Abstract
Targeted anticancer drugs block cancer cell growth by interfering with specific signaling pathways vital to carcinogenesis and tumor growth rather than harming all rapidly dividing cells as in cytotoxic chemotherapy. The Response Evaluation Criteria in Solid Tumor (RECIST) system has been used to assess tumor response to therapy via changes in the size of target lesions as measured by calipers, conventional anatomically based imaging modalities such as computed tomography (CT), and magnetic resonance imaging (MRI), and other imaging methods. However, RECIST is sometimes inaccurate in assessing the efficacy of targeted therapy drugs because of the poor correlation between tumor size and treatment-induced tumor necrosis or shrinkage. This approach might also result in delayed identification of response when the therapy does confer a reduction in tumor size. Innovative molecular imaging techniques have rapidly gained importance in the dawning era of targeted therapy as they can visualize, characterize, and quantify biological processes at the cellular, subcellular, or even molecular level rather than at the anatomical level. This review summarizes different targeted cell signaling pathways, various molecular imaging techniques, and developed probes. Moreover, the application of molecular imaging for evaluating treatment response and related clinical outcome is also systematically outlined. In the future, more attention should be paid to promoting the clinical translation of molecular imaging in evaluating the sensitivity to targeted therapy with biocompatible probes. In particular, multimodal imaging technologies incorporating advanced artificial intelligence should be developed to comprehensively and accurately assess cancer-targeted therapy, in addition to RECIST-based methods.
Similar content being viewed by others
Introduction
Cancer is the leading cause of mortality globally. It was estimated that approximately 2,370,000 and 4,820,000 new cancer cases and 640,000 and 3,210,000 cancer deaths would occur in the United States and China in 2022, respectively.1 Surgery and radiotherapy (RT) are two primary treatment cornerstones of locoregional and nonmetastatic cancers, while chemotherapeutics can be used in all cancer stages. Current chemotherapeutics are often limited by undesirable side effects due to an inability to distinguish between tumorous and normal tissues. To mitigate these side effects, it is possible to develop targeted therapy using monoclonal antibodies or small-molecule inhibitors directed against specific signal transduction pathways for angiogenesis, proliferation, survival, and invasiveness, which are often dysregulated in tumor cells.2 The development of targeted therapies is thus a valuable advance for cancer treatment.
To evaluate the efficacy of anticancer treatment, both the World Health Organization (WHO) response criteria3 and Response Evaluation Criteria in Solid Tumors (RECIST)4 utilize changes in tumor size as determined using imaging techniques such as CT, MRI, and positron emission tomography (PET). RECIST 1.1 is currently the gold standard for assessing treatment response in solid tumors in a clinical context, but it is insufficient for some targeted medications such as antiangiogenic agents and immunotherapy.5 For immunotherapy, a 5% rate of pseudo-progression, involving the tumor regressing after initial disease progression or the appearance of new lesions, was reported in non-small-cell lung cancer (NSCLC) patients treated with nivolumab.6 According to reports, this phenomenon was caused by an insufficient immune response or edema and necrosis related to immune-cell infiltration into tumor tissue.7 RECIST 1.1 relies solely on tumor size and does not consider changes in cellular events such as apoptosis, inhibition of proliferation, cell cycle arrest, tumor metabolism within the tumor microenvironment, and the density and number of intra-tumoral vessels. Thus, it remains unclear how best to evaluate the efficacy of targeted therapies and optimize the therapeutic strategy.
New functional and molecular imaging biomarkers are being developed to better evaluate targeted therapy’s effects. Molecular imaging combines biomedical imaging and molecular biology to visualize and quantify the spatiotemporal distribution of biological processes within living organisms in a noninvasive way for biochemical, biologic, diagnostic, and therapeutic applications.8,9 Representative examples of molecular imaging techniques include radionuclide imaging (PET), single-photon emission computed tomography (SPECT), molecular magnetic resonance imaging (mMRI), magnetic resonance spectroscopy (MRS), optical imaging (optical bioluminescence, optical fluorescence), photoacoustic imaging, and multimodal imaging. Some modalities, such as radionuclide and optical imaging, require the injection of molecular probes to acquire the imaging signal. In contrast, mMRI and photoacoustic imaging can track drug effectiveness through endogenous molecules or exogenous molecular probes.
This review focuses on how to use novel imaging modalities to visualize the response to cancer-targeted therapies. Signaling pathway-based targeted therapies are concisely summarized. In addition, functional and molecular imaging modalities are discussed in detail about basic principles, imaging probes, and their application in targeted therapies against different molecular pathways. Lastly, future directions for molecular imaging in targeted therapies are prospectively reviewed.
Targeted therapies and anticancer drugs
Dysregulation of oncogenic signaling pathways plays a key role in the occurrence and progression of cancer. Substantial efforts have been made in treating cancer through “targeted” therapies that specifically disrupt pro-oncogenic signaling pathways. Specifically, there are two types of targeted therapy: small-molecule (enzyme)-based therapies,10 such as with tyrosine kinase inhibitors (TKIs) like sunitinib, and antibody-based targeted therapies,11 such as with vascular endothelial growth factor (VEGF)-targeted antibodies including bevacizumab. Cancer immunotherapies, such as immune checkpoint inhibitors (ICIs), targeting the interaction between cancer and immune cells, broaden the scope of targetable tumors.12 In this review, we introduce targeted therapies according to each signaling pathway.
VEGF/VEGFR signaling pathway
Angiogenesis is a crucial step in the successful growth, invasion, and metastasis of tumors, without which tumors could not grow beyond 1–2 mm in diameter.13 Multiple growth factors and their receptors are dysregulated in the complex process of tumor angiogenesis. The VEGF/VEGF receptor (VEGFR) signaling pathway is the pivotal inducer of angiogenesis, so antiangiogenic approaches have primarily focused on inhibiting this pathway.
A large number of drugs have been developed for targeting the VEGF/VEGFR signaling pathway14: (1) ligand binding agents that block the binding of VEGF ligands to receptors, such as bevacizumab,15 which binds to VEGF alone, and aflibercept,16 which binds to VEGF and placental growth factor (PlGF); (2) antibodies that block signaling through VEGFR, such as ramucirumab17 that targets VEGFR2; and (3) small‐molecule TKIs that block the kinase activity of VEGFR,18 such as lenvatinib, sorafenib, sunitinib, pazopanib, and regorafenib. TKIs can often inhibit the activity of other receptor tyrosine kinases, such as platelet-derived growth factor receptors (PDGFRs), fibroblast growth factor receptors (FGFRs), and epidermal growth factor receptors (EGFRs).
Besides, integrin αVβ3 is highly expressed in tumors and neovascular endothelial cells19 and is recognized as an ideal marker for distinguishing between cancerous and normal states. RGD (Arg-Gly-Asp) is a polypeptide including cyclic and linear categories screened by phage peptide display technology, which can specifically bind to the extracellular region of the α chain of αVβ3. Recently, many studies have reported that RGD peptides can carry drugs to tumor sites precisely and described that radionuclide-labeled RGD peptides have many uses for tumor imaging and therapy.20
EGFR signaling pathway
The epidermal growth factor receptor (EGFR) is a member of the ERBB receptor tyrosine kinase family consisting of EGFR/human epidermal growth factor receptor 1 (HER1), HER2, HER3, and HER4.21 EGF ligand binding to EGFR results in EGFR dimerization and activation of intracellular kinase activity. Autophosphorylation of tyrosine residues of EGFR activates two main downstream signaling pathways,22 PI3K/AKT and RAS/RAF/MEK/ERK, which regulates cell proliferation, differentiation, and survival.23
Inhibitors of the EGFR signaling pathway include small-molecule TKIs and anti-EGFR monoclonal antibodies (MoAbs). TKIs act on the ATP binding pocket of EGFR, inhibit EGFR autophosphorylation and antagonize tyrosine kinase signal transduction.24 First-generation (erlotinib, gefitinib, and icotinib) and second-generation (afatinib and dacomitinib) EGFR TKIs have been approved for the treatment of advanced NSCLC patients harboring EGFR-activating mutations.25 Unfortunately, resistance is inevitably acquired in most patients, at a median of 10–14 months after treatment.26 The most common reason for acquired resistance is the T790M mutation in exon 20 of EGFR. Third-generation mutation-selective EGFR TKIs,27 such as rociletinib,28 osimertinib,29 and almonertinib,30 have been developed to overcome this resistance mutation.
The related anti-EGFR antibodies target the EGFR extracellular domain and competitively bind to receptors, which impedes dimer formation, thereby inhibiting intracellular signal transduction.31 The antibodies against EGFR include nimotuzumab,32 panitumumab,33 matuzumab,34 and cetuximab.35 Antibodies are specific to EGFR, while TKIs can cross-link with other EGFR tyrosine kinases (HER2 and HER4).
HER2 signaling pathway
Unlike other members of the EGFR family, HER2 has no identified ligand. The HER2 signaling pathway is mediated by its heterodimeric form, created by HER2’s binding to other members of the EGFR family. This heterodimer can transactivate HER2 tyrosine kinase activity, further activating its downstream signaling pathways like PI3K/Akt/mTOR, MAPK, phospholipase C, and protein kinase C.36
Numerous HER2 inhibitors had been developed in the last few decades, primarily consisting of monoclonal antibodies (MoAbs), small-molecule TKIs, and antibody–drug conjugates (ADCs).37 Trastuzumab, pertuzumab, ertumaxomab, and margetuximab are common monoclonal antibodies targeting HER2. Various HER1/HER2 TKIs, pan-HER TKIs, and dual HER2/VEGF TKIs are in different stages of clinical trials or clinical practice.38 Currently, the most commonly used TKI drugs include lapatinib, neratinib, pyrotinib, and tucatinib. HER2 ADCs direct drug delivery to HER2-expressing cancer cells while limiting exposure to normal tissue.39 The currently available ADCs for HER2-positive cancer include trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd).
In recent years, several novel emerging groups of anti-HER2 agents, including antibody-based fragments (Fabs), diabodies, minibodies, nanobodies (Nbs), and affibodies, have been explored for HER2-positive breast cancer imaging and targeted radionuclide therapy.40
CDK4/6 signaling pathway
The binding of cyclin D1 to CDKs (CDK4 and CDK6) drives G1 to S phase transition and disease progression in tumors.41 CDK4/6 inhibitors prevent RB1 phosphorylation and E2F transcription and thereby induce G1 cell cycle arrest and block cancer progression. There are three CDK4/6 inhibitors, palbociclib, ribociclib, and abemaciclib, that are approved for treating advanced HR-positive (HR+), HER2-negative (HER2−) breast cancer, either combined with an aromatase inhibitor (AI) as a first-line treatment option or combined with fulvestrant as a second-line treatment option. Among these, abemaciclib is the first US Food and Drug Administration (FDA)-approved CDK4/6 inhibitor for adjuvant therapy in HR+ HER2− early-stage breast cancer.42
PI3K/Akt/mTOR pathway
The phosphatidylinositol-3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway is frequently activated in response to various extracellular stimuli, such as growth factors, hormones, and cytokines.43,44,45 PI3K is mainly triggered by the two largest groups of membrane receptors: receptor tyrosine kinases (RTKs) and G-protein-coupled receptors (GPCRs).46 Activated PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2), which is converted to phosphatidylinositol 3,4,5-triphosphate (PIP3). PIP3 binds to Akt and promotes Akt phosphorylation.47 Then, phosphorylated Akt triggers the downstream effector mTOR and results in gene transcription to facilitate cell growth and metabolism, motility, and angiogenesis and suppress apoptosis. Besides, activated mTORC2 (mTOR complex 2) can also promote the hyperactivation of Akt by phosphorylating Akt.48 Two tumor suppressors [phosphatase and tensin homolog (PTEN)49 and inositol polyphosphate 4-phosphatase type II (INPP4B)]50 prevent the activation of downstream of PI3K by dephosphorylating PIP3 and PIP2.51,52
The PI3K/AKT/mTOR pathway is often genetically altered in different human cancers.53,54 Although many developed small-molecule inhibitors target this pathway, only a few have been approved by the FDA for therapeutic use.
Some isoform-specific inhibitors of PI3K have been approved for treating lymphoma/leukemia and breast cancer,55 including a pan-PI3K inhibitor (copanlisib/BAY 80-6946/Aliqopa),56 a dual PI3Kγ/δ inhibitor (duvelisib/IPI-145/Copiktra), an α-selective PI3K inhibitor (alpelisib/NVP-BYL719/Piqray),57 and a δ-selective PI3K inhibitor (umbralisib/TGR-1202, idelalisib/CAL-101/GS-1101/Zydelig).58
As the critical effector of the PI3K/Akt/mTOR pathway, genetic alterations of Akt or its abnormal expression initiate tumor development and lead to resistance to chemotherapy and radiotherapy.59 Many small-molecule inhibitors of Akt have been evaluated in clinical trials,60 but none have been approved for clinical use as of July 2022.
As a downstream effector of PI3K/Akt, mTOR is usually hyperactive in various tumor types. mTOR-selective or dual mTOR/PI3K small-molecule inhibitors61 have been developed, and four anticancer mTOR-specific inhibitors have been approved by the FDA: (1) sirolimus (rapamycin) to treat lymphangioleiomyomatosis62; (2) everolimus to treat advanced renal cell carcinoma (RCC), renal angiomyolipoma (AML), postmenopausal advanced HR-positive, HER2-negative breast cancer, progressive neuroendocrine tumors of pancreatic origin, and subependymal giant cell astrocytoma (SEGA)63; (3) temsirolimus to treat advanced RCC64; and (4) Fyarro (sirolimus albumin-bound nanoparticles, nab-sirolimus, ABI-009), the latest mTOR inhibitor approved in November 2021, to treat patients with locally advanced unresectable or metastatic malignant perivascular epithelioid cell tumor.65 PI3K/mTOR dual inhibitors, combining multiple therapeutic effects in a single molecule, reduce the activity of PI3K and mTOR by competitive interaction with the ATP-binding cleft of these enzymes. Recently, an array of inhibitors,66,67,68,69 such as dactolisib (BEZ235), samotolisib (LY3023414), and bimiralisib (PQR309), have been studied in clinical trials, but none has yet been approved for use clinically.
Immunomodulatory signaling pathways
As newcomers to the human body, tumor cells generate and express specific antigens on their surface, which can be recognized and eliminated by immune cells such as cytotoxic T lymphocytes cells (CTLs). However, malignant tumor cells develop multiple escape mechanisms to evade immune recognition and surveillance. Reversing these immune evasion strategies is a promising approach for antitumor therapy. Immune checkpoint blockade (ICB) therapies targeting the programmed cell death protein 1 (PD1)/programmed cell death ligand 1 (PD-L1)70,71 pathway or cytotoxic T lymphocyte antigen 4 (CTLA4)72,73,74 have revolutionized the treatment landscape for multiple cancer types.
PD-1/PD-L1 signaling pathway
PD-1 is expressed on the surface of activated T cells and acts as an immunosuppressant, while its ligand PD-L1 is mainly overexpressed on tumor cells. The binding of PD-1 to PD-L1 suppresses T-cell-mediated immune responses by inhibiting T-cell proliferation, limiting cytokine production, and ultimately resulting in immune evasion of tumors.75,76,77
Blockade of the PD-1/PD-L1 interaction with specific antibodies results in the rescue of functionally exhausted T cells and the reactivation of immune responses. As of 2022, based on highly successful clinical trials, the FDA, European Medicines Agency (EMA), and National Medical Products Administration (NMPA) have approved 10 anti-PD-1 (nivolumab, pembrolizumab, cemiplimab, sintilimab, camrelizumab, toripalimab, tislelizumab, zimberelimab, prolgolimab, and dostarlimab) and three anti-PD-L1 antibodies (atezolizumab, durvalumab, and avelumab) for various hematological and solid malignancies.78
CTLA-4 signaling pathway
During the process of T-cell activation, membrane CTLA-4 and secreted soluble CTLA-4 are upregulated on CD8+ T cells and CD4+ T cells. Then, CD28 binds to the costimulatory molecules B7-1 (CD80) and B7-2 (CD86) as a secondary signal. CTLA-4 competitively binds to B7 to block the B7-CD28 signaling pathway.79 Moreover, the intracellular domain of CTLA-4 becomes phosphorylated, which generates a negative signal blocking the activation and function of T cells.80 In addition, CTLA-4 is abundantly found in forkhead box p3 (Foxp3)+ regulatory T cells (Tregs), which mainly works to suppress T-cell activity.81 Therefore, CTLA4 is considered a negative regulator of T-cell activation.
CTLA-4 inhibitors interfere with the interaction of CTLA-4 and B7 to erase the suppressive impact of CTLA-4 on T-cell activity and promote antitumor immune response, leading to tumor regression. Considerable evidence has also shown that CTLA-4 inhibitors function through antibody-dependent cell-mediated cytotoxicity (ADCC) via the Fc receptor (FcR) to remove Treg and downregulate the immunosuppressive effect effectively.82 Ipilimumab is a commonly used CTLA-4-blocking antibody approved by the FDA.83
The signaling pathways discussed above are deemed as potentials therapeutic targets. We summarized the main signal transduction pathways in Fig. 1 by illustrating key signal transduction processes. Besides, based on the defined signal pathways, these FDA-approved and commonly used targeted anti-cancer drugs were summarized in Table 1, as well as their related targets, indications, and categories (i.e., antibody or small-molecule inhibitors or ADCs).
Molecular imaging
Molecular imaging is a noninvasive medical imaging method that enables the visualization, characterization, and measurement of biological processes at the molecular and cellular levels in tumors.84,85 In contrast to conventional imaging modalities that primarily image differences in the structure of tissues or organs, molecular imaging reveals the physiological activities or expression status of specific molecules within a tissue or organ by employing medical imaging modalities with or without tracers.
From the phenomenon of magnetic resonance spectroscopy observed in 1966,86 to the first SPECT instrument developed in 1976,87 to the first whole-body MRI scanner in 1977,88 to luciferase (Luc) used as a reporter of gene expression in vivo in 1986,89 to near-infrared fluorescence (NIRF) imaging developed in 1994,90 to the first PET-CT completing the unity of both function and anatomical imaging in clinical practice in 1998,91 to the photoacoustic imaging first used in human in 2002,92 to the NIR-II imaging proposed in 2009.93 Some other historic steps promote the development of molecular imaging (Fig. 2). Given this excellent work, in September 1999, Weissleder and other imaging authorities held an international imaging conference in Jackson, the capital of Mississippi. The participating experts agreed that molecular imaging has emerged as a new field. Since then, molecular imaging has accelerated oncology detection, surgical guidance, targeted drug delivery, imaging-guided therapy, and efficacy evaluation.94 Given that several valuable reviews on molecular imaging have recently been published,95,96,97,98,99,100 here we only highlight the modalities most commonly used and their application for evaluating the efficacy of cancer-targeted therapy.
