both showing excellent levels of agreement.
Sample demographics
The average age for 44 participants was 50 ± 7 and there was no significant age difference between the subgroups of different BMD status (p-value = 0.069). Distribution of DXA Z-scores vs. age was random, which is depicted in figure S1. Out of 44 participants, 8 participants (18%) had cervical spine injury, 24 (55%) thoracic spine injury, and 12 (27%) lumbar spine injury. The mean duration of SCI was 28 ± 4 years, which did not differ significantly across the subgroups of different BMD status (p-value = 0.349).
Out of 44 participants, 4 (9.0%) walked independently, 8 (18%) walked using unilateral or bilateral canes, 28 (64%) moved independently by utilizing wheelchairs, and 4 (9.0%) were dependent for movement completely on others. Ambulatory status did not differ significantly across the subgroups with different BMD (p-value = 0.698).
Overall, 15 participants (34%) had motor complete SCI whereas 29 (66%) had motor incomplete SCI. Status of motor complete or incomplete injury did not differ significantly across the different BMD subgroups (p-value = 0.452) (Tables 1 and 2).
BMD comparability
We categorized qCT and DXA results based on the previous studies and the suggestions which were specified in methods section. Performing Fisher’s exact test to assess whether the two methods differed in general assessment of BMD revealed no significant difference with a p-value of 0.237 and R = 0.188. Further measures of diagnostic comparability are presented in Table 3.
ROC analysis was performed for each region of the spine separately as each region differs from the other in the range of normal HU values.
Due to small numbers of participants in each subset, coordinates of ROC curves are given in the supplements (Table S1) since these coordinates provide more information in comparison with the curve itself when the sample size is small. The curves are presented in Fig. 2. Areas under curves (AUC) for cervical, thoracic, and lumbar spine were 0.27, 0.53, and 0.83 with p-values of 0.297, 0.876, and 0.062, respectively. As it can be seen in Table S1, a cutoff point of 375 HU in the cervical spine would provide a sensitivity of 0.67 and a specificity of 0.40. In the thoracic spine, the respective cutoff of 230 HU would provide a sensitivity of 0.5 and a specificity of 0.23, while in the lumbar spine a cutoff of 150 HU would give a sensitivity of 0.80 and a specificity of 0.86. p-Values for all AUC measures were non-significant (p > 0.05).
ROC curves demonstrating HU comparability with DXA-based results at each region of the spinal column. For information on the coordinates of the curve please refer to Table S1
Discussion
Osteoporosis is a serious complication of SCI which imposes increased costs on health-care system and endangers patients with various consequences [1,2,3,4,5,6]. Patients with chronic SCI may be investigated with CT scans throughout the years for complications associated with the condition, such as vertebral fractures, facet joints degenerations, and preoperative studies for mal-alignment correction or other surgeries. It has recently been suggested that due to thin sectioning and automatic exposure adjustments implemented in new CT scanning modalities, HU measurements provide a method readily available to assess aBMD with comparable accuracy to DXA [9, 14]. In this study, we aimed to investigate how well the proposed HU normal ranges performed in comparison with DXA in patients with chronic SCI. Results of this study demonstrate that considering qCT to be superior or inferior in assessing BMD in comparison with DXA is rather a simplistic view on this matter. As we can see from Table 3 and Fig. 2, the comparability of the two methods increases from the cervical spine to lumbar spine. This may be due to BMD heterogeneity in SCI setting. It has been previously suggested that while supralesional BMD is fairly preserved in patients with SCI, severe sublesional osteoporosis can occur [17]. This pattern of bone loss is the reason we chose to measure HU in sublesional vertebral bodies. However, while DXA and qCT may not show desirable comparability, we believe in the future HU measurement may prove to be a valuable tool to assess regional BMD, which in turn is an important prognostic predictor of device implantation success [18,19,20,21,22]. Coordinates of the ROC curve provide a better insight into this matter by showing sensitivity and specificity of a single HU measurement as is mentioned in the results section and can be further explored in Table S1.
Measuring HU in the planned trajectory of screw fixations may provide important insight for surgeons about strength of bone–screw interface before operation. This correlation has been tested in dentistry to see if bone strength assessed by using qCT with automatic exposure control correlates with insertion torque and stability of metal implants both in vivo and in vitro [18,19,20,21,22]. For this purpose, further correlations between HU measurements and screw fixation outcomes may prove beneficial. Another advantage of this method is that qCT measures mineral density of trabecular bone which is more metabolically dynamic and responds to treatments faster [23,24,25,26]. Present study however has following limitations: first limitation was lack of normative data for the cervical spine. In this study, we decided to choose an average of 250 HU for measurements done in cervical region which was referenced on work by Weishaupt et al. and similar study by Yoganandan et al. [1, 15]. However, because our sample was averagely older and all-male, this value might not be a proper representative of average HU in the cervical spine. For the thoracic and lumbar spine previously normative data have been suggested; however, establishing conclusive worldwide normative values represented following STARD guidelines is yet to be done. Another important limitation of this study was the small sample size. This is due to physicians’ preference of requesting spinal MRI, wherever applicable, mainly because MRI does not subject patients to X-ray beam and also because it visualizes the cord itself in which multiple abnormalities can occur in chronic SCI such as cord tethering, cyst formation, and cord atrophy [27, 28].
In conclusion, this study suggests using qCT, done throughout the spine, can become a useful tool in assessing BMD. Preferably, large prospective cohort studies which use fracture rates as the main outcome will assist in determining normal HU values for the spinal column and provide a better insight into the comparability of DXA and qCT. However, qCT can be done on any vertebral body, and with the growing pool of normal values, it can be used for pre-operative assessment about screw implantations stability and to serve as a regional measure of spinal strength.
