Abstract
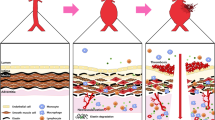
Aortic aneurysm is a chronic aortic disease affected by many factors. Although it is generally asymptomatic, it poses a significant threat to human life due to a high risk of rupture. Because of its strong concealment, it is difficult to diagnose the disease in the early stage. At present, there are no effective drugs for the treatment of aneurysms. Surgical intervention and endovascular treatment are the only therapies. Although current studies have discovered that inflammatory responses as well as the production and activation of various proteases promote aortic aneurysm, the specific mechanisms remain unclear. Researchers are further exploring the pathogenesis of aneurysms to find new targets for diagnosis and treatment. To better understand aortic aneurysm, this review elaborates on the discovery history of aortic aneurysm, main classification and clinical manifestations, related molecular mechanisms, clinical cohort studies and animal models, with the ultimate goal of providing insights into the treatment of this devastating disease. The underlying problem with aneurysm disease is weakening of the aortic wall, leading to progressive dilation. If not treated in time, the aortic aneurysm eventually ruptures. An aortic aneurysm is a local enlargement of an artery caused by a weakening of the aortic wall. The disease is usually asymptomatic but leads to high mortality due to the risk of artery rupture.
Similar content being viewed by others
Discovery of aneurysms
Galen is credited with being the first to define and describe aneurysms, mainly those that are visible and palpable on the surface of the body, while deep-seated aneurysms are the most common. In the 2nd century, Antyllos was a pioneer in the field of aneurysm surgery and discovered the differences between false and true aneurysms. Aetius, in the 6th century, described the clinical symptoms of aneurysms and revealed that they could occur anywhere in the body, even the head. Before the 16th century, aneurysm research seems to have stalled. Aortic aneurysms (AA) were first described by Saporta in 1554 and first diagnosed by Vesalius the following year. Subsequently, from the 17th century to the 18th century, many scholars explored the pathological features and etiology of aneurysm formation, mainly including the following: (1) Sennertus in 1628 believed that it was the rupture of the inner coat that led to the expansion of the external coat; (2) Iseman in 1676 dismissed this idea, arguing that aneurysms were formed mainly because of a rupture in the aortic wall that allows blood to diffuse to extravasation tissues; and (3) Bourdelot discovered nontraumatic aneurysms in 1681, but aneurysms formed by external trauma remained the most common and familiar. Lancisi, in 1728, was the first to mention a congenital vascular defect as a possible cause of vessel dilation under pressure. Alexander Monro in 1733 described the difference between true and false aneurysms in forming vascular wall damage but mistakenly believed that true aneurysm was rare and did not recognize the phenomenon of elastic tissue being replaced by fibrous tissue in the early stages of an aneurysm. Home and John Hunter believed that preexisting arterial disease was the cause of aneurysm. Donald Monro, reflecting on a macroscopic dissection in 1760, said that aneurysms were not caused by the rupture of the inner layer and the expansion of the outer layer but by the expansion of all layers. In the 19th century, due to the advent of the microtome and microscope as well as the pathologist, the world had a deeper understanding of the pathological mechanism of aneurysm. Welch isolated syphilis from aneurysmal tissue in 1875, thinking that syphilis was an additional factor in the etiology of aneurysms. Coats and Auld in 1893 discovered early aneurysms developed by the blood passing through atheromatous ulcers, and elastic fibers were found to break suddenly or disappear gradually by the staining method.1 Until the 20th century, atherosclerosis and syphilis were recognized as the two most important causes of aneurysm.2
In the past 30 years, considerable progress has been made in the research of the pathogenesis of aneurysms under multidisciplinary efforts involving molecular and cellular biology and solid and fluid mechanics.3 Throughout a healthy person’s life, the active components of the aortic wall must be constantly regenerated and modified to maintain the integrity and function of the system and to withstand repeated wall stresses. Unfortunately, in some cases, this perfectly stable system becomes destabilized by disease or other complex processes, and part of the aortic wall can permanently weaken and swell, forming an aneurysm, primarily in the abdominal and thoracic portions of the aorta and in the intracranial artery surrounding the circle of Willis. Aneurysms rupture when the dilated wall of an artery cannot support the stress created by the flow of blood inside. Ruptured aneurysms often result in sudden death or severe disability.3 The exact cause of the disease is still unknown, but a widely proposed hypothesis has been that specific changes in the hemodynamic forces acting on the aortic walls are a key contributor to the origin and progression of the disease.4,5
Classification
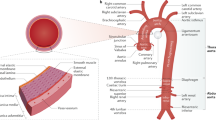
According to where they occur, aortic aneurysms (AAs) are often classified as abdominal aortic aneurysms (AAAs), thoracic aortic aneurysms (TAAs), intracranial aneurysms, and so on. Since smooth muscle cells in the thoracic aorta originate from the neural crest and the somitic mesoderm, whereas smooth muscle cells in the abdominal aorta originate from the splanchnic mesoderm, this distinction leads to a different pathogenesis of TAA versus AAA.6
A dissecting aneurysm, also called aortic dissection (AD), is caused by rupture of the aorta, which flows from the inner hole to the middle layer, causing the wall to be disformed and the blood flow to extend along the longitudinal axis.7 Clinical classification of AD and AA are mainly formed on the basis of anatomical location. AD is more likely to occur in the descending aorta with aortic dilatation, which may be because parts of vessels are differentiated from cells of different layers during embryonic development.8 According to the De Bakey fractal, AD is divided into the following three types. Type I lesions extend from the ascending aorta to the abdominal aorta, and the lining of the wall is much closer to the ascending aorta. Aortic valve closure is often caused by aortic valve expansion. Type II: the disease is limited to the ascending aorta, which extends to the beginning of the unknown artery. The endometrium is often above the aortic valve, and the aortic valve is not fully closed. Type IIIa: the aorta of the lesion from the lower artery of the left clavicle to the upper lobe of the diaphragm. Type IIIb: lesions from the descending aorta to the abdominal aorta and the iliac artery. According to the Stanford type, it can be divided into two types: Type A: the aortic wall and the ascending aorta are removed; Type B: aortic wall stripping begins with the lower artery opening of the left clavicle, which extends to the descending aorta9 (Fig. 1). Because of the heterogeneity in the segmental development of the aorta, the pathogenesis and targeted treatment of AA in different parts of the aorta needs further exploration to achieve better therapeutic results.