Nuclear imaging
PET imaging
PET is a molecular imaging technique that uses radiotracers to visualize and quantify the biological characteristics of tumors. PET is based on the principle that radionuclides emit positrons when decaying. The emitted positron is annihilated with an electron to create two 511 keV gamma rays at an angle of 180°.101 A ring of detectors is used to detect these emitted gamma rays. Radionuclides that are available for PET imaging in clinical and research applications include fluorine-18 [18F], carbon-11 [11C], zirconium-89 [89Zr], gallium-68 [68Ga], and copper-64 [64Cu]. Among these, 18F is most commonly used for clinical applications because of its beneficial half-life (T1/2 = 1.8 h), positron yield, and associated detection sensitivity.102
Owing to the development of nuclear medicine, there is now a wide variety of radiopharmaceuticals available in clinical practice to evaluate the biological features of tumors, such as 18F-FDG for tumor metabolism,101 18F-FMISO for hypoxia,103 18F-FLT for tumor cell proliferation,104 18F-labeled amino acids for protein synthesis,105 and 15O–water for blood flow.106 Furthermore, PET, in combination with tumor-specific monoclonal antibodies (immune-PET), has broadened the application of PET imaging. Various monoclonal antibodies and radionuclides have been explored to develop immune-PET tracers.107 In addition, peptides and other receptor-targeting compounds, such as nanobodies or bispecific antibodies, are being used to design novel immune-PET tracers.107,108 By dynamically monitoring tumor antigens’ expression, immune-PET imaging is a promising technique for evaluating the efficacy of cancer-targeted therapy.
Inherent advantages of PET imaging include its high sensitivity and quantifiable imaging parameters, such as standardized uptake value (SUV). PET-CT combining anatomical and functional imaging information becomes possible to assess the molecular features of tumors with highly accurate anatomical structure correction. Furthermore, PET–MRI combining PET with MRI extends the scope of multimodality imaging and reduces patients’ exposure to radiation.109
SPECT imaging
SPECT is another nuclear imaging modality that uses radionuclides that emit single photons, such as technetium-99m (99mTc), iodine-123 (123I), and indium-111 (111In). The emitted single photons are subsequently detected by a gamma camera to image the organs of interest or the whole body. SPECT has limited spatial resolution and lower sensitivity in tumor detection compared to PET. The quantification of SPECT is also more challenging. However, despite these limitations, it is more commonly used than PET in clinical practice. This is mainly due to its advantage of having a large number of radiopharmaceuticals that are readily available for clinical use. Specifically, ~85% of radiopharmaceuticals can be detected in clinical practice by SPECT imaging.110 Tumor-specific biological compounds, such as antibodies or peptides, can be used for radiolabeling to produce tumor-targeted SPECT radiopharmaceuticals, e.g., 123I-VEGF for VEGFR targeting, 111In-bevacizumab for VEGF targeting, 111In-trastuzumab for HER2 targeting, and 111In-EGF for EGFR targeting.111,112,113 These approaches have been explored for monitoring the responses to anticancer treatment in humans.110,111
Magnetic resonance imaging
MRI is a noninvasive imaging technique often classified as an anatomical imaging modality. When placed in a strong magnetic field, specific atomic nuclei can absorb radiofrequency energy and align like small magnets because of their spin. The absorption of energy by the nuclei causes a transition from a high- to a low-energy state. This further induces a voltage that can be detected, amplified, and displayed as “free-induction decay (FID),” which can be resolved by a mathematical process to generate high-resolution anatomical images.114 Moreover, using specific techniques, MRI demonstrates the ability to image molecular processes within a tumor. This provides functional information on tumor vascular permeability, perfusion,115 vascular volume and flow, tortuosity of extracellular space,115 and hypoxia.116 As such, these MRI techniques can be classified as forms of functional imaging.
Dynamic contrast-enhanced MRI
As suggested by its name, dynamic contrast-enhanced MRI (DCE-MRI) demonstrates the temporal enhancement pattern of a tissue following the injection of a paramagnetic contrast agent (CA), such as gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA). Factors influencing the tumor uptake of CA include blood perfusion, tissue vascularization, vessel permeability, cell density, extravascular extracellular volume fraction, and extracellular matrix density.117 A CA has two important physicochemical properties, namely, the relaxation effect and the susceptibility effect. MRI sequences studying the relaxation effect are termed DCE-MRI or T1-W DCE, while those assessing the susceptibility effect are termed dynamic susceptibility contrast (DSC)-MRI or T2*-W DCE.
T1-W DCE is sensitive to the presence of CA in the extravascular extracellular space. Tofts’ standard pharmacokinetic model is the mathematical model most commonly used in clinical studies for analyzing human DCE-MRI data.118 Tofts’ model introduces three key parameters, namely, Ktrans, Ve, and Vp.119,120 Ktrans is the bulk transfer coefficient, which reflects the leakage of contrast from the vascular to the extravascular compartment.119 Ve is the fractional volume of the extravascular extracellular space (EES),119 while Vp represents the concentration of CA in plasma space.120 The efflux rate constant from EES to plasma (Kep), which is the ratio of Ktrans to Ve, is also frequently described in DCE-MRI.120 DCE-MRI has been used to evaluate the treatment response and demonstrate prognostic value in patients receiving cancer-targeted therapy. This is elaborated on in the following section.
T2*-W DCE is sensitive to the vascular phase of CA and is used to evaluate tissue perfusion and blood volume. The following parameters are measured in T2*-W DCE: (1) regional blood volume (rBV), which is defined as the volume (ml) of blood perfusing vessels in a voxel divided by the tissue mass in that voxel (g); (2) mean transit time (MTT), which is the average transit time of a CA particle through the capillary bed; and (3) regional blood flow (rBF), which reflects the tissue perfusion and is measured in milliliters per minute.
Diffusion-weighted imaging
Diffusion-weighted imaging (DWI) is an MRI technique that generates signal contrast based on differences in the diffusion of water molecules. Within the body, water is distributed in the intracellular and extracellular compartments. The water molecules in the extracellular compartments diffuse relatively freely, while those within the intracellular compartments experience more restricted diffusion. The diffusion of water molecules can be quantitatively evaluated by the apparent diffusion coefficient (ADC). A lower ADC value reflects restricted diffusion. In tumor tissue, especially those with a high histological grade, the ADC value is lower than that in surrounding normal tissue. This is due to the high cellularity of tumors, as cellularity is positively correlated with the degree to which the diffusion of water is restricted.121,122,123,124 The use of DWI for evaluating the response to anticancer treatments has been explored. An increase in the ADC value can be observed at an earlier stage than a decrease in tumor size.125
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) uses the same principles of signal acquisition as other MRI techniques. MRS studies in a medical context usually involve the detection of radiofrequency electromagnetic signals that are produced by chemical compounds. As a result, this technique provides chemical information on tissue metabolites.126 Therefore, MRS can be used to monitor the metabolic variations caused by treatments and treatment efficacy.127
Chemical exchange saturation transfer
Chemical exchange saturation transfer (CEST) is an advanced MRI technique in which the exchangeable protons on target metabolites are selectively saturated and exchanged with water protons. The relative concentration of target metabolites can be measured with enhanced sensitivity by detecting the attenuation of the water proton signal indirectly.112 Amide proton transfer (APT) imaging is the CEST technique most commonly used in a clinical context to indirectly detect proteins and peptides in tissue. This technique provides important information for the diagnosis and monitoring of tumors.
Targeted magnetic resonance imaging
Like other tumor-targeted imaging modalities, targeted magnetic resonance imaging (TMRI) can visualize tumor-specific molecular markers with targeted magnetic nanoparticles. This provides the opportunity to classify patients, deliver individualized therapy directly to tumors, and monitor the treatment response through MRI for tumors expressing specific biomarkers.128,129
Optical imaging
Optical imaging is a noninvasive technique that uses light and optical properties of protons to image tissues, cells, and even molecules within the body.130 One of the major advantages of optical imaging is that it uses nonionizing radiation, making it much safer than techniques that use ionizing radiation such as X-rays. This makes optical imaging suitable for being repeatedly used to monitor gene expression, disease progression, or treatment response. Optical imaging includes multiple submodalities, such as bioluminescence imaging (BLI), chemiluminescence, Cherenkov imaging, and fluorescence imaging (FLI).131
Bioluminescence imaging makes use of the reaction between luciferases and their substrates to produce light.131 Luciferases, such as firefly luciferase, can be constitutively or inducibly expressed, and as such used for tracking the expression of targeted genes or monitoring tumor growth or regression to evaluate drug efficacy. These applications of BLI have been routinely used in preclinical studies.132,133,134,135 However, the drawbacks of BLI, such as the need for cell transfection and administration of a reactive substrate, prevent its clinical translation.
Fluorescence imaging provides images of tumors by detecting the emitted light that is generated from genetically encoded fluorescent proteins or fluorescent dyes after excitation by light of a different wavelength.131 The fluorescent dyes can be used to label tumor-specific antibodies, peptides, or nanobodies, enabling tumor-targeted molecular imaging. In comparison with BLI, a significant disadvantage of FLI is that its signal-to-background ratio is lower due to the auto-fluorescent noise coming from endogenous fluorophores within the tissue, which absorbs the excitation light. Other fundamental factors influencing the image quality of FLI are diffraction and diffusion.131 Diffraction reduces the spatial resolution of fluorescent images, while diffusion is caused by the tissue scattering of light, which limits the tissue penetration depth. Near-infrared (NIR) fluorescence imaging, including the first NIR (NIR-I) window (650–950 nm) and second NIR (NIR-II) window (1000–1700 nm), was emerged recently as an attractive imaging modality with high sensitivity, relative safety, and low cost. Compared with traditional NIR-I imaging, NIR-II imaging has less autofluorescence, absorption and scattering of light, higher penetration depths, and spatiotemporal resolution for biological tissues.136 FLI is primarily used in preclinical studies for tumor detection, fluorescence image-guided surgery, and monitoring of response to therapy.137,138,139,140 Along with the development of good manufacturing practice (GMP) tracers and clinical imaging systems, NIR FLI has been actively explored in early clinical trials for guiding cancer surgeries.141,142,143,144 However, the clinical application of FLI for evaluating the efficacy of targeted treatment has not been reported.
Photoacoustic imaging
Photoacoustic imaging (PAI) is a novel noninvasive molecular imaging modality, which generates an ultrasound signal based on the photoacoustic effect. When laser pulses are delivered to a material, some of the energy is absorbed and converted to heat, resulting in a thermoelastic expansion that generates an ultrasonic signal from which images can be produced.145 As a hybrid of optical imaging and ultrasound imaging, PAI combines the high contrast and sensitivity of the optical property and high ultrasonic spatial resolution in a single imaging modality. In addition, the unprecedented imaging depth (up to centimeters) makes this a promising technique for various clinical applications.146,147 PAI can be used to analyze various endogenous contrast agents such as oxygenated and deoxygenated hemoglobin, lipids, melanin, and water.147 By using multiwavelength measurement, PAI can simultaneously quantify the concentrations of these endogenous chromophores and further provide biological information on tissues that reflects their different physiological or pathophysiological status.148 Furthermore, by using tumor-specific exogenous contrast agents, PAI can identify tumor cells and monitor the expression of tumor-specific biomarkers.149,150,151,152,153 Examples of these agents include gold nanoparticle-conjugated peptides or antibodies for EGFR and HER2 molecular imaging.152,154 The ability to perform both functional (by endogenous contrast) and molecular (by exogenous contrast) imaging makes PAI an attractive technique for evaluating tumor-targeted therapy.
Ultrasound imaging
Ultrasound is a technique that uses high-frequency sound waves to produce anatomical images. It possesses several advantages, such as high availability, lack of radioactivity, and cost-effectiveness. These merits make it suitable to be repeatedly used in clinical practice. The Doppler technique allows ultrasound to be used to assess the blood flow in tumors. More importantly, with the use of contrast agents such as microbubbles, dynamic contrast-enhanced ultrasound (DCE-US) can measure longitudinal changes in hemodynamic parameters (e.g., perfusion, flow velocity) and morphological parameters (e.g., blood volume, vascular heterogeneity) of a given tumor relative to the findings in a pretreatment baseline assessment. The value of information on the changes in these parameters for monitoring the therapeutic response induced by anti-angiogenic therapies has been explored.155
Molecular imaging probes
Among the above-mentioned imaging modalities, some modalities, such as PET, SPECT, and optical imaging, require the injection of imaging probes into the studied subjects to acquire an imaging signal. On the other hand, other modalities, such as photoacoustic imaging and MRI, can monitor the biological change associated with diseases either through the injection of exogenous molecular probes or by using endogenous molecules.156 Tumor nonspecific imaging probes, such as 18F-FDG for PET imaging and 99mTc-sulfur colloid for SPECT imaging, have been widely used in clinical practice for detecting lesions or lymph nodes in cancer patients and for evaluating the efficacy of anticancer treatments.157,158,159 However, concerning tumor-specific imaging probes, many are still under development and are being tested in preclinical or early-stage clinical studies.160,161,162 A comprehensive review of all molecular imaging probes is beyond the scope of this paper. Therefore, here we only briefly describe the tumor-specific imaging probes that have been employed to evaluate the efficacy of cancer-targeted therapy. The mechanism of action of tumor-specific imaging probes is based on the concept that a carrier molecule that is labeled with a positron emitter for PET imaging, a single-photon emitter for SPECT imaging, or a fluorophore for fluorescent imaging specifically binds to a certain tumor target. Carrier molecules can be monoclonal antibodies, monoclonal antibody fragments, affibody molecules, small peptides, or small molecules that specifically target certain cell surface markers that are overexpressed in tumors.163,164,165 Moreover, carrier molecules can also be small molecules that detect the acidic microenvironment of tumors.142
Monoclonal antibody
Monoclonal antibody (mAb)-based probes have been most commonly studied in evaluations of cancer-targeted therapy using molecular imaging, mainly due to their high specificity and high binding affinity. In addition, the relative stability and tolerance of chemical modifications to mAbs make them desirable for creating moieties targeting radionuclides or fluorophores. For PET and SPECT imaging, owing to the prolonged circulation time of mAbs in the body, radionuclides with long physical half-lives, such as 124I (t½ = 100.3 h),166 89Zr (t½ = 78.4 h),166 and 111In (t½ = 2.8 days),167 should be chosen for radiolabeling. PET imaging of 89Zr-mAb has shown it to be a biomarker for predicting the efficacy of cancer-targeted treatments in xenograft models and early clinical trials.163,168 Nevertheless, the potential of mAb PET imaging or fluorescent imaging is limited by the slow clearance of intact antibodies from the blood, which causes undesirable high background signals and excessive nonspecific tissue accumulation such as in the liver.169
Monoclonal antibody fragments
To reduce the undesirable high background signal and nonspecific tissue accumulation of mAb probes, specifically in PET and SPECT imaging, there is a demand for the use of antibody fragments that are cleared more rapidly from circulation. Furthermore, the shorter circulation time of antibody fragments requires shorter-lasting radionuclides such as 68Ga (t½ = 68 min),170 99mTc (t½ = 6 h),167 and 64Cu (t½ = 12.7 h) for radiolabeling, which in turn decreases radiation exposure in patients. All of these advantages make antibody fragments an attractive alternative to employ for molecular imaging.
Various antibody-derived fragments with different sizes, serum half-lives, bio-distributions, and levels of tumor penetration have been developed.169 Full-length antibodies can be digested by enzymes to produce F(ab’)2 (110 kDa) and F(ab) (50 kDa) fragments, or genetically engineered to generate a variety of antibody derivatives such as minibodies (75 kDa), scFvs (26 kDa), diabodies (50 kDa), and nanobodies (12–15 kDa).167,169 These antibody fragments retain the specificity of binding to the molecular targets of their parental IgG. However, much of the data on their application in molecular imaging has been obtained from preclinical studies.167 Clinical translational studies on them have been performed in only limited numbers of cancer types and patients.167 Examples include the use of a 68Ga-labeled anti-HER2 VHH for detecting lesions in patients with breast cancer.171
Affibody molecules
Affibody molecules are engineered scaffold proteins consisting of 58 amino acids with a molecular weight of 6–7 kDa, which meet the requirements for an optimal balance between clearance from circulation and extravasation.172 This ensures the high absolute tumor uptake of affibody molecules and further increases the signal-to-background ratio. Several affibody molecules with high affinity to VEGFR2, EGFR, HER2, HER3, and IGF-1R have been demonstrated as probes for radionuclide or fluorescent molecular imaging in preclinical settings.167,173 Clinical evaluation of radiolabeled affibody molecules has been explored for HER2 imaging174,175,176,177 and fluorescent dye-labeled affibody molecules (ABY-029) for EGFR imaging have been tested in patients with recurrent glioma.178
Peptides
According to the definition by the United States Food and Drug Administration (FDA), peptides are proteins consisting of fewer than 40 amino acids. Although their binding affinity is lower than that of antibodies, they are small, easy to synthesize, and capable of flexible chemical modification.179 With these distinctive advantages over antibodies, peptides have been increasingly used as probes for tumor receptor imaging, such as peptides for PD-L1 imaging,180,181,182,183 integrin αvβ3 imaging,184,185,186 and somatostatin receptor imaging.187 To date, many peptide-based probes have been developed and clinically used for cancer diagnosis and treatment.187,188,189
Application of molecular imaging in targeted therapy
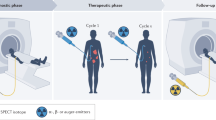
As discussed above, both conventional imaging modalities and standard-of-care assessment of tumor responses to treatment are based on morphological indicators such as tumor size. Molecular imaging is more sensitive and may detect changes earlier than morphological changes in tumors because it can visualize the expression of a specific molecule when used to monitor therapeutic efficacy.190 Figure 3 shows the representative diagram of how molecular imaging probes are designed and how responses to targeted therapy are evaluated.