References
Weishaupt D, Schweitzer ME, DiCuccio MN, Whitley PE. Relationships of cervical, thoracic, and lumbar bone mineral density by quantitative CT. J Comput Assist Tomogr. 2001;25:146–50.
Biering-Sorensen F, Bohr H, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–5.
Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, et al. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–80.
Jiang SD, Dai LY, Jiang LS. Osteoporosis after spinal cord injury. Osteoporos Int. 2006;17:180–92.
Froztler A, Cheikh-Sarraf B, Pourtehrani M, Krebs J, Lippnuner K. Long-bone fractures in persons with spinal cord injury. Spinal Cord. 2015;53:701–4.
Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39:208–14.
Gifre L, Vidal J, Carrasco J, Portell E, Puig J, Monegal A, et al. Incidence of skeletal fractures after traumatic spinal cord injury: a 10-year follow-up study. Clin Rehabil. 2014;28:361–9.
Lala D, Craven BC, Thabane L, Papaioannou A, Adachi JD, Popovic MR, et al. Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporos Int. 2014;25:177–85.
Schreiber JJ, Anderson P, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011;93:1057–63.
Morse LR, Battaglino RA, Stolzmann KL, Hallet LD, Waddimba A, Gagnon D, et al. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int. 2009;20:385–92.
Comarr AE, Hutchinson RH, Bors E. Extremity fractures of patients with spinal cord injuries. Am J Surg. 1962;103:732–9.
Freehafer AA. Limb fractures in patients with spinal cord injury. Arch Phys Med Rehabil. 1995;76:823–7.
Rogers T, Shokes L, Woodworth P. Pathologic extremity fracture care in spinal cord injury. Spinal Cord Inj Rehabil. 2005;11:70–8.
Marinova M, Edon B, Wolter K, Katsimbari B, Schild HH, Strunk HM. Use of routine thoracic and abdominal computed tomography scans for assessing bone mineral density and detecting osteoporosis. Curr Med Res Opin. 2015;31:1871–81.
Yoganandan N, Pintar F, Stemper BD, Baisden JL, Aktay R, Shender BS, et al. Bone mineral density of human female cervical and lumbar spines from quantitative computed tomography. Spine. 2006;31:73–6.
Le Chapelian L, Perrouin-Verbe B, Fattal C. SOFMER French Society for Physical Medicine and Rehabilitation. Chronic neuropathic pain in spinal cord injury patients: what relevant additional clinical exams should be performed. Ann Phys Rehabil Med. 2009;52:103–10.
Dauty M, Perrouin-Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord injured patients. Bone. 2000;27:305–9.
Lund T, Oxland TR, Jost B, Cripton P, Grassmann S, Etter C, et al. Interbody cage stabilization in the lumbar spine: biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg Br. 1998;80:351–9.
Hasegawa K, Abe M, Washio T, Hara T. An experimental study on the interface strength between titanium mesh cage and vertebra in reference to vertebral bone mineral density. Spine. 2001;26:956–63.
Lim TH, Kwon H, Kim JG, Sokolowski M, Natarajan R, An HS, et al. Effect of endplate conditions and bone mineral density on the compressive strength of the graft-endplate interface in anterior cervical spine fusion. Spine. 2001;26:951–6.
Shapurian T, Damoulis PD, Reiser GM, Griffin TJ, Rand WM. Quantitative evaluation of bone density using the Hounsfield index. Int J Oral Maxillofac Implants. 2006;21:290–7.
Turkyilmaz I, Ozan O, Yilmaz B, Ersoy AE. Determination of bone quality of 372 implant recipient sites using Hounsfield unit from computerized tomography: a clinical study. Clin Implant Dent Relat Res. 2008;10:238–44.
Froztler A, Berger M, Knecht H, Eser P. Bone steady-state is established at reduced bone strength after spinal cord injury: a longitudinal study using peripheral quantitative computed tomography (pQCT). Bone. 2008;43:549–55.
Eswaran SK, Gupta A, Adams MF, Keaveny TM. Cortical and trabecular load sharing in the human vertebral body. Bone Miner Res. 2006;21:307–14.
Tsuzuku S, Ikegami Y, Yabe K. Bone mineral density differences between paraplegic and quadriplegic patients: a cross-sectional study. Spinal Cord. 1999;37:358–61.
Gifre L, Vidal J, Carrasco J, Muxi A, Portell E, Monegal A, et al. Risk factors for the development of osteoporosis after spinal cord injury. A 12-month follow-up study. Osteoporos Int. 2015;26:2273–80.
Jaovisidha S, Sartoris DJ, Martin EM, De Maeseneer M, Szollar SM, Deftos LJ. Influence of spondylopathy on bone densitometry using dual energy x-ray absorptiometry. Calcif Tissue Int.1997;60:424–9.
Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D. Regional osteoporosis in women who have a complete spinal cord injury. J Bone Joint Surg Am. 2001;83-A:1195–200.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Haghighat Khah, H.R., Moradi, N., Taheri, T. et al. Investigating comparability of quantitative computed tomography with dual energy x-ray absorptiometry in assessing bone mineral density of patients with chronic spinal cord injury. Spinal Cord 56, 487–493 (2018). https://doi.org/10.1038/s41393-017-0041-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-017-0041-3
This article is cited by
-
Time to augment?! Impact of cement augmentation on pedicle screw fixation strength depending on bone mineral density
European Spine Journal (2018)