Etiology and risk factors
Etiology
Most AAAs are nonspecific, meaning there is no known cause.10 A few aneurysms have a clear etiology and are secondary to other diseases, such as atherosclerotic disease, trauma, connective tissue disease (Marfan Syndrome, Ehlers‒Danlos Type IV), infectious disease (tuberculosis, syphilis, bacteria, fungi), and inflammatory diseases.11
The causal relationship between aneurysm and atherosclerosis has always been controversial. Atherosclerotic diseases and AA have similar risk factors, and atherosclerotic lesions are considered to be the cause of some aneurysms. To address this issue, Dwayne Reed conducted a 20-year follow-up of more than 8000 Japanese men in Hawaii, accumulated clinical and autopsy data, and analyzed the causality of atherosclerosis in the development of AAs. From a preventive perspective, risk factors for aortic atherosclerosis and atherosclerosis itself appear to be essential for causal pathways for the vast majority of AAs in this cohort.12 In recent years, an increasing number of studies have suggested that atherosclerotic obstructive disease and AA should be distinguished. Xu et al. evaluated the relationship between atherosclerotic plaque deposition and aortic wall reaction and the development of aneurysmal and occlusive disease of the inferior renal aorta. Morphologic differences at five standardized locations in the infrarenal aorta in 67 male cadaver aortas were studied. The results indicate that there may be different local responses to atherosclerosis in humans. Plaque deposition with local dilation, thinning of the middle membrane, and loss of the medial elastic plate may predispose this aorta to subsequent aneurysm formation. Plaque deposition without thinning of the middle membrane, loss of elastic plates, and dilation of the aortic wall may lead to lumen narrowing of the aorta in the event of continued plaque accumulation.13 The vascular response to atherosclerotic lesions is different and may degenerate into dilation or stenosis, with only a small percentage developing into aneurysms.
There could be permanent and abnormal dilation of the aortic wall after trauma to the aorta. CT showed local widening of the aorta with an irregular wall edge. The density of the hematoma on the plain scan was slightly higher than that in the aortic cavity, and the enhancement was relatively low. Different degrees of calcification could be seen between some aortic walls. A portion of blunt abdominal aortic trauma may produce pseudoaneurysm. In one study, 40 children aged 1–16 who experienced blunt abdominal trauma were analyzed. Major aortic injuries included complete aortic wall rupture among 12.5% of the subjects, 70% experienced endometrial transection, and 15% exhibited pseudoaneurysm formation. Symptomatic lesions and complete rupture should be repaired immediately. Peri-circumferential transplantation has a high risk of complications and should also be treated. Some endometrial transection and delayed pseudoaneurysm can be observed by clinical examination and imaging. Patients with the latter pathology should be operated on at any sign of deterioration.14
Infectious diseases such as syphilis and tuberculosis and bacterial and fungal infections can cause arteritis in the middle layer, where elastic fibers breakdown and dilate or form localized cystic hematomas. Inflammatory arteritis mainly includes Takayasu’s arteritis, giant cell arteritis and Behcet’s disease. Inflammatory aortitis is the main cause. Aortitis may develop into dilation or even aneurysm, with a high risk of rupture.15
Connective tissue diseases include Marfan Syndrome and Ehlers‒Danlos Type IV, and most of them are associated with aneurysm. Marfan syndrome is a systematic connective tissue disorder caused by mutations in the extracellular matrix protein fibrillin 1. Major clinical manifestations include proximal AA, lens dislocation, and long bone overgrowth. Fibrillin 1 is a major component of extracellular matrix microfibers. In patients with Marfan’s syndrome, the aorta demonstrates reduced elastin content and rupture of elastic fibers, resulting in aortic aneurysm or dissection.16
Risk factors
There are many risk factors for AA, including poor lifestyle habits and other chronic diseases, such as smoking, age, high blood pressure, chronic obstructive pulmonary disease, hyperlipidemia, and genetic factors, such as male sex, white race and family history.