The diagram of molecular imaging and functional imaging in cancer-targeted therapy. The probes are constructed by imageable agent and their targets, respectively. On the cellular level, probes can bind to cell surface receptors or targets in the cytoplasm or nucleus to visualize and measure the target. After the probes are injected into animals or the human body, quantitative measures of probe uptake are used as predictive or evaluative assays for response to targeted therapy by different molecular imaging technologies
Nuclear imaging
Because of temporal and spatial heterogeneity and discordance in gene expression status between primary and distant metastatic lesions,191 the reproducible, noninvasive, whole-body evaluation of the efficacy of targeted therapy is critical for determining optimal treatment options. Among various types of molecular imaging, radionuclide imaging is advantageous for clinical usage because of the excellent sensitivity and tissue penetration of radionuclides. This approach would facilitate treatment optimization, prevent useless prescriptions, avoid unnecessary side effects, and, more importantly, prevent treatment failure in nonresponding patients.
Although nuclear imaging has been mainly utilized for monitoring the pharmacokinetics and pharmacodynamics of targeted drugs noninvasively,192 a discussion of this is outside the scope of this review. We instead discuss the application of nuclear imaging, including PET/CT and SPECT, in evaluating the response and predicting the prognosis after targeted therapy, among which the clinical application is summarized in Table 2.
Evaluation of therapeutic responses
In clinical practice, 18F-labeled fluorodeoxyglucose (18F-FDG) is the most commonly used radiotracer for PET-based imaging. PET/CT with 18F-FDG has been a proven staging modality for various neoplasms for many years. Besides staging, it is used increasingly frequently to categorize the metabolic response to antineoplastic therapy, called PET Response Criteria in Solid Tumors (PERCIST).193 Several studies have confirmed that 18F-FDG PET or PET/CT can predict the response to EGFR-TKI treatment in 1–2 weeks, while conventional CT requires 2–3 months in patients with lung cancer.194,195,196,197,198 Similar results were also seen upon the use of HER2-targeted therapy,199 antiangiogenic agents,200,201,202 CDK4/6 kinase inhibitor,203 and mTOR inhibitor.204 Therefore, by using 18F-FDG, it is possible to evaluate the efficacy of targeted therapy at an early stage without waiting until the middle or end of treatment. For example, in patients with metastatic renal cell cancer treated with an mTOR inhibitor everolimus, the 2-week relative changes of 18F-FDG uptake (SUVmax) from baseline were predictive of the 8-week change in tumor size as evaluated by conventional computed tomography.204
18F-FLT is another PET tracer for assessing cell proliferation in vivo. It is also a potential candidate for evaluating response to targeted therapy, such as VEGFR TKI sunitinib,205 EGFR inhibitors,206 HER2 inhibitor trastuzumab,207 PI3K inhibitor GDC-0941, and the MEK inhibitor PD 0325901,208 mTOR inhibition everolimus,209 but most of the studies were in preclinical models.
Despite the optimal performance of 18F-FDG or 18F-FLT in a clinical context, they suffer from certain limitations in evaluating therapeutic response as it only detects the glucose metabolism of tumors and cannot reflect all biometric features of tumors. Against this background, many studies are underway to explore and develop specific radiotracers capable of binding to particular targets and to identify efficacy as accurately as possible.
In addition to 18F, some other PET radioisotopes are commonly used, including 124I, 89Zr, 68Ga, and 64Cu.210 Among these, 124I and 89Zr with long half-lives have been used for radiolabeling intact antibodies. In contrast, short half-life nuclides have been used for antibody fragments, nanobodies, peptides, affibodies, and small molecules.211
For antiangiogenic therapy, iodinated VEGF is the most studied radiolabeled VEGF tracer.212,213,214,215 VEGF, VEGFR, and related integrins can also be labeled with 18F,216,217 99mTc,218,219,220 111In,220,221 64Cu,218,222,223 and 89Zr,224 but most of these are simply for imaging of the tumor vasculature and can help to visualize tumors and metastatic lesions usually overexpressing VEGFR or VEGF. Recently, reports have been published on several clinical trials on the efficacy of 89Zr-labeled bevacizumab imaging for monitoring various cancer-targeted therapy such as the mTOR inhibitor everolimus,225 bevacizumab/interferon-α,226 multi-targeted tyrosine kinase inhibitors like sunitinib,226 and a similar result was found in 111In-bevacizumab imaging for evaluation sorafenib.111 In 70 evaluable lesions of 10 patients with metastatic renal cell carcinoma, the uptake of 89Zr-bevacizumab (SUVmax) decreased by a mean of 9.1% (P < 0.0001) at 2 weeks and 23.4% (P < 0.0001) at 6 weeks after everolimus treatment. All 10 patients continued the treatment and had stable disease at 3 months.225 Similar data were found in patients with metastatic renal cell carcinomas treated with bevacizumab/interferon-α.226 In patients who received sunitinib from the same study, the uptake of 89Zr-bevacizumab (SUVmax) decreased by 14.3% (P = 0.006) at 2 weeks but increased by 72.6% (P < 0.0001) at 6 weeks after treatment compared with that at baseline.226 These data indicate the role of 89Zr-bevacizumab imaging in reflecting the biological effects of cancer-targeted therapy.
Evaluation of the success of HER2-targeted therapy depends on the precise determination of HER2 expression. Full-length monoclonal antibodies are usually labeled with long-half-life radionuclides, such as 64Cu,227,228,229,230,231 89Zr,232,233,234 111In,235,236 and 124I.237 Besides, imaging tracers targeting HER2 by shorter fragments (antibody fragments [Fab or F(ab)2], nanobodies, or affibodies) have been introduced into clinical trials, such as 68Ga-DOTA-F(ab0)2-trastuzumab,238 68Ga-NOTA-2Rs15d,171 ABY-002 labeled with 111In and 68Ga,239 111In-ABY-025,174 68Ga-ABY-025,175 99mTc-ADAPT6,240 and 99mTc-(HE)3-G3,241 to assess HER2 expression in breast carcinoma.
Most anti-HER2 probes exhibit the potential advantages of enabling the noninvasive and specific identification of HER2-positive tumors. In a study by Guo et al. 242, PET imaging of 124I-trastuzumab showed a difference in SUVmax (7.83 ± 0.55 vs. 1.75 ± 0.29, P < 0.0001) between HER2-positive and -negative lesions and recognized 18 out of 18 HER2-positive lesions in both primary and metastatic gastric cancer patients. In addition, Ulaner and colleagues published a series of articles about 89Zr-trastuzumab243,244 and 89Zr-pertuzumab245,246 to identify HER2-positive metastases in patients with HER2-negative primary breast cancer. Similar results were confirmed for 64Cu-DOTA-trastuzumab228,247,248 in breast cancer. Besides screening HER2-positive lesions, some novel molecular imaging probes were designed to monitor the response to anti-HER2 treatment directly.249,250,251 In a preclinical study, 89Zr-pertuzumab accurately detected changes in tumor volume from 243.80 ± 40.91 mm3 before T-DM1 therapy to 78.4 ± 40.43 mm3 after this therapy in mice bearing BT-474 tumors. In contrast to the findings with 89Zr-pertuzumab, no apparent changes were observed in 18F-FDG.249 Similar preclinical results were obtained with 99mTc-HYNIC-H10F, which can assess trastuzumab response at the earlier stage of day 4 post-treatment.250 In addition, in 2021, a study reported that 64Cu-DOTA-trastuzumab could predict the response of metastatic breast cancer patients receiving T-DM1.252 When compared with those in nonresponding patients, higher minimum SUVmax (5.6 vs. 2.8, P < 0.02) at day 1, higher average SUVmax (8.5 vs. 5.4, P < 0.05), and higher minimum SUVmax (8.1 vs. 3.2, P < 0.01) at day 2 were found in responding patients.
For trastuzumab-related cardiotoxicity, Perik et al. found that myocardial 111In-DTPA-trastuzumab uptake was not associated with cardiotoxicity in all 15 evaluable HER2-positive metastatic breast cancer patients.253 Next, they discovered that myocardial 111In-DTPA-trastuzumab uptake was observed in 50% of anthracycline-treated patients without symptomatic cardiac dysfunction, while none was found in non-anthracycline-related heart failure patients. They thus considered that 111In-DTPA-trastuzumab potentially recognized patients susceptible to trastuzumab-related cardiotoxicity.254
For EGFR-targeted therapy, cetuximab, panitumumab, and their analogs were labeled with different radionuclides. Most of the tracers, such as 18F-FBEM-cEGF (ligand),255 64Cu-panitumumab,256 111In-cetuximab,257,258 64Cu-cetuximab,258 89Zr-cetuximab,259 111In-cetuximab-F(ab’)2 (antibody fragment),260 64Cu-cetuximab-F(ab’)2 (antibody fragment),261 and 89Zr-DFO-ZEGFR:2377 (affibody),262 were designed to image the expression of EGFR in vivo. In 10 advanced colorectal cancer (mCRC) patients without K-RAS mutation, 89Zr-cetuximab uptake was found in 6 patients, 4 of whom benefited from cetuximab treatment. Disease progression was detected in 3 of the remaining 4 patients without uptake of 89Zr-cetuximab. The results suggested that 89Zr-cetuximab uptake is correlated with response, but this warrants further clinical validation.263 Besides, N-(3-chloro-4-fluorophenyl)-7-(2-(2-(2-(2-18F-fluoroethoxy) ethoxy) ethoxy) ethoxy)-6-methoxyquinazolin-4-amine (18F-MPG) precisely quantified EGFR-activating mutation status, meanwhile monitored the response to EGFR-TKIs in NSCLC patients.264
To predict the treatment response of CDK4/6 inhibitors combined with endocrine therapy in ER+/HER2-metastatic breast cancer (MBC) patients, 18F-FES-PET/CT was undergone. 90% 18F-FES-negative patients developed progressive disease (PD), while only 8.7% 18F-FES-positive patients had PD. 18F-FES-positive patients had longer PFS than 18F-FES-negative patients (23.6 months vs. 2.4 months, P < 0.001).265
Immunotherapy is associated with pseudo-progression, which limits the application of conventional anatomically based imaging modalities for treatment evaluation. Recently, some studies have demonstrated the use of radiolabeled PD-L1 antibodies (68Ga-NOTA-Nb10989, 89Zr-atezolizumab, 89Zr-durvalumab)168,266,267 and PD-1 antibodies (89Zr-pembrolizumab, 89Zr-nivolumab)268,269,270 to assess the efficacy of PD-1/PD-L1 blockade in cancer patients. The first-in-human whole-body PD-L1 imaging study by Niemeijer et al. used 18F-BMS-986192 and 89Zr-nivolumab in 13 NSCLC patients with nivolumab treatment269(see Fig. 4). They showed that median SUVpeak values of 18F-BMS-986192 (6.5 vs. 3.2, P = 0.03) and 89Zr-nivolumab (6.4 vs. 3.9, P = 0.019) were higher in responding lesions than in nonresponding ones. Similar results were obtained for using 89Zr-atezolizumab to assess atezolizumab response in 22 patients, including 9 bladder cancer patients, 9 NSCLC patients, and 4 triple-negative breast cancer patients.168
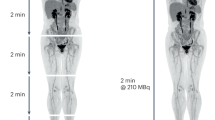
Tracer uptake and correlation with PD1/PDL1 treatment response. a Study design. b Immunohistochemical staining of PD-L1 in patient 2. Biopsy of the tumor in the left lower lobe. PD-L1 expression is expressed in 95% of the tumor cells. Scale bar, 100 µm. c Immunohistochemical staining of PD-1 in patient 2. PD-1 expression in aggregates was scored as IC1. Scale bar, 100 µm. d 18F-FDG PET (225 MBq) (18F-FDG PET scan images of both patients were used from archival PET scans) demonstrates high glucose metabolism of tumors in both lungs and mediastinal lymph nodes. 18F-BMS-986192 PET (145.7 MBq, imaging time point 1 h post-injection (p.i.)) and 89Zr-labeled Nivolumab PET (37.09 MBq, 162 h p.i.) demonstrate heterogeneous tracer uptake within and between tumors. e Patient 3 with tumor PD-L1 expression < 1%: 18F-FDG PET (268 MBq) (18F-FDG PET scan images of both patients were used from archival PET scans) demonstrates high glucose metabolism in the left-sided tumor. 18F -BMS-986192 PET (214.62 MBq, 1 h p.i.) demonstrates low tumor tracer uptake. 89Zr-labeled Nivolumab PET (37.27 MBq, 160 h p.i.) demonstrates heterogeneous tracer uptake in the tumor. f SUVpeak of the 18F-BMS-986192 tracer is higher in responding lesions than non-responding lesions (comparison of lesions with a diameter of 20 mm or more). The p-value is 0.02, as determined by the Mann–Whitney U-test. SUVpeak of the 89Zr-nivolumab tracer is numerically higher in responding lesions (comparison of lesions with a diameter of 20 mm or more). The p-value is 0.019, as determined by the Mann–Whitney U-test. For all the boxplots, the lower edge of the box represents the first quartile, and the upper edge represents the third quartile. The horizontal line inside the box indicates the median. Whiskers identify the minimum and the maximum value. (Reproduced from Niemeijer, A. N. et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 9, 4664 (2018))
Therefore, 18F-FDG PET is potentially useful for the early evaluation of therapeutic response. Tumor-specific nuclear imaging holds promise to assess the target expression and to predict the targeted treatment response early.
Prognosis prediction
Radionuclide imaging can also predict the prognosis of patients prescribed targeted therapy. As the most widely used tracer in oncology, 18F-FDG has been investigated in the early prediction of outcomes after targeted treatment, including that with anti-EGFR agents such as the monoclonal antibody cetuximab.271 It was reported that an increase in peak tumor metabolism at the end of the first week of therapy implied poor PFS (P = 0.001) and OS (P < 0.001) at the end of the first week of third-line cetuximab-based therapy in metastatic colorectal cancer patients. Similar results were also found for the EGFR-TKIs erlotinib and gefitinib,272,273,274,275 the antiangiogenic agent bevacizumab,202,276,277,278 HER2-targeted therapy with trastuzumab,279 multiple TKIs such as regorafenib,280,281 the CDK4/6 kinase inhibitors ribociclib, palbociclib, and abemcaciclib,203,282,283 and the mTOR inhibitor everolimus,204,284 as well as the ICIs nivolumab and pembrolizumab.285,286 In a meta-analysis,287 865 participants from 26 studies receiving inspection of FDG-PET or FLT-PET were included. In comparison with the PET nonresponsive group, the PET responsive group showed a decline in SUVmax, which was related to prolonged PFS (HR = 0.41, P < 0.00001), OS (HR = 0.52, P < 0.00001), and time to progression (TTP) (HR = 0.30, P = 0.003). However, some other clinical trials obtained inconsistent results on this issue. Rinzivillo et al. found no significant difference in clinical outcomes between 18F-FDG-PET-positive and -negative groups in patients with advanced neuroendocrine neoplasia receiving everolimus therapy (median PFS of 24 and 18 months, respectively; P = 0.337).284 In general, the prognostic value of 18F-FDG-PET in patients who received targeted therapy is inconclusive. The prediction based on 18F-FDG-PET imaging may be cancer-specific or treatment-specific.
To clarify the accuracy of PET or SPECT for predicting prognosis, especially in patients who have simultaneously received two or more combined targeted therapies or ICIs, a few specific radiotracers are under pre-clinical and clinical investigations. Rainer et al. investigated the predictive value of 123I-VEGF165 scintigraphy in patients with glioma.213 Their results showed that 123I-VEGF165 may provide relevant prognostic information in glioma, as patients with a tumor-to-normal brain uptake ratio (T/N ratio) of <1.32 showed significantly longer survival (2680 days vs. 295 days; P < 0.05). Similar results were seen in a grade IV glioma subgroup, as patients with a T/N ratio < 1.75 had longer OS (720 vs. 183 days; P < 0.05).288 89Zr-bevacizumab226 was used to predict differential effects of antiangiogenic treatment (bevacizumab with interferon-α or sunitinib) in metastatic RCC (mRCC). High baseline tumor SUVmax before antiangiogenic therapy in the three most intense lesions was correlated with longer time to disease progression (89.7 vs. 23.0 weeks; HR = 0.22; P = 0.050).
For HER2-targeted therapy, in 2016, Gebhart et al. 251 conducted a prospective clinical trial (ZEPHIR study) using 89Zr-trastuzumab PET/CT and FDG PET/CT to predict the efficacy of T-DMl therapy in patients with HER2-positive metastatic breast cancer. The results showed that, among the 55 evaluable patients, the negative predictive value was 100% when combining 89Zr-trastuzumab-PET with early FDG-PET response after one cycle of T-DM1, which can predict response to T-DM1 and differentiate patients with a median time to treatment failure (TTF) of 2.8 months from those with a TTF of 15 months.
For EGFR-targeted therapy, even though FDG-PET/CT is better than CT at assessing the benefit of cetuximab in incurable squamous cell carcinoma of the head and neck,289 no relationship was identified between uptake on 89Zr-cetuximab PET/CT and PFS (3.6 vs. 5.7 months, P = 0.15) or OS (7.1 vs. 9.4 months, P = 0.29) in patients with RAS wild-type advanced colorectal cancer receiving cetuximab monotherapy.
For immunotherapy, it was reported that high tumor uptake of 89Zr-atezolizumab was correlated with a better response to atezolizumab treatment. Those with low uptake were more likely to progress or die, whereas PD-L1 IHC failed to predict the treatment outcome.168 Similar results were seen for 89Zr-pembrolizumab.268
Taking together, immuno-PET imaging, as a non-invasive method for the early detection of tumor receptor blocking by anti-cancer targeted drugs, may serve as an effective technique in predicting patient prognosis.
MRI imaging
Regarding the clinical application of MRI in cancer-targeted therapy, the focus has been particularly placed on three aspects: (1) predicting the response to targeted therapy by using information obtained by MRI performed pretreatment; (2) evaluating the treatment response to targeted therapy by analyzing the changes in MRI parameters; and (3) predicting patient outcome in those receiving targeted therapy. Data on the clinical application of MRI in cancer-targeted therapy are summarized in Table 3.
DCE-MRI and DSC-MRI
Among the different MRI techniques, DCE-MRI is the approach most commonly studied in evaluating treatment response to targeted therapies. In an early study of 21 patients with inflammatory and locally advanced breast cancer treated with bevacizumab, the DCE-MRI parameters Ktrans, Ve, and Kep significantly decreased compared with those at baseline after one cycle of bevacizumab.290 This was accompanied by a decrease in the tumor expression of p-VEGFR2 and an increase in tumor apoptosis, as evaluated by the TUNEL assay.290 Similar reductions in Ktrans, Ve, and Kep from baseline were observed in 70 patients with early breast cancer who received one cycle of bevacizumab as neoadjuvant therapy.291 Besides, the changes in DCE-MRI parameters were significantly correlated to the changes in the SUV of FLT-PET imaging.291 In patients with NSCLC under gefitinib or erlotinib therapy, Ktrans, Ve, and Vp decreased significantly at day 7 post-treatment.292 These results indicate that the changes in DCE-MRI parameters reflect the biological effects of bevacizumab or EGFR tyrosine kinase inhibitors on tumor cells and may serve as early noninvasive imaging biomarkers for evaluating the response to VEGFR-targeted agents.
Regarding anti-HER2 therapy, in a preclinical study, Syed and coworkers found that, in a mouse model with a HER2-positive tumor, both vascular heterogeneity and cellularity heterogeneity increased after trastuzumab treatment, as indicated by increases in the mean K–S distance for the Ktrans distribution and Ve distribution, respectively.293 An increase in cellularity heterogeneity in trastuzumab-treated tumors is expected with increased tumor cell death. Therefore, the changes in these MRI parameters could potentially be used for evaluating the treatment efficacy of trastuzumab. In a study with 51 HER2-positive breast cancer patients who received neoadjuvant HER2-targeted therapy, the pretreatment initial enhancement ratio, defined as the percentage signal increase relative to the baseline at the first postcontrast acquisition, was not associated with tumor pathological complete response after treatment.294 Therefore, the changes in the DCE-MRI parameters of tumors after treatment may better reflect the impact of trastuzumab on tumors and serve as a more helpful tool for evaluating treatment efficacy.
The data from DCE-MRI imaging have also been shown to correlate with patient prognosis. For example, in a study of 22 patients with breast cancer that had metastasized to the brain, small reductions in Ktrans and Peak in DCE-MRI scanning 3 weeks after bevacizumab therapy were independently correlated with shorter central nervous system-specific progression-free survival (PFS) and shorter overall survival (OS), respectively.295 Similar results were observed in patients with advanced hepatocellular carcinoma. Significant early reductions in Ktrans, Peak, and AUC in the tumor were associated with longer PFS and OS than in those with smaller reductions.296 In addition, in patients with metastatic colorectal cancer, a drop in KEF, derived from Ktrans and enhancing fraction (EF) (Ktrans × EF), 15 days after regorafenib treatment was correlated with reduced CD31 expression (a marker of vascular density) in the tumor tissue, indicating the biological effect of the treatment.297 Moreover, patients with a >70% reduction in KEF had a higher disease control rate and longer PFS and OS than the remaining patients.297 The post-treatment contrast-enhancing volumetric change has been demonstrated to be prognostic. In patients with advanced-stage hepatocellular carcinoma treated with sorafenib, the difference in the enhancing lesion volume after treatment could discriminate patients with tumor progression from those with tumor control. Furthermore, this patient classification was proven to predict the prognosis as defined by OS independently.298 The similar predictive value of post-treatment enhancing volumetric change was confirmed in patients with recurrent glioblastoma receiving cabozantinib.299 The pretreatment contrast-enhancing volume could also be predictive. In post hoc analysis of the randomized ARTE trial, in which patients newly diagnosed with glioblastoma were treated with radiotherapy with or without bevacizumab, larger pretreatment contrast-enhancing volume was associated with inferior OS in both treatment arms.300 This result was supported by data from the ACRIN 6677/RTOG 0625 trial and the EORTC 26101 trial, which included patients with recurrent glioblastoma treated with bevacizumab and chemotherapy.301,302 In addition, in both of these trials, early decreases in the relative cerebral blood volume (rCBV), derived from DSC-MRI, after treatment was associated with improved OS.302,303 Moreover, in pediatric patients with supratentorial high-grade glioma treated with radiotherapy plus erlotinib, the CBV ratio of tumor to normal brain tissue remained relatively constant after treatment.304 Nevertheless, patients with a CBV ratio above 1.15 at 8 weeks after treatment had a shorter time to death than the other patients.304 Another study evaluated the predictive value of baseline rCBV before bevacizumab treatment in the ACRIN 6686 trial. Patients with newly diagnosed glioblastoma who had high pretreatment rCBV demonstrated improved OS in the bevacizumab-treated group compared with that in the placebo group.305 These findings indicate that monitoring the change in the blood volume of tumors may be more critical than merely learning about their absolute value in evaluating their prognostic significance. Contradictory data regarding the prognostic value of Ktrans and rCBV have been reported in the literature, with the changes in these variables being reported not to be predictive for OS in patients newly diagnosed with glioblastoma.305 Moreover, in patients with NSCLC who were treated with bevacizumab and erlotinib, Ktrans was not associated with PFS. However, an increase in Ktrans heterogeneity 3 weeks after treatment was found to be associated with worse PFS.200 This discrepancy may have been due to the small number of patients in most of the studies and the different approaches used for defining the changes in Ktrans among the studies.295,305
Diffusion-weighted imaging (DWI)
DWI is another commonly used MRI technique for evaluating the response to anticancer treatments. Tang and colleagues demonstrated that in patients with gastrointestinal stromal tumor (GIST) treated by neoadjuvant imatinib or sunitinib, the percentage change in ADC 2 weeks after therapy differed significantly between responders and nonresponders (increase by 30% in responders vs. an increase by 1% in nonresponders, P < 0.001).306 Additionally, the predictive value of pretreatment ADC was confirmed in patients with unresectable intrahepatic cholangiocarcinoma (ICC) who received first-line systemic therapy with lenvatinib plus PD1 antibody.307 Another DWI-related parameter, the pretreatment fractional order parameter β (which correlates with intravoxel tissue heterogeneity), was proven to be predictive of treatment response in another study of GIST patients treated with second-line sunitinib.308
The prognostic value of DWI-related parameters has been explored in patients with multiple cancer types who received targeted therapies, such as those with unresectable ICC receiving lenvatinib plus PD1 antibody,307 recurrent glioblastomas receiving anti-VEGF monotherapy,309 colorectal liver metastases receiving bevacizumab,310 and NSCLC brain metastases treated with whole-brain radiotherapy and gefitinib.311 In general, a higher baseline or post-treatment percentage change of ADC was associated with improved patient outcomes.300,307,309,310,311,312,313,314,315 These findings indicate that the pretreatment ADC value or post-treatment percentage change of ADC could accurately reflect the therapeutic efficacy of tumor-targeted therapies and predict patient survival. Therefore, the monitoring of ADC could potentially support the optimization of strategies in anticancer treatment.313
Magnetic resonance spectroscopy
The most common metabolites detected by MRS are choline (Cho), lipids, N-acetyl aspartate (NAA), creatine/phosphocreatine (Cr), lactate, and glutamine.316 In cancer tissues, the relative concentrations of these compounds are abnormal; for example, in brain tumors, Cho is generally increased, and NAA is decreased compared with the levels in normal brain tissue.94,317 Thus, MRS has been commonly used for diagnosing brain tumors and evaluating the therapeutic response. The RTOG 0625/ACRIN 6677 trial, which included patients with recurrent glioblastoma treated with bevacizumab plus chemotherapy, demonstrated that the NAA/Cho level increased and the Cho/Cr level decreased within the enhancing tumor at 2 weeks after treatment compared with the pretreatment levels, indicating potential treatment efficacy.317 Similarly, these metabolites demonstrated predictive value in patients with recurrent malignant glioma treated with tamoxifen.318,319 In a critical preclinical study, Ros et al. showed that MRS could rapidly assess treatment response to PI3K inhibition in ER+ breast cancer mouse models by detecting the changes in lactate and pyruvate levels.320 Therefore, this imaging method could help to identify patients who would benefit from current treatments and design new drug combination strategies to counteract treatment resistance.
Chemical exchange saturation transfer
The application of CEST in evaluating treatment response is still in its infancy. In a preclinical study of a human mantle cell lymphoma xenograft model, the acidoCEST technique was used to monitor changes in the tumor extracellular pH (pHe) in response to an mTOR inhibitor, everolimus.321 This study showed a significant increase in tumor pHe within 1 day of initiating treatment. Subsequently, acidoCEST MRI identified a decrease in tumor pHe 7 days after initiating treatment.321 This contrasts with the findings of an untreated control group from another study performed by the same research group, which showed a consistent decrease in tumor pHe in the same tumor xenograft model.321 This reflects the reduction in lactate production due to the inhibition of cellular metabolism by everolimus, as demonstrated in in vitro experiments.321 The clinical application of CEST has been explored in small studies of patients with recurrent glioblastoma treated with bevacizumab.322,323 Both pH-weighted amine CEST echoplanar imaging322 and APT imaging323 were potentially useful for predicting the treatment response to bevacizumab and the PFS of patients. Moreover, residual or emerging regions of acidity, as assessed by pH-weighted amine CEST echoplanar imaging, may colocalize to the site of tumor recurrence, which may provide important information for site-specific treatment (see Fig. 5).322
Three patient examples (a: patient #4, b: patient #8, c: patient #11) are demonstrated, with anatomic images (FLAIR and post-contrast T1-weighted images, T1 + C) and pH-weighted CEST-EPI images of MTRasym at 3.0 ppm at three-time points: pre-bevacizumab (baseline), post-bevacizumab (follow-up), and the time of tumor recurrence. Baseline T2 lesion ROIs used for volume and median MTRasym calculation are outlined in red (on FLAIR and post-contrast T1-weighted images) and black (on MTRasym images). The red arrows demonstrate co-localization of residual or newly emerging areas of high acidity (MTRasym at 3 ppm) at the post-treatment time point and the corresponding location of tumor recurrence ~2 months later. CEST-EPI: chemical exchange saturation transfer echoplanar imaging. ROIs: region of interest. FLAIR: fluid-attenuated inversion recovery. (Reproduced from Yao, J. et al. pH-weighted amine chemical exchange saturation transfer echoplanar imaging (CEST-EPI) as a potential early biomarker for bevacizumab failure in recurrent glioblastoma. J. Neurooncol. 142, 587–595 (2019).)
Other techniques
Several other novel techniques have been explored for evaluating the efficacy of treatment in cancer-targeted therapies, such as intravoxel incoherent motion (IVIM) MRI,324,325 vascular architecture mapping (VAM) MRI,326 arterial spin-labeled (ASL) MRI,327 and magnetic resonance elastography (MRE).328 In general, these techniques demonstrated the potential to predict the treatment response or long-term outcome of patients. Additionally, MRI biomarkers of the tumor microenvironment, for example, necrosis, hypoxia with/without neovascularization, oxidative phosphorylation, and aerobic glycolysis, are predictive of the treatment response to bevacizumab in patients with glioblastoma.329 Gadolinium ethoxybenzyl diethylenetriamine (Gd-EOB-DTPA; a liver-specific contrast agent)-enhanced MRI is an imaging biomarker of OATP1B3, a transporter of Gd-EOB-DTPA.330 The expression of OATP1B3 is induced by Wnt/β-catenin mutation. Therefore, EOB-MRI is also considered an imaging biomarker of Wnt/b-catenin mutation/activation, which has been demonstrated to be an important mechanism of resistance to ICIs.330 In patients with hepatocellular carcinoma treated with ICIs, those with tumors with hyperintensity in EOB-MRI had significantly shorter PFS than patients with hypointense tumors.331,332 Since these studies involved early trials with a small number of patients, the definitive predictive and prognostic value of these new techniques needs to be validated in future studies.
Thus, the MRI parameters, especially their change after treatment, are sensitive to the biological changes induced by cancer-targeted therapies. Together with their radiation-free nature, these MRI techniques are attractive tools in clinical practice for early treatment response assessment and patient prognosis prediction. Their clinical significance in improving patients’ outcomes is worthy of further evaluation in future prospective studies.
Optical imaging
The evidence on the application of optical imaging for predicting the efficacy of cancer-targeted treatment is from preclinical research but not human studies. BLI has been widely used in the preclinical setting for cancer detection, monitoring disease progression, and assessing the efficacy of anticancer treatment in vivo. The rapid and quantitative assessment of response to cancer treatment by this technique has accelerated drug discovery and development. However, a comprehensive review of the application of this technique in drug development is outside the scope of this paper. Here, we only present some examples to demonstrate how this technique can be used for evaluating the efficacy of cancer-targeted therapy.
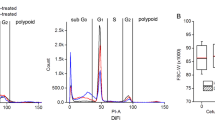
Guo and colleagues developed genetically engineered bioluminescent reporters that reflected the G1 phase alternation of the cell cycle.333 In vitro and in vivo experiments, this reporter system was shown to monitor G1 phase arrest caused by a clinically used CDK4/6 inhibitor, palbociclib.333 In another preclinical study of a syngeneic murine triple-negative breast cancer model subjected to PD-1 inhibition, BLI could monitor the volume change of luciferase-tagged murine 4T1 tumors after treatment.334 Although minimal data from the literature demonstrated the use of FLI for assessing the efficacy of anticancer treatment, several FLI probes have been developed for monitoring the expression of HER2,335,336,337 VEGF/VEGFR,337,338 and EGFR.339,340,341 These probes have the potential for the early detection of blocking of these receptors and may be used for early assessment of the efficacy of targeted treatment. Additionally, in a recently published study, Gao and colleagues developed an FLI probe for the real-time monitoring of CDK4 activity. In a hormone receptor-positive/HER2-negative breast cancer xenograft model, they demonstrated that the probe could reflect the therapeutic efficacy of palbociclib before an apparent change in the tumor size (see Fig. 6).342 FLI probes have also been constructed for noninvasive, preclinical in vivo evaluation of the efficacy of cancer immunotherapeutics by detecting the presence of either the immune activation-related biomarker granzyme B343 or CD8+ cytotoxic T lymphocytes.344
CPP30-Lipo/CDKACT4 reports the CDK4 inhibitor pharmacodynamics in vivo. a The chemical structures of molecular probes of CPP30-Lipo/CDKA4. b The activation of CPP30-Lipo/CDKA4 within cells. c Fluorescence images of mice bearing MCF-7 tumor injected with CPP30-Lipo/CDKACT4 before and after gavage of sterile water for 7 days, or before and after treatment with 150 mg/kg palbociclib daily for 7 days. The fluorescence signals were measured in radiance counts per cm2 per second per steradian (p/s/cm2/sr) (left). Magnetic resonance imaging (MRI) of mice in (right). (Reproduced from Gao, Y. Y. et al. In vivo visualization of fluorescence reflecting CDK4 activity in a breast cancer mouse model. MedComm (2020) 3, e136 (2022).)
Interestingly, the clinical application of optical imaging techniques for evaluating the efficacy of cancer-targeted treatment has been explored using a diffuse optical tomography breast imaging system (DOTBIS).345 In a proof-of-principle study with seven postmenopausal women with early-stage breast cancer who received pre-surgical treatment with an AKT inhibitor (MK-2206) or an aromatase inhibitor (exemestane or letrozole), DOTBIS was performed on the tumor at baseline and post-therapy. Consistent decreases in DOTBIS-measured total hemoglobin, oxyhemoglobin, deoxyhemoglobin, and water fraction were observed in the tumor after treatment.345 This supports the value of further investigation of DOTBIS as a potential tool for assessing the response to cancer-targeted therapies in early-stage breast cancer.
In general, the exploration of optical imaging for treatment efficacy evaluation is in the very early stage. The clinical translation of BLI and FLI is challenging mainly due to their limited tissue penetration and the need for luciferase gene transfection in BLI. On the contrary, the clinical translation of DOTBIS is more feasible as it is a cheap, user-friendly, and repeatable technique.
Photoacoustic imaging (PAI)
As PAI can use endogenous contrast medium, such as hemoglobin, for real-time and noninvasive imaging, it is well suited for delineating the anatomy of the vasculature and evaluating tumor responses to antiangiogenic therapy.346 In a study by Yang et al. 347, PAI was used to measure early tumor response in a breast cancer mouse model treated with bevacizumab by clearly identifying the vessels surrounding tumors. By quantitative analysis, PAI parameters including MAP 760, MAP 840, hemoglobin (HbT), and deoxyhemoglobin (HbR) were shown to be significantly reduced 5 days after both high-dose and low-dose bevacizumab treatments compared with the levels in the control group, while no noticeable change in tumor volume was found. In the high-dose group, hypoxia showed negative correlations with these four parameters and CD31 (a marker of the maturation state) showed positive correlations with HbT, HbR, and MAP 760, while VMI (another marker of the maturation state) showed positive correlations with MAP 760 and HbR. Similar conclusions were reached by using photoacoustic tomography (PAT) in breast cancer mouse models treated with bevacizumab348 and an ovarian tumor mouse model treated with Trebananib.349 Moreover, Pham et al. 350 used contrast-enhanced ultrasound (CEUS) and PAI to perform the preclinical evaluation of the efficacy of bevacizumab in combination with CRLX101 (an investigational nanoparticle–drug conjugate). Therefore, PAI has been used as a noninvasive method in preclinical models to measure the effect of antiangiogenic therapy by visualizing vascular regression, normalization, and tumor hypoxia. Its clinical translation in cancer-targeted treatment efficacy evaluation is worthy of exploring, given the fact that PAI has been employed for the early detection of multiple cancers, including breast cancer, melanoma, and prostate cancer.351
Multimodal imaging
Each imaging technique discussed above is associated with certain limitations.352 For example, PET/SPECT are associated with poor spatial resolution and risks posed by exposure to radiation, while MRI has relatively low specificity and a long imaging time. In contrast, optical imaging has low spatial resolution and a small penetration depth. Meanwhile, US imaging has poor resolution and subjective results dependent on the particular operator. To overcome these limitations, researchers have attempted to fuse two or more different imaging techniques to create a new imaging mode, also known as multimodal molecular imaging, to obtain more consistent and accurate information.
Currently, most multimodal imaging techniques feature dual modes, involving optical imaging combined with MRI, PET, or SPECT, or PET and SPECT combined with CT or MRI, among others. Multimodal molecular imaging has been utilized in preclinical and clinical research for early diagnosis, disease staging, assessment of therapeutic response, surgical navigation, and prognosis evaluation.
In studies on the application of multimodal molecular imaging in predicting the efficacy of targeted therapy, the focus has mainly been placed on antiangiogenic drugs in preclinical studies. Using a novel metal-based imaging probe, Fe3O4-DMSA-SMCC-BCZM-99mTc, with the monoclonal antibody bevacizumab radiolabeled with 99mTc for dual-modality SPECT/MR imaging of angiogenesis by targeting VEGF-A was reported.353 This approach could be utilized to evaluate the efficacy of potential antiangiogenic drugs. Similar results were found for NIR830-bevacizumab-IONPs with MR and optical imaging.354 In a mouse model, Chen et al. detected cancer in vivo using multimodal imaging with photoacoustic and computed tomography (CT), which targeted epidermal growth factor receptors (EGFRs) and ErbB2 and may be used as a new platform for evaluating the response to EGFR- and HER2-targeted therapy.355
Conclusion and future perspectives
This paper reviews studies performed to date on the application of molecular imaging for early prediction of the efficacy of cancer-targeted therapy. Conventional predictive biomarkers for targeted therapy mainly rely on invasive tissue biopsy and subsequent pathological analysis. Since only a limited amount of tissue can be biopsied, as well as the issue of tumor heterogeneity, the conventional histopathological biomarkers obtained from a single lesion are not always predictive of the response to targeted therapy, especially for patients with multiple metastatic tumors. For post-therapy assessment of the efficacy of targeted treatment, the current gold standard is the use of the RECIST criteria, a method based on the change in the tumor size. However, such change often occurs over weeks, or even months, after treatment initiation, which rules out the possibility of detecting treatment resistance soon after it develops.
Against the above background, the novel molecular imaging technique demonstrates more potential than conventional imaging techniques for cancer-targeted therapy in the following aspects: (1) It provides whole-body imaging, such as by immuno-PET,168 which better demonstrates the intra-tumoral and inter-tumoral heterogeneity of targeted molecule expression. This enables the prediction of targeted treatment efficacy at the lesion level.168 (2) It enables the imaging of early changes (usually several days after treatment) in the functional status of a tumor, which could reflect the response to targeted therapy and enable early detection of treatment resistance and prediction of long-term efficacy. (3) It allows noninvasive monitoring of the changes in the expression of targeted molecules, which is essential for optimizing the treatment strategy during therapy.
To date, almost all studies reporting the application of molecular imaging in cancer-targeted therapy were in early-stage clinical trials with a small number of patients. Although these studies are essential for obtaining preliminary findings on the performance of these advanced imaging techniques for evaluating the efficacy of targeted treatment, their definitive value needs to be confirmed in future studies with larger sample sizes. There is also heterogeneity in the data acquisition among the applied approaches, such as the scanning protocol and timing of scanning, strategy for data analysis, and study endpoints. This makes it difficult to directly compare the data among studies and draw definitive conclusions on the performance of any technique or any parameter for evaluating the efficacy of targeted treatment. There is an urgent need to develop a uniform scanning protocol, data analysis strategy, and study endpoints for each imaging technique, as this is the only way to increase uniformity among studies. This would in turn allow direct data comparison and pooled data analysis and should eventually accelerate the clinical application of molecular imaging for evaluating the efficacy of targeted therapy.
At present, 18F-FDG PET-CT, DCE-MRI, and DWI-MRI are the most commonly used techniques for assessing cancer-targeted therapy in clinical practice. These techniques also hold great promise as they enable noninvasive whole-body imaging with deep tissue penetration and high sensitivity for detecting the biological changes after targeted treatment, such as changes in the metabolic activity, perfusion, and diffusion of tumors. Although immune-PET provides target-specific information on tumors and maybe a more helpful tool for evaluating the efficacy of targeted treatment. VEGF, HER2, EGFR, ER, and PD-1/PD-L1 are the most commonly studied targets in this field. Immuno-PET binding to these targets has been applied in early clinical trials to evaluate the anti-cancer targeted treatment efficacy and to predict patient prognosis. Early results demonstrated excellent potential translation of these tools to clinical practice. However, clinical translation of new radiotracers is difficult in many countries, including China, due to regulatory restrictions. Multimodality molecular imaging could be a promising approach to improve the predictive accuracy in evaluating the efficacy of targeted treatment.300,356,357,358 The development of this approach is an essential task in future work.
Along with the increasing use of molecular imaging in cancer management, artificial intelligence (AI) techniques have the potential for several related applications, such as automated interpretation of images and predicting treatment efficacy and patient survival. Information derived from AI may help select the most appropriate treatment for patients and thus improve patient care. An early attempt toward this goal has been reported by Mu and colleagues.359 They found that 18F-FDG PET-CT images could be used to predict the EGFR mutation status using the AI technique of deep learning. A higher EGFR deep learning score (EGFR-DLS) was associated with longer PFS in patients treated with EGFR-TKIs, and a shorter PFS in patients receiving immune checkpoint inhibitors.359 The utilization of AI to evaluate the efficacy of targeted treatment and guide clinical decision-making regarding appropriate treatment is expected to increase soon.
Targeted therapy has been one of the cornerstones in treating cancer patients for decades. The rapid development of molecular imaging should improve the early prediction of treatment response to allow early adaptation of patient management and eventually improve patient outcomes. From a perspective point of view, molecular imaging approaches will provide valuable tools to optimize the dosing schedule, determine therapeutic regimes, and monitor the therapeutic response to guide the change in treatment protocol timely if resistance happens. These are important in assisting the clinical decision-making of multidisciplinary treatment, including targeted therapy.
References
Xia, C. et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin. Med. J. (Engl.). 135, 584–590 (2022).
Joo, W. D., Visintin, I. & Mor, G. Targeted cancer therapy-are the days of systemic chemotherapy numbered? Maturitas 76, 308–314 (2013).
Miller, A. B., Hoogstraten, B., Staquet, M. & Winkler, A. Reporting results of cancer treatment. Cancer 47, 207–214 (1981).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Ratain, M. J. & Eckhardt, S. G. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J. Clin. Oncol. 22, 4442–4445 (2004).
Gettinger, S. N. et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 33, 2004–2012 (2015).
Guaitoli, G. et al. Are we ready to describe response or progression to immunotherapy in lung cancer? Crit. Rev. Oncol. Hematol. 138, 112–119 (2019).
Weissleder, R. & Mahmood, U. Molecular imaging. Radiology 219, 316–333 (2001).
Thakur, M. & Lentle, B. C. Report of a summit on molecular imaging. Am. J. Roentgenol. 186, 297–299 (2006).
Zhong, L. et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 6, 201 (2021).
Jin, S. et al. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct. Target. Ther. 7, 39 (2022).
van den Bulk, J., Verdegaal, E. M. & de Miranda, N. F. Cancer immunotherapy: broadening the scope of targetable tumours. Open Biol. 8, 180037 (2018).
Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl Cancer Inst. 82, 4–6 (1990).
Zirlik, K. & Duyster, J. Anti-angiogenics: current situation and future perspectives. Oncol. Res. Treat. 41, 166–171 (2018).
Ferrara, N., Hillan, K. J. & Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 333, 328–335 (2005).
Saoudi Gonzalez, N. et al. Up-to-date role of aflibercept in the treatment of colorectal cancer. Expert Opin. Biol. Ther. 21, 1315–1324 (2021).
Roviello, G., Sohbani, N., Petrioli, R. & Rodriquenz, M. G. Ramucirumab as a second line therapy for advanced HCC: a significant achievement or a wasted opportunity for personalised therapy? Investig. N. Drugs 37, 1274–1288 (2019).
Jeltsch, M., Leppanen, V. M., Saharinen, P. & Alitalo, K. Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harb. Perspect. Biol. 5, a009183 (2013).
Hood, J. D. & Cheresh, D. A. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2, 91–100 (2002).
Zou, Y. et al. Cyclic RGD-functionalized and disulfide-crosslinked iodine-rich polymersomes as a robust and smart theranostic agent for targeted CT imaging and chemotherapy of tumor. Theranostics 9, 8061–8072 (2019).
da Cunha Santos, G., Shepherd, F. A. & Tsao, M. S. EGFR mutations and lung cancer. Annu. Rev. Pathol. 6, 49–69 (2011).
Wee, P. & Wang, Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 9, 52 (2017).
Morrison, D. K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 4, a011254 (2012).
Jiao, Q. et al. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 17, 36 (2018).
Lu, X. et al. Targeting EGFR(L858R/T790M) and EGFR(L858R/T790M/C797S) resistance mutations in NSCLC: current developments in medicinal chemistry. Med. Res. Rev. 38, 1550–1581 (2018).
Hsu, W. H., Yang, J. C., Mok, T. S. & Loong, H. H. Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol. 29, i3–i9 (2018).
Yun, C. H. et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl Acad. Sci. USA 105, 2070–2075 (2008).
Sequist, L. V. et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N. Engl. J. Med. 372, 1700–1709 (2015).
Kashima, K. et al. CH7233163 overcomes osimertinib-resistant EGFR-Del19/T790M/C797S mutation. Mol. Cancer Ther. 19, 2288–2297 (2020).
Yang, J. C. et al. Safety, efficacy, and pharmacokinetics of almonertinib (HS-10296) in pretreated patients with EGFR-mutated advanced NSCLC: a Multicenter, Open-label, Phase 1 Trial. J. Thorac. Oncol. 15, 1907–1918 (2020).
Li, S. et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7, 301–311 (2005).
Schultheis, B. et al. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen in KRAS wildtype patients with locally advanced or metastatic pancreatic cancer: a multicenter, randomized phase IIb study. Ann. Oncol. 28, 2429–2435 (2017).
Van Cutsem, E. et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 25, 1658–1664 (2007).
Kollmannsberger, C. et al. A phase I study of the humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody EMD 72000 (matuzumab) in combination with paclitaxel in patients with EGFR-positive advanced non-small-cell lung cancer (NSCLC). Ann. Oncol. 17, 1007–1013 (2006).
Xu, M. J., Johnson, D. E. & Grandis, J. R. EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev. 36, 463–473 (2017).
Thomas, P. J. G. et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell 11, 495–505 (2003).
Yan, M., Parker, B. A., Schwab, R. & Kurzrock, R. HER2 aberrations in cancer: implications for therapy. Cancer Treat. Rev. 40, 770–780 (2014).
Witton, C. J. et al. Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. J. Pathol. 200, 290–297 (2003).
Corti, C. et al. Antibody–drug conjugates for the treatment of breast cancer. Cancers (Basel) 13, 84 (2021).
Hrynchak, I. et al. Nanobody-based theranostic agents for HER2-positive breast cancer: radiolabeling strategies. Int. J. Mol. Sci. 22, 10745 (2021).
Gao, X., Leone, G. W. & Wang, H. Cyclin D-CDK4/6 functions in cancer. Adv. Cancer Res. 148, 147–169 (2020).
Raheem, F., Ofori, H., Simpson, L. & Shah, V. Abemaciclib: the first FDA-approved CDK4/6 inhibitor for the adjuvant treatment of HR+ HER2− early breast cancer. Ann. Pharmacother. 10600280211073322, (2022).
Arafeh, R. & Samuels, Y. PIK3CA in cancer: the past 30 years. Semin. Cancer Biol. 59, 36–49 (2019).
Murugan, A. K., Munirajan, A. K. & Tsuchida, N. Genetic deregulation of the PIK3CA oncogene in oral cancer. Cancer Lett. 338, 193–203 (2013).
Iksen, Pothongsrisit, S. & Pongrakhananon, V. Targeting the PI3K/AKT/mTOR signaling pathway in lung cancer: an update regarding potential drugs and natural products. Molecules 26, 4100 (2021).
Lemmon, M. A. & Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 (2010).
Fedele, C. G. et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc. Natl Acad. Sci. USA 107, 22231–22236 (2010).
Zhao, L. & Vogt, P. K. Class I PI3K in oncogenic cellular transformation. Oncogene 27, 5486–5496 (2008).
Papa, A. & Pandolfi, P. P. The PTEN-PI3K axis in cancer. Biomolecules 9, 153 (2019).
Chen, Y. et al. INPP4B restrains cell proliferation and metastasis via regulation of the PI3K/AKT/SGK pathway. J. Cell. Mol. Med. 22, 2935–2943 (2018).
Bonneau, D. & Longy, M. Mutations of the human PTEN gene. Hum. Mutat. 16, 109–122 (2000).
Alvarez-Garcia, V., Tawil, Y., Wise, H. M. & Leslie, N. R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 59, 66–79 (2019).
Hao, Y. et al. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nat. Commun. 7, 11971 (2016).
Lee, J. J., Loh, K. & Yap, Y. S. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol. Med. 12, 342–354 (2015).
Vanhaesebroeck, B. et al. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 20, 741–769 (2021).
Le, T., Jerel, D. & Bryan, L. J. Update on the role of copanlisib in hematologic malignancies. Ther. Adv. Hematol. 12, 20406207211006027 (2021).
André, F. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380, 1929–1940 (2019).
Zirlik, K. & Veelken, H. Idelalisib. Recent Results Cancer Res. 212, 243–264 (2018).
West, K. A., Castillo, S. S. & Dennis, P. A. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist. Updat. 5, 234–248 (2002).
He, Y. et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target Ther. 6, 425 (2021).
Sabbah, D. A., Brattain, M. G. & Zhong, H. Dual inhibitors of PI3K/mTOR or mTOR-selective inhibitors: which way shall we go? Curr. Med. Chem. 18, 5528–5544 (2011).
McCarthy, C. et al. Lymphangioleiomyomatosis: pathogenesis, clinical features, diagnosis, and management. Lancet Respir. Med. 9, 1313–1327 (2021).
Hasskarl, J. Everolimus. Recent Results Cancer Res. 211, 101–123 (2018).
Bhatia, S. & Thompson, J. A. Temsirolimus in patients with advanced renal cell carcinoma: an overview. Adv. Ther. 26, 55–67 (2009).
Cohen, P. R. et al. Cutaneous perivascular epithelioid cell tumor (PEComa): case report and world literature review of clinical and molecular characteristics. Dermatol. Online J. 28, https://doi.org/10.5070/D328157058 (2022).
Calero, R., Morchon, E., Martinez-Argudo, I. & Serrano, R. Synergistic anti-tumor effect of 17AAG with the PI3K/mTOR inhibitor NVP-BEZ235 on human melanoma. Cancer Lett. 406, 1–11 (2017).
Yang, X. et al. Autophagy inhibition enhances colorectal cancer apoptosis induced by dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Oncol. Lett. 12, 102–106 (2016).
Wise-Draper, T. M. et al. A Phase Ib Study of the dual PI3K/mTOR inhibitor dactolisib (BEZ235) combined with everolimus in patients with advanced solid malignancies. Target Oncol. 12, 323–332 (2017).
Salazar, R. et al. Phase II Study of BEZ235 versus everolimus in patients with mammalian target of rapamycin inhibitor-naive advanced pancreatic neuroendocrine tumors. Oncologist 23, 766–790 (2018).
Ribas, A. et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 16, 908–918 (2015).
Robert, C. et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384, 1109–1117 (2014).
Schadendorf, D. et al. Pooled analysis of long-term survival data from Phase II and Phase III Trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 33, 1889–1894 (2015).
Lipson, E. J. & Drake, C. G. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin. Cancer Res. 17, 6958–6962 (2011).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Baumeister, S. H., Freeman, G. J., Dranoff, G. & Sharpe, A. H. Coinhibitory pathways in immunotherapy for cancer. Annu. Rev. Immunol. 34, 539–573 (2016).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Ribas, A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 5, 915–919 (2015).
Yi, M. et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol. Cancer 21, 28 (2022).
Rowshanravan, B., Halliday, N. & Sansom, D. M. CTLA-4: a moving target in immunotherapy. Blood 131, 58–67 (2018).
Walker, L. S. & Sansom, D. M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 11, 852–863 (2011).
Wing, K. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008).
Arce Vargas, F. et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell 33, 649–663.e644 (2018).
Lisi, L. et al. Clinical experience with CTLA-4 blockade for cancer immunotherapy: from the monospecific monoclonal antibody ipilimumab to probodies and bispecific molecules targeting the tumor microenvironment. Pharm. Res. 175, 105997 (2022).
Mankoff, D. A. A definition of molecular imaging. J. Nucl. Med. 48, 21N (2007).
Floyd, E. & McShane, T. M. Development and use of biomarkers in oncology drug development. Toxicol. Pathol. 32, 106–115 (2004).
Casy, A. F. Applications of nuclear magnetic resonance spectroscopy in medicinal and pharmaceutical chemistry. J. Pharm. Sci. 56, 1049–1063 (1967).
Kuhl, D. E. et al. The Mark IV system for radionuclide computed tomography of the brain. Radiology 121, 405–413 (1976).
Mansfield, P., Pykett, I. L. & Morris, P. G. Human whole body line-scan imaging by NMR. Br. J. Radiol. 51, 921–922 (1978).
Legocki, R. P., Legocki, M., Baldwin, T. O. & Szalay, A. A. Bioluminescence in soybean root nodules: demonstration of a general approach to assay gene expression in vivo by using bacterial luciferase. Proc. Natl Acad. Sci. USA 83, 9080–9084 (1986).
Kang, K. A., Bruley, D. F., Londono, J. M. & Chance, B. Highly scattering optical system identification via frequency response analysis of NIR-TRS spectra. Ann. Biomed. Eng. 22, 240–252 (1994).
Zasadny, K. R., Kison, P. V., Francis, I. R. & Wahl, R. L. FDG-PET determination of metabolically active tumor volume and comparison with CT. Clin. Positron Imaging 1, 123–129 (1998).
Viator, J. A. et al. Clinical testing of a photoacoustic probe for port wine stain depth determination. Lasers Surg. Med. 30, 141–148 (2002).
Smith, A. M., Mancini, M. C. & Nie, S. Bioimaging: second window for in vivo imaging. Nat. Nanotechnol. 4, 710–711 (2009).
Rowe, S. P. & Pomper, M. G. Molecular imaging in oncology: current impact and future directions. CA Cancer J. Clin. 72, 333–352 (2022).
Wu, M. & Shu, J. Multimodal molecular imaging: current status and future directions. Contrast Media Mol. Imaging 2018, 1382183 (2018).
Anderson, C. J. & Lewis, J. S. Current status and future challenges for molecular imaging. Philos. Trans. A Math. Phys. Eng. Sci. 375, 20170023 (2017).
James, M. L. & Gambhir, S. S. A molecular imaging primer: modalities, imaging agents, and applications. Physiol. Rev. 92, 897–965 (2012).
Abrantes, A. M. et al. Tumour functional imaging by PET. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165717 (2020).
Vaidya, T. et al. The continuing evolution of molecular functional imaging in clinical oncology: the road to precision medicine and radiogenomics (Part I). Mol. Diagn. Ther. 23, 1–26 (2019).
Vaidya, T. et al. The continuing evolution of molecular functional imaging in clinical oncology: the road to precision medicine and radiogenomics (Part II). Mol. Diagn. Ther. 23, 27–51 (2019).
Basu, S. et al. The basic principles of FDG-PET/CT imaging. PET Clin. 9, 355–370 (2014).
Sanchez-Crespo, A. Comparison of Gallium-68 and Fluorine-18 imaging characteristics in positron emission tomography. Appl. Radiat. Isot. 76, 55–62 (2013).
Abdo, R. A., Lamare, F., Fernandez, P. & Bentourkia, M. Quantification of hypoxia in human glioblastoma using PET with 18F-FMISO. Nucl. Med. Mol. Imaging 55, 107–115 (2021).
Peck, M. et al. Applications of PET imaging with the proliferation marker [18F]-FLT. Q. J. Nucl. Med. Mol. Imaging 59, 95–104 (2015).
Laverman, P., Boerman, O. C., Corstens, F. H. & Oyen, W. J. Fluorinated amino acids for tumour imaging with positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 29, 681–690 (2002).
Lodge, M. A., Jacene, H. A., Pili, R. & Wahl, R. L. Reproducibility of tumor blood flow quantification with 15O-water PET. J. Nucl. Med. 49, 1620–1627 (2008).
Wei, W. et al. ImmunoPET: concept, design, and applications. Chem. Rev. 120, 3787–3851 (2020).
Marik, J. & Junutula, J. R. Emerging role of immunoPET in receptor targeted cancer therapy. Curr. Drug Deliv. 8, 70–78 (2011).
Hernot, S. et al. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 20, e354–e367 (2019).
Naqvi, S. & Imran, M. B. Single-Photon Emission Computed Tomography (SPECT) Radiopharmaceuticals (Medical Isotopes, 2021).
Desar, I. M. et al. 111In-bevacizumab imaging of renal cell cancer and evaluation of neoadjuvant treatment with the vascular endothelial growth factor receptor inhibitor sorafenib. J. Nucl. Med. 51, 1707–1715 (2010).
Wu, B. et al. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. 3, 19 (2016).
Ward, K. M., Aletras, A. H. & Balaban, R. S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 143, 79–87 (2000).
Grover, V. P. et al. Magnetic resonance imaging: principles and techniques: lessons for clinicians. J. Clin. Exp. Hepatol. 5, 246–255 (2015).
Kauczor, H. U., Zechmann, C., Stieltjes, B. & Weber, M. A. Functional magnetic resonance imaging for defining the biological target volume. Cancer Imaging 6, 51–55 (2006).
Howe, F. A. et al. Issues in flow and oxygenation dependent contrast (FLOOD) imaging of tumours. NMR Biomed. 14, 497–506 (2001).
Tofts, P. S. et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J. Magn. Reson. Imaging 10, 223–232 (1999).
Port, R. E. et al. Multicompartment analysis of gadolinium chelate kinetics: Blood-tissue exchange in mammary tumors as monitored by dynamic MR imaging. J. Magn. Reson. Imaging 10, 233–241 (1999).
Tofts, P. S. & Kermode, A. G. Measurement of the blood–brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn. Reson. Med. 17, 357–367 (1991).
Tofts, P. S. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J. Magn. Reson. Imaging 7, 91–101 (1997).
Guo, Y. et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J. Magn. Reson. Imaging 16, 172–178 (2002).
Gauvain, K. M. et al. Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. Am. J. Roentgenol. 177, 449–454 (2001).
Lang, P. et al. Osteogenic sarcoma: noninvasive in vivo assessment of tumor necrosis with diffusion-weighted MR imaging. Radiology 206, 227–235 (1998).
Berthezène, Y. et al. Prospective comparison of MR lung perfusion and lung scintigraphy. J. Magn. Reson. Imaging 9, 61–68 (1999).
Yuan, W. et al. Efficacy of diffusion-weighted imaging in neoadjuvant chemotherapy for osteosarcoma: a systematic review and meta-analysis. Acad. Radiol. 29, 326–334 (2022).
Daly, P. F. & Cohen, J. S. Magnetic resonance spectroscopy of tumors and potential in vivo clinical applications: a review. Cancer Res. 49, 770–779 (1989).
van der Graaf, M. In vivo magnetic resonance spectroscopy: basic methodology and clinical applications. Eur. Biophys. J. 39, 527–540 (2010).
Chenevert, T. L. et al. Diffusion MRI: a new strategy for assessment of cancer therapeutic efficacy. Mol. Imaging 1, 336–343 (2002).
Lanza, G. M. et al. Targeted antiproliferative drug delivery to vascular smooth muscle cells with a magnetic resonance imaging nanoparticle contrast agent: implications for rational therapy of restenosis. Circulation 106, 2842–2847 (2002).
Muller, J., Wunder, A. & Licha, K. Optical imaging. Recent Results Cancer Res. 187, 221–246 (2013).
Serkova, N. J. et al. Preclinical applications of multi-platform imaging in animal models of cancer. Cancer Res. 81, 1189–1200 (2021).
Zhang, G. J. et al. Bioluminescent imaging of Cdk2 inhibition in vivo. Nat. Med. 10, 643–648 (2004).
Zhang, G. J. et al. Visualization of mitotic arrest of cell cycle with bioluminescence imaging in living animals. Mol. Imaging Biol. 15, 431–440 (2013).
Koessinger, A. L. et al. Quantitative in vivo bioluminescence imaging of orthotopic patient-derived glioblastoma xenografts. Sci. Rep. 10, 15361 (2020).
Zobel, M. J. et al. Initiation of immunotherapy with activated natural killer cells and anti-GD2 antibody dinutuximab prior to resection of primary neuroblastoma prolongs survival in mice. J. Immunother. Cancer 8, e001560 (2020).
Vahrmeijer, A. L. et al. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 10, 507–518 (2013).
Leblond, F., Davis, S. C., Valdes, P. A. & Pogue, B. W. Pre-clinical whole-body fluorescence imaging: review of instruments, methods and applications. J. Photochem. Photobiol. B 98, 77–94 (2010).
Suurs, F. V. et al. Fluorescent image-guided surgery in breast cancer by intravenous application of a quenched fluorescence activity-based probe for cysteine cathepsins in a syngeneic mouse model. EJNMMI Res. 10, 111 (2020).
Zhao, T. et al. A transistor-like pH nanoprobe for tumour detection and image-guided surgery. Nat. Biomed. Eng. 1, 0006 (2016).
Kramer-Marek, G., Longmire, M. R., Choyke, P. L. & Kobayashi, H. Recent advances in optical cancer imaging of EGF receptors. Curr. Med. Chem. 19, 4759–4766 (2012).
Koller, M. et al. Implementation and benchmarking of a novel analytical framework to clinically evaluate tumor-specific fluorescent tracers. Nat. Commun. 9, 3739 (2018).
Voskuil, F. J. et al. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nat. Commun. 11, 3257 (2020).
Rosenthal, E. L. et al. Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin. Cancer Res. 21, 3658–3666 (2015).
van Dam, G. M. et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat. Med. 17, 1315–1319 (2011).
Wang, L. V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 3, 503–509 (2009).
Attia, A. B. E. et al. A review of clinical photoacoustic imaging: current and future trends. Photoacoustics 16, 100144 (2019).
Steinberg, I. et al. Photoacoustic clinical imaging. Photoacoustics 14, 77–98 (2019).
Laufer, J., Delpy, D., Elwell, C. & Beard, P. Quantitative spatially resolved measurement of tissue chromophore concentrations using photoacoustic spectroscopy: application to the measurement of blood oxygenation and haemoglobin concentration. Phys. Med. Biol. 52, 141–168 (2007).
Li, L. et al. Photoacoustic imaging of lacZ gene expression in vivo. J. Biomed. Opt. 12, 020504 (2007).
De la Zerda, A. et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 3, 557–562 (2008).
Copland, J. A. et al. Bioconjugated gold nanoparticles as a molecular based contrast agent: implications for imaging of deep tumors using optoacoustic tomography. Mol. Imaging Biol. 6, 341–349 (2004).
Mallidi, S. et al. Molecular specific optoacoustic imaging with plasmonic nanoparticles. Opt. Express 15, 6583–6588 (2007).
Razansky, D. & Ntziachristos, V. Hybrid photoacoustic fluorescence molecular tomography using finite-element-based inversion. Med. Phys. 34, 4293–4301 (2007).
A, Agarwal, S. et al. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. J. Appl. Phys. 102, 064701 (2007).
Hudson, J. M. et al. Dynamic contrast enhanced ultrasound for therapy monitoring. Eur. J. Radiol. 84, 1650–1657 (2015).
Chen, K. & Chen, X. Design and development of molecular imaging probes. Curr. Top. Med. Chem. 10, 1227–1236 (2010).
Duch, J. et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 36, 1551–1557 (2009).
Sarikaya, I. et al. Combined use of preoperative 18F FDG-PET imaging and intraoperative gamma probe detection for accurate assessment of tumor recurrence in patients with colorectal cancer. World J. Surg. Oncol. 5, 80 (2007).
Wang, X. et al. Clinical Application of 99mTc-sulfur colloid preoperative lymphoscintigraphy in sentinel lymph node biopsy in breast cancer patients[J]. Chinese J. Clin. Oncol. 37(21): 1227–1231 (2010).
Nagengast, W. B. et al. Near-infrared fluorescence molecular endoscopy detects dysplastic oesophageal lesions using topical and systemic tracer of vascular endothelial growth factor A. Gut 68, 7–10 (2019).
Nagengast, W. B. et al. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J. Nucl. Med. 48, 1313–1319 (2007).
Nagengast, W. B. et al. VEGF-PET imaging is a noninvasive biomarker showing differential changes in the tumor during sunitinib treatment. Cancer Res. 71, 143–153 (2011).
Teng, F. F., Meng, X., Sun, X. D. & Yu, J. M. New strategy for monitoring targeted therapy: molecular imaging. Int. J. Nanomed. 8, 3703–3713 (2013).
Lee, S., Xie, J. & Chen, X. Peptide-based probes for targeted molecular imaging. Biochemistry 49, 1364–1376 (2010).
Hentzen, J. et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: a narrative review. J. Surg. Oncol. 118, 332–343 (2018).
Tijink, B. M. et al. (124)I-L19-SIP for immuno-PET imaging of tumour vasculature and guidance of (131)I-L19-SIP radioimmunotherapy. Eur. J. Nucl. Med. Mol. Imaging 36, 1235–1244 (2009).
Garousi, J., Orlova, A., Frejd, F. Y. & Tolmachev, V. Imaging using radiolabelled targeted proteins: radioimmunodetection and beyond. EJNMMI Radiopharm. Chem. 5, 16 (2020).
Bensch, F. et al. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 24, 1852–1858 (2018).
Dammes, N. & Peer, D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics 10, 938–955 (2020).
Martiniova, L., Palatis, L., Etchebehere, E. & Ravizzini, G. Gallium-68 in medical imaging. Curr. Radiopharm. 9, 187–207 (2016).
Keyaerts, M. et al. Phase I Study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J. Nucl. Med. 57, 27–33 (2016).
Schmidt, M. M. & Wittrup, K. D. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol. Cancer Ther. 8, 2861–2871 (2009).
Frejd, F. Y. & Kim, K. T. Affibody molecules as engineered protein drugs. Exp. Mol. Med. 49, e306 (2017).
Sorensen, J. et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 55, 730–735 (2014).
Sörensen, J. et al. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga]ABY-025 Affibody PET/CT. Theranostics 6, 262–271 (2016).
Sandberg, D. et al. Intra-image referencing for simplified assessment of HER2-expression in breast cancer metastases using the Affibody molecule ABY-025 with PET and SPECT. Eur. J. Nucl. Med. Mol. Imaging 44, 1337–1346 (2017).
Sandstrom, M. et al. Biodistribution and radiation dosimetry of the anti-HER2 Affibody molecule 68Ga-ABY-025 in breast cancer patients. J. Nucl. Med. 57, 867–871 (2016).
Samkoe, K. S. et al. Measuring microdose ABY-029 fluorescence signal in a primary human soft-tissue sarcoma resection. Proc. SPIE Int. Soc. Opt. Eng. 10862, 1086212 (2019).
Wang, W. & Hu, Z. Targeting peptide-based probes for molecular imaging and diagnosis. Adv. Mater. 31, e1804827 (2019).
Chang, H. N. et al. Blocking of the PD-1/PD-L1 interaction by a D-peptide antagonist for cancer immunotherapy. Angew. Chem. Int. Ed. Engl. 54, 11760–11764 (2015).
Chatterjee, S. et al. Corrigendum to “Rapid PD-L1 detection in tumors with PET using a highly specific peptide” [Biochemical and Biophysical Research Communications 483/1 (2017) 258–263]. Biochem. Biophys. Res. Commun. 491, 1125 (2017).
Maute, R. L. et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc. Natl Acad. Sci. USA 112, E6506–E6514 (2015).
Li, C. et al. Peptide blocking of PD-1/PD-L1 interaction for cancer immunotherapy. Cancer Immunol. Res. 6, 178–188 (2018).
Zhang, D. et al. In situ formation of nanofibers from purpurin18-peptide conjugates and the assembly induced retention effect in tumor sites. Adv. Mater. 27, 6125–6130 (2015).
Lee, H. Y. et al. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J. Nucl. Med. 49, 1371–1379 (2008).
Lee, J. et al. RGD peptide-conjugated multimodal NaGdF4:Yb3+/Er3+ nanophosphors for upconversion luminescence, MR, and PET imaging of tumor angiogenesis. J. Nucl. Med. 54, 96–103 (2013).
Sun, X. et al. Peptide-based imaging agents for cancer detection. Adv. Drug Deliv. Rev. 110-111, 38–51 (2017).
Li, D. et al. First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using (68)Ga-IRDye800CW-BBN. Theranostics 8, 2508–2520 (2018).
Burggraaf, J. et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat. Med. 21, 955–961 (2015).
Wu, J., Yuan, Y. & Tao, X. F. Targeted molecular imaging of head and neck squamous cell carcinoma: a window into precision medicine. Chin. Med. J. (England) 133, 1325–1336 (2020).
Schrijver, W. et al. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J. Natl Cancer Inst. 110, 568–580 (2018).
Gupta, N., Price, P. M. & Aboagye, E. O. PET for in vivo pharmacokinetic and pharmacodynamic measurements. Eur. J. Cancer 38, 2094–2107 (2002).
Wahl, R. L., Jacene, H., Kasamon, Y. & Lodge, M. A. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 50, 122s–150s (2009).
Zander, T. et al. Early prediction of nonprogression in advanced non-small-cell lung cancer treated with erlotinib by using [(18)F]fluorodeoxyglucose and [(18)F]fluorothymidine positron emission tomography. J. Clin. Oncol. 29, 1701–1708 (2011).
Takahashi, R. et al. Early [18F]fluorodeoxyglucose positron emission tomography at two days of gefitinib treatment predicts clinical outcome in patients with adenocarcinoma of the lung. Clin. Cancer Res. 18, 220–228 (2012).
Fledelius, J. et al. (18)F-FDG PET/CT for very early response evaluation predicts CT response in erlotinib-treated non-small cell lung cancer patients: a comparison of assessment methods. J. Nucl. Med. 58, 1931–1937 (2017).
Ho, K. C. et al. TLG-S criteria are superior to both EORTC and PERCIST for predicting outcomes in patients with metastatic lung adenocarcinoma treated with erlotinib. Eur. J. Nucl. Med. Mol. Imaging 43, 2155–2165 (2016).
van Gool, M. H. et al. FDG-PET/CT response evaluation during EGFR-TKI treatment in patients with NSCLC. World J. Radiol. 6, 392–398 (2014).
Gebhart, G. et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO. J. Nucl. Med. 54, 1862–1868 (2013).
de Langen, A. J. et al. Monitoring response to antiangiogenic therapy in non-small cell lung cancer using imaging markers derived from PET and dynamic contrast-enhanced MRI. J. Nucl. Med. 52, 48–55 (2011).
Goshen, E., Davidson, T., Zwas, S. T. & Aderka, D. PET/CT in the evaluation of response to treatment of liver metastases from colorectal cancer with bevacizumab and irinotecan. Technol. Cancer Res. Treat. 5, 37–43 (2006).
Avallone, A. et al. (18)F-FDG PET/CT is an early predictor of pathologic tumor response and survival after preoperative radiochemotherapy with bevacizumab in high-risk locally advanced rectal cancer. J. Nucl. Med. 60, 1560–1568 (2019).
Taralli, S. et al. Response evaluation with (18)F-FDG PET/CT in metastatic breast cancer patients treated with Palbociclib: first experience in clinical practice. Ann. Nucl. Med. 33, 193–200 (2019).
Chen, J. L. et al. FDG-PET as a predictive biomarker for therapy with everolimus in metastatic renal cell cancer. Cancer Med. 2, 545–552 (2013).
Horn, K. P. et al. FDG and FLT-PET for early measurement of response to 37.5 mg daily sunitinib therapy in metastatic renal cell carcinoma. Cancer Imaging 15, 15 (2015).
Iommelli, F. et al. Inositol trisphosphate receptor type 3-mediated enhancement of EGFR and MET cotargeting efficacy in non-small cell lung cancer detected by (18)F-fluorothymidine. Clin. Cancer Res. 24, 3126–3136 (2018).
Shah, C. et al. Imaging biomarkers predict response to anti-HER2 (ErbB2) therapy in preclinical models of breast cancer. Clin. Cancer Res. 15, 4712–4721 (2009).
Haagensen, E. J. et al. The enhanced in vivo activity of the combination of a MEK and a PI3K inhibitor correlates with [18F]-FLT PET in human colorectal cancer xenograft tumour-bearing mice. PLoS ONE 8, e81763 (2013).
Aide, N. et al. 18F-FLT PET as a surrogate marker of drug efficacy during mTOR inhibition by everolimus in a preclinical cisplatin-resistant ovarian tumor model. J. Nucl. Med. 51, 1559–1564 (2010).
Crișan, G. et al. Radiopharmaceuticals for PET and SPECT Imaging: a literature review over the last decade. Int. J. Mol. Sci. 23, 5023 (2022).
Fu, R. et al. Antibody fragment and affibody ImmunoPET imaging agents: radiolabelling strategies and applications. ChemMedChem 13, 2466–2478 (2018).
Li, S. et al. Imaging gastrointestinal tumours using vascular endothelial growth factor-165 (VEGF165) receptor scintigraphy. Ann. Oncol. 14, 1274–1277 (2003).
Rainer, E. et al. The prognostic value of [(123)I]-vascular endothelial growth factor ([(123)I]-VEGF) in glioma. Eur. J. Nucl. Med. Mol. Imaging 45, 2396–2403 (2018).
Li, S. et al. Characterization of (123)I-vascular endothelial growth factor-binding sites expressed on human tumour cells: possible implication for tumour scintigraphy. Int. J. Cancer 91, 789–796 (2001).
Yoshimoto, M. et al. Radioiodinated VEGF to image tumor angiogenesis in a LS180 tumor xenograft model. Nucl. Med. Biol. 33, 963–969 (2006).
Beer, A. J. et al. Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin alpha(v)beta3 expression in man. Clin. Cancer Res. 12, 3942–3949 (2006).
Chen, X. et al. MicroPET imaging of brain tumor angiogenesis with 18F-labeled PEGylated RGD peptide. Eur. J. Nucl. Med. Mol. Imaging 31, 1081–1089 (2004).
Backer, M. V. et al. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat. Med. 13, 504–509 (2007).
Blankenberg, F. G. et al. Tumor imaging using a standardized radiolabeled adapter protein docked to vascular endothelial growth factor. J. Nucl. Med. 45, 1373–1380 (2004).
Chan, C., Cai, Z., Su, R. & Reilly, R. M. 111In- or 99mTc-labeled recombinant VEGF bioconjugates: in vitro evaluation of their cytotoxicity on porcine aortic endothelial cells overexpressing Flt-1 receptors. Nucl. Med. Biol. 37, 105–115 (2010).
Chan, C. et al. A human transferrin-vascular endothelial growth factor (hnTf-VEGF) fusion protein containing an integrated binding site for (111)In for imaging tumor angiogenesis. J. Nucl. Med. 46, 1745–1752 (2005).
Wang, H. et al. A new PET tracer specific for vascular endothelial growth factor receptor 2. Eur. J. Nucl. Med. Mol. Imaging 34, 2001–2010 (2007).
Cai, W. et al. PET of vascular endothelial growth factor receptor expression. J. Nucl. Med. 47, 2048–2056 (2006).
Gaykema, S. B. et al. 89Zr-bevacizumab PET imaging in primary breast cancer. J. Nucl. Med. 54, 1014–1018 (2013).
van Es, S. C. et al. (89)Zr-Bevacizumab PET: potential early indicator of everolimus efficacy in patients with metastatic renal cell carcinoma. J. Nucl. Med. 58, 905–910 (2017).
Oosting, S. F. et al. 89Zr-bevacizumab PET visualizes heterogeneous tracer accumulation in tumor lesions of renal cell carcinoma patients and differential effects of antiangiogenic treatment. J. Nucl. Med. 56, 63–69 (2015).
Sasada, S. et al. 64Cu-DOTA-trastuzumab PET imaging for HER2-specific primary lesions of breast cancer. Ann. Oncol. 28, 2028–2029 (2017).
Mortimer, J. E. et al. Tumor uptake of (64)Cu-DOTA-Trastuzumab in patients with metastatic breast cancer. J. Nucl. Med. 59, 38–43 (2018).
Sasada, S. et al. Visualization of HER2-specific breast cancer intratumoral heterogeneity using (64)Cu-DOTA-trastuzumab PET. Eur. J. Nucl. Med. Mol. Imaging 44, 2146–2147 (2017).
Guo, X. et al. Noninvasive detection of HER2 expression in gastric cancer by (64)Cu-NOTA-Trastuzumab in PDX mouse model and in patients. Mol. Pharm. 15, 5174–5182 (2018).
Lee, I. et al. A preliminary clinical trial to evaluate (64)Cu-NOTA-Trastuzumab as a positron emission tomography imaging agent in patients with breast cancer. EJNMMI Res. 11, 8 (2021).
Dehdashti, F. et al. Evaluation of [(89)Zr]trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res. Treat. 169, 523–530 (2018).
Bensch, F. et al. (89)Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging 45, 2300–2306 (2018).
Dijkers, E. C. et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharm. Ther. 87, 586–592 (2010).
Chan, C. et al. A comparison of 111In- or 64Cu-DOTA-trastuzumab Fab fragments for imaging subcutaneous HER2-positive tumor xenografts in athymic mice using microSPECT/CT or microPET/CT. EJNMMI Res. 1, 15 (2011).
Kurdziel, K. A. et al. First-in-human phase 0 study of (111) In-CHX-A”-DTPA trastuzumab for HER2 tumor imaging. J. Transl. Sci. 5, https://doi.org/10.15761/JTS.1000269. (2019).
Robinson, M. K. et al. Quantitative immuno-positron emission tomography imaging of HER2-positive tumor xenografts with an iodine-124 labeled anti-HER2 diabody. Cancer Res. 65, 1471–1478 (2005).
Beylergil, V. et al. Pilot study of 68Ga-DOTA-F(ab’)2-trastuzumab in patients with breast cancer. Nucl. Med. Commun. 34, 1157–1165 (2013).
Baum, R. P. et al. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J. Nucl. Med. 51, 892–897 (2010).
Bragina, O. et al. Phase I Study of (99m)Tc-ADAPT6, a scaffold protein-based probe for visualization of HER2 expression in breast cancer. J. Nucl. Med. 62, 493–499 (2021).
Bragina, O. et al. Phase I Trial of (99m)Tc-(HE)3-G3, a DARPin-based probe for imaging of HER2 expression in breast cancer. J. Nucl. Med. 63, 528–535 (2022).
Guo, X. et al. Construction of (124)I-trastuzumab for noninvasive PET imaging of HER2 expression: from patient-derived xenograft models to gastric cancer patients. Gastric Cancer 23, 614–626 (2020).
Ulaner, G. A. et al. 89Zr-Trastuzumab PET/CT for detection of human epidermal growth factor receptor 2-positive metastases in patients with human epidermal growth factor receptor 2-negative primary breast cancer. Clin. Nucl. Med. 42, 912–917 (2017).
Ulaner, G. A. et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-trastuzumab PET/CT. J. Nucl. Med. 57, 1523–1528 (2016).
Ulaner, G. A. et al. Identification of HER2-positive metastases in patients with HER2-negative primary breast cancer by using HER2-targeted (89)Zr-pertuzumab PET/CT. Radiology 296, 370–378 (2020).
Ulaner, G. A. et al. First-in-human human epidermal growth factor receptor 2-targeted imaging using (89)Zr-pertuzumab PET/CT: dosimetry and clinical application in patients with breast cancer. J. Nucl. Med. 59, 900–906 (2018).
Tamura, K. et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 54, 1869–1875 (2013).
Mortimer, J. E. et al. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using (64)Cu-DOTA-trastuzumab PET. J. Nucl. Med. 55, 23–29 (2014).
Massicano, A. V. F. et al. Imaging of HER2 with [(89)Zr]pertuzumab in Response to T-DM1 Therapy. Cancer Biother. Radiopharm. 34, 209–217 (2019).
Wu, Y. et al. Imaging and monitoring HER2 expression in breast cancer during trastuzumab therapy with a peptide probe (99m)Tc-HYNIC-H10F. Eur. J. Nucl. Med. Mol. Imaging 47, 2613–2623 (2020).
Gebhart, G. et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann. Oncol. 27, 619–624 (2016).
Mortimer, J. E. et al. Use of (64)Cu-DOTA-trastuzumab PET to predict response and outcome of patients receiving trastuzumab emtansine (T-DM1) for metastatic breast cancer: a Pilot Study. J. Nucl. Med. 63, 1145–1148 (2021).
Perik, P. J. et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 24, 2276–2282 (2006).
de Korte, M. A. et al. 111Indium-trastuzumab visualises myocardial human epidermal growth factor receptor 2 expression shortly after anthracycline treatment but not during heart failure: a clue to uncover the mechanisms of trastuzumab-related cardiotoxicity. Eur. J. Cancer 43, 2046–2051 (2007).
Li, W. et al. PET imaging of EGF receptors using [18F]FBEM-EGF in a head and neck squamous cell carcinoma model. Eur. J. Nucl. Med. Mol. Imaging 39, 300–308 (2012).
Niu, G. et al. PET of EGFR antibody distribution in head and neck squamous cell carcinoma models. J. Nucl. Med. 50, 1116–1123 (2009).
Hoeben, B. A. et al. Radiolabeled cetuximab: dose optimization for epidermal growth factor receptor imaging in a head-and-neck squamous cell carcinoma model. Int. J. Cancer 129, 870–878 (2011).
Yamaguchi, A. et al. Immuno-PET imaging for non-invasive assessment of cetuximab accumulation in non-small cell lung cancer. BMC Cancer 19, 1000 (2019).
Even, A. J. et al. Quantitative assessment of Zirconium-89 labeled cetuximab using PET/CT imaging in patients with advanced head and neck cancer: a theragnostic approach. Oncotarget 8, 3870–3880 (2017).
van Dijk, L. K. et al. Imaging of epidermal growth factor receptor expression in head and neck cancer with SPECT/CT and 111In-labeled cetuximab-F(ab’)2. J. Nucl. Med. 54, 2118–2124 (2013).
van Dijk, L. K. et al. PET of EGFR with (64) Cu-cetuximab-F(ab’)2 in mice with head and neck squamous cell carcinoma xenografts. Contrast Media Mol. Imaging 11, 65–70 (2016).
Garousi, J. et al. PET imaging of epidermal growth factor receptor expression in tumours using 89Zr-labelled ZEGFR:2377 affibody molecules. Int. J. Oncol. 48, 1325–1332 (2016).
Menke-van der Houven van Oordt, C. W. et al. 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget 6, 30384–30393 (2015).
Sun, X. et al. A PET imaging approach for determining EGFR mutation status for improved lung cancer patient management. Sci. Transl. Med. 10, eaan8840 (2018).
Liu, C. et al. Evaluation of tumour heterogeneity by (18)F-fluoroestradiol PET as a predictive measure in breast cancer patients receiving palbociclib combined with endocrine treatment. Breast Cancer Res. 24, 57 (2022).
Qin, S. et al. A preclinical study: correlation between PD-L1 PET imaging and the prediction of therapy efficacy of MC38 tumor with (68)Ga-labeled PD-L1 targeted nanobody. Aging (Albany, NY) 13, 13006–13022 (2021).
Smit, J. et al. PD-L1 PET/CT imaging with radiolabeled durvalumab in patients with advanced-stage non-small cell lung cancer. J. Nucl. Med. 63, 686–693 (2022).
Kok, I. C. et al. (89)Zr-pembrolizumab imaging as a non-invasive approach to assess clinical response to PD-1 blockade in cancer. Ann. Oncol. 33, 80–88 (2022).
Niemeijer, A. N. et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 9, 4664 (2018).
Niemeijer, A. N. et al. Study of (89)Zr-pembrolizumab PET/CT in patients with advanced-stage non-small cell lung cancer. J. Nucl. Med. 63, 362–367 (2022).
Liu, F. Y., Yen, T. C., Wang, J. Y. & Yang, T. S. Early prediction by 18F-FDG PET/CT for progression-free survival and overall survival in patients with metastatic colorectal cancer receiving third-line cetuximab-based therapy. Clin. Nucl. Med. 40, 200–205 (2015).
Kahraman, D. et al. Tumor lesion glycolysis and tumor lesion proliferation for response prediction and prognostic differentiation in patients with advanced non-small cell lung cancer treated with erlotinib. Clin. Nucl. Med. 37, 1058–1064 (2012).
Huang, Y. E. et al. (18)F-Fluorodeoxyglucose PET/CT for early prediction of outcomes in patients with advanced lung adenocarcinomas and EGFR mutations treated with first-line EGFR-TKIs. Cancers (Basel) 14, 1507 (2022).
Bhoil, A. et al. Can 3’-deoxy-3’-(18)F-fluorothymidine or 2’-deoxy-2’-(18)F-fluoro-d-glucose PET/CT better assess response after 3-weeks treatment by epidermal growth factor receptor kinase inhibitor, in non-small lung cancer patients? Preliminary results. Hell. J. Nucl. Med. 17, 90–96 (2014).
Winther-Larsen, A., Fledelius, J., Sorensen, B. S. & Meldgaard, P. Metabolic tumor burden as marker of outcome in advanced EGFR wild-type NSCLC patients treated with erlotinib. Lung Cancer 94, 81–87 (2016).
Hwang, S. H. et al. Prognostic value of pretreatment metabolic tumor volume and total lesion glycolysis using 18F-FDG PET/CT in patients with metastatic renal cell carcinoma treated with anti-vascular endothelial growth factor-targeted agents. Clin. Nucl. Med. 42, e235–e241 (2017).
De Bruyne, S. et al. Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br. J. Cancer 106, 1926–1933 (2012).
Colavolpe, C. et al. FDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan. Neuro Oncol. 14, 649–657 (2012).
Park, J. S. et al. The prognostic value of volume-based parameters using (18)F-FDG PET/CT in gastric cancer according to HER2 status. Gastric Cancer 21, 213–224 (2018).
Kim, J. E. et al. 3’-Deoxy-3’-(18)F-fluorothymidine and (18)F-fluorodeoxyglucose positron emission tomography for the early prediction of response to Regorafenib in patients with metastatic colorectal cancer refractory to all standard therapies. Eur. J. Nucl. Med. Mol. Imaging 46, 1713–1722 (2019).
Nakamura, M. et al. (18)F-FDG-PET/CT as an imaging biomarker for regorafenib efficacy in metastatic colorectal cancer (JACCRO CC-12). Oncol. Ther. 9, 635–645 (2021).
Boers, J. et al. Molecular imaging to identify patients with metastatic breast cancer who benefit from endocrine treatment combined with cyclin-dependent kinase inhibition. Eur. J. Cancer 126, 11–20 (2020).
Seifert, R. et al. [18F]-Fluorodeoxyglucose positron emission tomography/CT to assess the early metabolic response in patients with hormone receptor-positive HER2-negative metastasized breast cancer treated with cyclin-dependent 4/6 kinase inhibitors. Oncol. Res. Treat. 44, 400–407 (2021).
Rinzivillo, M. et al. [(18)F]FDG-PET/CT and long-term responses to everolimus in advanced neuroendocrine neoplasia. J. Endocrinol. Investig. 44, 811–818 (2021).
Kaira, K. et al. Metabolic activity by (18)F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur. J. Nucl. Med. Mol. Imaging 45, 56–66 (2018).
Kitajima, K. et al. The utility of (68)F-FDG PET/CT for evaluation of tumor response to immune checkpoint inhibitor therapy and prognosis prediction in patients with non-small-cell lung cancer. Hell. J. Nucl. Med. 24, 186–198 (2021).
Xie, X. et al. Predictive value of positron emission tomography for the prognosis of molecularly targeted therapy in solid tumors. Onco Targets Ther. 11, 8885–8899 (2018).
Li, S. et al. Iodine-123-vascular endothelial growth factor-165 (123I-VEGF165). Biodistribution, safety and radiation dosimetry in patients with pancreatic carcinoma. Q. J. Nucl. Med. Mol. Imaging 48, 198–206 (2004).
Adkins, D. et al. A prospective trial comparing FDG-PET/CT and CT to assess tumor response to cetuximab in patients with incurable squamous cell carcinoma of the head and neck. Cancer Med. 3, 1493–1501 (2014).
Wedam, S. B. et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J. Clin. Oncol. 24, 769–777 (2006).
López-Vega, J. M. et al. Early imaging and molecular changes with neoadjuvant bevacizumab in stage II/III breast cancer. Cancers (Basel) 13, 3511 (2021).
Kim, J. H. et al. Dynamic contrast-enhanced MRI for response evaluation of non-small cell lung cancer in therapy with epidermal growth factor receptor tyrosine kinase inhibitors: a pilot study. Ann. Palliat. Med. 10, 1589–1598 (2021).
Syed, A. K. et al. Characterizing trastuzumab-induced alterations in intratumoral heterogeneity with quantitative imaging and immunohistochemistry in HER2+ breast cancer. Neoplasia 21, 17–29 (2019).
Heacock, L. et al. Dynamic contrast-enhanced MRI evaluation of pathologic complete response in human epidermal growth factor receptor 2 (HER2)-positive breast cancer after HER2-targeted therapy. Acad. Radiol. 27, e87–e93 (2020).
Chen, B. B. et al. Imaging biomarkers from multiparametric magnetic resonance imaging are associated with survival outcomes in patients with brain metastases from breast cancer. Eur. Radiol. 28, 4860–4870 (2018).
Chen, B. B. et al. Early changes in DCE-MRI biomarkers may predict survival outcomes in patients with advanced hepatocellular carcinoma after sorafenib failure: two Prospective Phase II Trials. Cancers (Basel) 13, 4962 (2021).
Khan, K. et al. Functional imaging and circulating biomarkers of response to regorafenib in treatment-refractory metastatic colorectal cancer patients in a prospective phase II study. Gut 67, 1484–1492 (2018).
Doemel, L. A. et al. Reliable prediction of survival in advanced-stage hepatocellular carcinoma treated with sorafenib: comparing 1D and 3D quantitative tumor response criteria on MRI. Eur. Radiol. 31, 2737–2746 (2021).
Ellingson, B. M. et al. Volumetric response quantified using T1 subtraction predicts long-term survival benefit from cabozantinib monotherapy in recurrent glioblastoma. Neuro Oncol. 20, 1411–1418 (2018).
Wirsching, H. G. et al. MRI and (18)FET-PET predict survival benefit from bevacizumab plus radiotherapy in patients with isocitrate dehydrogenase wild-type glioblastoma: results from the randomized ARTE Trial. Clin. Cancer Res. 27, 179–188 (2021).
Ellingson, B. M. et al. Diffusion MRI quality control and functional diffusion map results in ACRIN 6677/RTOG 0625: a multicenter, randomized, phase II trial of bevacizumab and chemotherapy in recurrent glioblastoma. Int. J. Oncol. 46, 1883–1892 (2015).
Kickingereder, P. et al. Noninvasive characterization of tumor angiogenesis and oxygenation in bevacizumab-treated recurrent glioblastoma by using dynamic susceptibility MRI: secondary analysis of the European Organization for Research and Treatment of Cancer 26101 Trial. Radiology 297, 164–175 (2020).
Schmainda, K. M. et al. Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro Oncol. 17, 1148–1156 (2015).
Lucas, J. T. Jr. et al. Posttreatment DSC-MRI is predictive of early treatment failure in children with supratentorial high-grade glioma treated with erlotinib. Clin. Neuroradiol. 28, 393–400 (2018).
Schmainda, K. M. et al. Value of dynamic contrast perfusion MRI to predict early response to bevacizumab in newly diagnosed glioblastoma: results from ACRIN 6686 multicenter trial. Neuro Oncol. 23, 314–323 (2021).
Tang, L. et al. MRI in predicting the response of gastrointestinal stromal tumor to targeted therapy: a patient-based multi-parameter study. BMC Cancer 18, 811 (2018).
Sheng, R. et al. Apparent diffusion coefficient MRI shows association with early progression of unresectable intrahepatic cholangiocarcinoma with combined targeted-immunotherapy. J. Magn. Reson. Imaging 57, 275–284 (2023).
Tang, L. et al. Non-Gaussian diffusion imaging with a fractional order calculus model to predict response of gastrointestinal stromal tumor to second-line sunitinib therapy. Magn. Reson. Med. 79, 1399–1406 (2018).
Ellingson, B. M. et al. Diffusion MRI phenotypes predict overall survival benefit from anti-VEGF monotherapy in recurrent glioblastoma: converging evidence from Phase II Trials. Clin. Cancer Res. 23, 5745–5756 (2017).
Liu, L. H. et al. Identifying response in colorectal liver metastases treated with bevacizumab: development of RECIST by combining contrast-enhanced and diffusion-weighted MRI. Eur. Radiol. 31, 5640–5649 (2021).
Ye, C. et al. Predictive effect of DCE-MRI and DWI in brain metastases from NSCLC. Open Med. (Wars.) 16, 1265–1275 (2021).
Zhu, H. B. et al. Prediction of therapeutic effect to treatment in patients with colorectal liver metastases using functional magnetic resonance imaging and RECIST criteria: a pilot study in comparison between bevacizumab-containing chemotherapy and standard chemotherapy. Ann. Surg. Oncol. 29, 3938–3949 (2022).
Patel, K. S. et al. Diffusion Magnetic Resonance Imaging Phenotypes Predict Overall Survival Benefit From Bevacizumab Or Surgery In Recurrent Glioblastoma With Large Tumor Burden. Neurosurgery 87, 931–938 (2020).
Schell, M. et al. Validation of diffusion MRI phenotypes for predicting response to bevacizumab in recurrent glioblastoma: post-hoc analysis of the EORTC-26101 trial. Neuro Oncol. 22, 1667–1676 (2020).
Wen, Q. et al. Comparison of ADC metrics and their association with outcome for patients with newly diagnosed glioblastoma being treated with radiation therapy, temozolomide, erlotinib and bevacizumab. J. Neurooncol. 121, 331–339 (2015).
Rhodes, C. J. Magnetic resonance spectroscopy. Sci. Prog. 100, 241–292 (2017).
Ratai, E. M. et al. Magnetic resonance spectroscopy as an early indicator of response to anti-angiogenic therapy in patients with recurrent glioblastoma: RTOG 0625/ACRIN 6677. Neuro-Oncology 15, 936–944 (2013).
Preul, M. C. et al. Using proton magnetic resonance spectroscopic imaging to predict in vivo the response of recurrent malignant gliomas to tamoxifen chemotherapy. Neurosurgery 46, 306–318 (2000).
Sankar, T. et al. Prospective serial proton MR spectroscopic assessment of response to tamoxifen for recurrent malignant glioma. J. Neurooncol. 90, 63–76 (2008).
Ros, S. et al. Metabolic imaging detects resistance to PI3Kalpha inhibition mediated by persistent FOXM1 expression in ER(+) breast cancer. Cancer Cell 38, 516–533.e519 (2020).
Akhenblit, P. J. et al. Assessing metabolic changes in response to mTOR inhibition in a mantle cell lymphoma xenograft model using AcidoCEST MRI. Mol. Imaging 15, 1536012116645439 (2016).
Yao, J. et al. pH-weighted amine chemical exchange saturation transfer echoplanar imaging (CEST-EPI) as a potential early biomarker for bevacizumab failure in recurrent glioblastoma. J. Neurooncol. 142, 587–595 (2019).
Park, J. E. et al. Identification of early response to anti-angiogenic therapy in recurrent glioblastoma: amide proton transfer-weighted and perfusion-weighted MRI compared with diffusion-weighted MRI. Radiology 295, 397–406 (2020).
Karayama, M. et al. Intravoxel incoherent motion magnetic resonance imaging for predicting the long-term efficacy of immune checkpoint inhibitors in patients with non-small-cell lung cancer. Lung Cancer 143, 47–54 (2020).
Chen, B. B. et al. Dynamic contrast-enhanced and intravoxel incoherent motion MRI biomarkers are correlated to survival outcome in advanced hepatocellular carcinoma. Diagnostics (Basel) 11, 1340 (2021).
Stadlbauer, A. et al. Vascular hysteresis loops and vascular architecture mapping in patients with glioblastoma treated with antiangiogenic therapy. Sci. Rep. 7, 8508 (2017).
Tsai, L. L. et al. Arterial spin labeled perfusion MRI for the evaluation of response to tyrosine kinase inhibition therapy in metastatic renal cell carcinoma. Radiology 298, 332–340 (2021).
Kim, B. et al. Liver stiffness in magnetic resonance elastography is prognostic for sorafenib-treated advanced hepatocellular carcinoma. Eur. Radiol. 31, 2507–2517 (2021).
Stadlbauer, A. et al. Predicting glioblastoma response to bevacizumab through MRI biomarkers of the tumor microenvironment. Mol. Imaging Biol. 21, 747–757 (2019).
Kudo, M. Gd-EOB-DTPA-MRI could predict WNT/beta-catenin mutation and resistance to immune checkpoint inhibitor therapy in hepatocellular carcinoma. Liver Cancer 9, 479–490 (2020).
Aoki, T. et al. Higher enhancement intrahepatic nodules on the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI as a poor responsive marker of anti-PD-1/PD-L1 monotherapy for unresectable hepatocellular carcinoma. Liver Cancer 10, 615–628 (2021).
Sasaki, R. et al. Evaluating the role of hepatobiliary phase of gadoxetic acid-enhanced magnetic resonance imaging in predicting treatment impact of lenvatinib and atezolizumab plus bevacizumab on unresectable hepatocellular carcinoma. Cancers (Basel) 14, 827 (2022).
Guo, C. et al. The G1 phase optical reporter serves as a sensor of CDK4/6 inhibition in vivo. Int. J. Biol. Sci. 17, 728–741 (2021).
Han, M. G. et al. Combination of OX40 Co-stimulation, radiotherapy, and PD-1 inhibition in a syngeneic murine triple-negative breast cancer model. Cancers (Basel) 14, 2692 (2022).
Yasaman et al. In vivo assessment of HER2 receptor density in HER2-positive tumors by near-infrared imaging, using repeated injections of the fluorescent probe. Technol. Cancer Res. Treat. 13, 427–434 (2014).
Hassan, M. et al. In vivo method to monitor changes in HER2 expression using near-infrared fluorescence imaging. Mol. Imaging 11, 177–186 (2012).
Terwisscha van Scheltinga, A. G. et al. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J. Nucl. Med. 52, 1778–1785 (2011).
Backer, M. V. et al. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol. Cancer Ther. 4, 1423–1429 (2005).
Ke, S. et al. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 63, 7870–7875 (2003).
Miao, Z. et al. Cy5.5-labeled Affibody molecule for near-infrared fluorescent optical imaging of epidermal growth factor receptor positive tumors. J. Biomed. Opt. 15, 036007 (2010).
Zhao, P. et al. Molecular imaging of hepatocellular carcinoma xenografts with epidermal growth factor receptor targeted affibody probes. Biomed. Res. Int. 2013, 759057 (2013).
Gao, Y. Y. et al. In vivo visualization of fluorescence reflecting CDK4 activity in a breast cancer mouse model. MedComm (2020) 3, e136 (2022).
He, S. et al. Near-infrared fluorescent macromolecular reporters for real-time imaging and urinalysis of cancer immunotherapy. J. Am. Chem. Soc. 142, 7075–7082 (2020).
Zhong, Y. et al. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat. Biotechnol. 37, 1322–1331 (2019).
McGuinness, J. E. et al. Diffuse optical tomography breast imaging measurements are modifiable with pre-surgical targeted and endocrine therapies among women with early stage breast cancer. Breast Cancer Res. Treat. 189, 297–304 (2021).
Lin, L. & Wang, L. V. The emerging role of photoacoustic imaging in clinical oncology. Nat. Rev. Clin. Oncol. 19, 365–384 (2022).
Yang, J. et al. Photoacoustic imaging for the evaluation of early tumor response to antivascular treatment. Quant. Imaging Med. Surg. 9, 160–170 (2019).
Quiros-Gonzalez, I. et al. Photoacoustic tomography detects response and resistance to bevacizumab in breast cancer mouse models. Cancer Res. 82, 1658–1668 (2022).
Bohndiek, S. E. et al. Photoacoustic tomography detects early vessel regression and normalization during ovarian tumor response to the antiangiogenic therapy trebananib. J. Nucl. Med. 56, 1942–1947 (2015).
Pham, E. et al. Preclinical efficacy of bevacizumab with CRLX101, an investigational nanoparticle–drug conjugate, in treatment of metastatic triple-negative breast cancer. Cancer Res. 76, 4493–4503 (2016).
Zhang, J., Duan, F., Liu, Y. & Nie, L. High-resolution photoacoustic tomography for early-stage cancer detection and its clinical translation. Radiology 2, e190030 (2020).
Luengo Morato, Y. et al. Recent advances in multimodal molecular imaging of cancer mediated by hybrid magnetic nanoparticles. Polymers (Basel) 13, 2989 (2021).
Tsoukalas, C. et al. A novel metal-based imaging probe for targeted dual-modality SPECT/MR imaging of angiogenesis. Front. Chem. 6, 224 (2018).
Lin, R. et al. Bevacizumab and near infrared probe conjugated iron oxide nanoparticles for vascular endothelial growth factor targeted MR and optical imaging. Biomater. Sci. 6, 1517–1525 (2018).
Chen, J. et al. Thin layer-protected gold nanoparticles for targeted multimodal imaging with photoacoustic and CT. Pharmceuticals (Basel) 14, 1075 (2021).
Beppu, T. et al. MRI and 11C-methyl-l-methionine PET differentiate bevacizumab true responders after initiating therapy for recurrent glioblastoma. Clin. Nucl. Med. 41, 852–857 (2016).
Lombardi, G. et al. PET/MR in recurrent glioblastoma patients treated with regorafenib: [(18)F]FET and DWI-ADC for response assessment and survival prediction. Br. J. Radiol. 95, 20211018 (2022).
Jarrett, A. M. et al. Towards integration of (64)Cu-DOTA-trastuzumab PET-CT and MRI with mathematical modeling to predict response to neoadjuvant therapy in HER2 + breast cancer. Sci. Rep. 10, 20518 (2020).
Mu, W. et al. Non-invasive decision support for NSCLC treatment using PET/CT radiomics. Nat. Commun. 11, 5228 (2020).
Chen, W. et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J. Clin. Oncol. 25, 4714–4721 (2007).
Schwarzenberg, J. et al. 3’-deoxy-3’-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J. Nucl. Med. 53, 29–36 (2012).
Sohn, H. J. et al. [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin. Cancer Res. 14, 7423–7429 (2008).
Zhou, X. et al. First-in-humans evaluation of a PD-L1-binding peptide PET radiotracer in non-small cell lung cancer patients. J. Nucl. Med. 63, 536–542 (2022).
Thukral, A. et al. Inflammatory breast cancer: dynamic contrast-enhanced MR in patients receiving bevacizumab-initial experience. Radiology 244, 727–735 (2007).
Schmainda, K. M. et al. Quantitative Delta T1 (dT1) as a replacement for adjudicated central reader analysis of contrast-enhancing tumor burden: a subanalysis of the American College of Radiology Imaging Network 6677/Radiation Therapy Oncology Group 0625 Multicenter Brain Tumor Trial. Am. J. Neuroradiol. 40, 1132–1139 (2019).
Huang, S. et al. Assessment of tumor hypoxia and perfusion in recurrent glioblastoma following bevacizumab failure using MRI and (18)F-FMISO PET. Sci. Rep. 11, 7632 (2021).
El-Abtah, M. E. et al. Myo-inositol levels measured with MR spectroscopy can help predict failure of antiangiogenic treatment in recurrent glioblastoma. Radiology 302, 410–418 (2022).
Boxerman, J. L. et al. Prognostic value of contrast enhancement and FLAIR for survival in newly diagnosed glioblastoma treated with and without bevacizumab: results from ACRIN 6686. Neuro Oncol. 20, 1400–1410 (2018).
Bahrami, N. et al. Edge contrast of the FLAIR hyperintense region predicts survival in patients with high-grade gliomas following treatment with bevacizumab. Am. J. Neuroradiol. 39, 1017–1024 (2018).
Field, K. M. et al. The role of early magnetic resonance imaging in predicting survival on bevacizumab for recurrent glioblastoma: results from a prospective clinical trial (CABARET). Cancer 123, 3576–3582 (2017).
Nowosielski, M. et al. Imaging necrosis during treatment is associated with worse survival in EORTC 26101 study. Neurology 92, e2754–e2763 (2019).
Liu, T. T. et al. Magnetic resonance perfusion image features uncover an angiogenic subgroup of glioblastoma patients with poor survival and better response to antiangiogenic treatment. Neuro Oncol. 19, 997–1007 (2017).
Beuselinck, B. et al. Whole-body diffusion-weighted magnetic resonance imaging for the detection of bone metastases and their prognostic impact in metastatic renal cell carcinoma patients treated with angiogenesis inhibitors. Acta Oncol. 59, 818–824 (2020).
Hudson, J. M. et al. The prognostic and predictive value of vascular response parameters measured by dynamic contrast-enhanced-CT, -MRI and -US in patients with metastatic renal cell carcinoma receiving sunitinib. Eur. Radiol. 28, 2281–2290 (2018).
Ocal, O. et al. Gadoxetic acid uptake as a molecular imaging biomarker for sorafenib resistance in patients with hepatocellular carcinoma: a post hoc analysis of the SORAMIC trial. J. Cancer Res. Clin. Oncol. 148, 2487–2496 (2022).
Ocal, O. et al. Prognostic value of baseline imaging and clinical features in patients with advanced hepatocellular carcinoma. Br. J. Cancer 126, 211–218 (2022).
Mo, Z. et al. Gd-EOB-DTPA-enhanced magnetic resonance imaging may help identify patients with hepatocellular carcinoma eligible for treatment targeted at RAF1. Abdom. Radiol. (NY) 47, 209–220 (2022).
Dong, Z. et al. Prediction of sorafenib treatment-related gene expression for hepatocellular carcinoma: preoperative MRI and histopathological correlation. Eur. Radiol. 29, 2272–2282 (2019).
Andronesi, O. C. et al. Early changes in glioblastoma metabolism measured by MR spectroscopic imaging during combination of anti-angiogenic cediranib and chemoradiation therapy are associated with survival. NPJ Precis. Oncol. 1, 20 (2017).
Henderson, S. A. et al. Breast cancer: influence of tumour volume estimation method at MRI on prediction of pathological response to neoadjuvant chemotherapy. Br. J. Radiol. 91, 20180123 (2018).
van Ramshorst, M. S. et al. MRI predicts pathologic complete response in HER2-positive breast cancer after neoadjuvant chemotherapy. Breast Cancer Res. Treat. 164, 99–106 (2017).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 32171363, 82103614, and 81901801), Fujian Major Scientific and Technological Special Project for “Social Development” (No. 2020YZ016002), Natural Science Foundation of Fujian Province of China (No. 2021J05007), Natural Science Foundation of Guangdong Province (No. 2021A1515011180), Xiamen Municipal Bureau of Science and Technology (Nos. 3502Z20194040 and 3502Z20209101), Research Fund of Fujian Key Laboratory of Precision Diagnosis and Treatment in Breast Cancer & Xiamen Key Laboratory of Endocrine-Related Cancer Precision Medicine (XKLEC2020KF01), Youth Fund of Xiang’an Hospital of Xiamen University (PM202103050001), Xiamen’s Key Laboratory of Precision Medicine for Endocrine-Related Cancers, and start-up fund from Xiamen University and Shantou Central Hospital Research Incubation Program (201905). We thank Drs. Kang-Lang Lou, Min Wei, Yi-Yang Gao, Xue-Qi Fan, Qiu-Min Deng, Yi-Fei Pei, Hong-Yu Chen, Zhong Luo, Xiao Shen, and Cheng-Xi Li for their kind help in this study.
Author information
Authors and Affiliations
Contributions
G-JZ designed the review and revised the draft. J-WB and S-QQ drafted the manuscript and prepared the tables and figures. All authors have read and approved the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bai, JW., Qiu, SQ. & Zhang, GJ. Molecular and functional imaging in cancer-targeted therapy: current applications and future directions. Sig Transduct Target Ther 8, 89 (2023). https://doi.org/10.1038/s41392-023-01366-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-023-01366-y
This article is cited by
-
Latest advances in hepatocellular carcinoma management and prevention through advanced technologies
Egyptian Liver Journal (2024)
-
A mathematical model for simulating photoacoustic signal generation and propagation in biological tissues
Optical and Quantum Electronics (2024)
-
Revolutionizing bone tumor management: cutting-edge breakthroughs in limb-saving treatments
European Journal of Orthopaedic Surgery & Traumatology (2024)