Older men are more likely to have AA. Deaths from AA ruptures begin to increase significantly in people over age 65. The prevalence of AAA is six times higher in men than in women, with a 40% increase in risk every five years after age 65.17 Studies also found that Blacks, Hispanics, and Asians had a lower risk of AA than Whites and Native Americans.18,19
There is a strong clinical correlation between smoking and the occurrence and development of AA. Wilmink T.B.M. studied the exact effect of smoking, duration of smoking, and smoking cessation on the risk of developing AAA and found that smokers were 7.6 times more likely to develop aneurysms than nonsmokers, and former smokers were 3 times more likely to develop aneurysms than nonsmokers.20 The duration of smoking was significantly correlated with the risk of aneurysm.21 Among all populations, annual smoking increases the risk of rupture of AAA by 4% (95% CI 2%).20 According to these clinical observations, long-term smoking may be the most important environmental risk factor for the occurrence and development of AA, but the specific pathophysiological mechanism of smoking affecting the occurrence and development of AA is still not clear. Possible theories include disruption of collagen synthesis, altered expression of matrix metalloproteinases (MMPs), and oxidative stress.22
Hypertension is generally considered to be a risk factor for AA, and elevated mean blood pressure is considered to be an independent risk factor for aneurysm rupture, reflecting the ongoing hemodynamic burden on the aortic wall, which leads to wall weakness.23 In experimental animal models, hypertension accelerates the progression of experimental aneurysms by upregulating nuclear factor kappa-B (NF-κB) and erythroblast transformation specific (ETS). AAA was produced by elastase perfusion in hypertensive and normal rats. The size of AAA increased rapidly in hypertensive rats compared with normal rats. Western blot analysis showed that the expression of MMP-2, MMP-3, MMP-9, MMP-12 and intercellular adhesion molecules was increased in hypertensive AAA rats, accompanied by upregulation of NF-κB and ETS.24 However, the association between hypertension and aneurysm seems to be weak and needs further investigation.25,26
Hyperlipidemia is a risk factor for AA, whereas high HDL levels are a protective factor. Iribarren et al. reported that serum cholesterol increases (>240 mg/dl) were associated with an odds ratio (OR) of 2.82 for AAA (95% CI 2.13–3.72).27 Pleumeeker et al. reported a protective effect of elevated HDL in plasma on AA.28 Diabetes has a protective effect on AA, and Jonathan Golermer found that diabetes is negatively correlated with aneurysms.29 Ning et al. studied incident AAA according to baseline glycemic status. Diabetes was independently associated with a lower AAA risk. In addition, with a longer duration of diabetes, the inverse association was more evident.30 The possible mechanism is that diabetes mellitus (DM) can also alter the production, degradation and deposition of other glycosaminoglycans in the aorta, with additional consequences on extracellular matrix (ECM) remodeling as well as the structural and physical properties of the aortic wall.31 It can be observed that the etiology and risk factors for AA mostly come from other vascular-related diseases. Genetic factors and key pathogenic genes remain to be explored to establish patient pathogenic gene profiles so that AA screening and prevention in high-risk populations can be implemented (Fig. 1).
Clinical manifestations
Symptoms
Unruptured aneurysms are usually asymptomatic in most patients and are mostly detected during physical examination, especially in those with coronary, peripheral or cerebrovascular diseases.32 Unruptured AAAs may have complications such as distal embolization and acute thrombosis, but acute thrombosis is rare. There are also complications due to pressure on adjacent structures caused by swelling, including lumbar pain when the spine is compressed. Hydronephrosis of the ureter may also occur when the aneurysm is inflammatory or involves iliac bifurcation. Some asymptomatic aneurysms may be detected because of complications.
AAA rupture is a clinical emergency. The patient presents with severe abdominal or chest and back pain that cannot be relieved, accompanied by hypotensive shock, abdominal pulsing mass and other symptoms. This is the typical triad of ruptured AAA, but clinically, typical symptoms are often few, and only 25–50% of patients have this typical triad.33,34 The degree of shock varies depending on the location and size of the rupture and when the patient is admitted to the emergency department for evaluation. Rupture of the anterior lateral wall into the peritoneal cavity is more serious and usually causes death. In the event of a rupture of the posterior lateral wall into the retroperitoneal cavity, a small tear can temporarily seal the rupture, and initial bleeding is less likely. However, within a few hours, there will be a larger rupture. This two-phase evolution underscores the importance of the intermediate phase after the initial event, which applies to medical transfers and emergency repairs. Differential diagnosis is needed for acute myocardial infarction, kidney stones, and gastrointestinal diseases such as ulcerative perforation.35
Diagnosis
Most of the patients admitted to the emergency department complain of abdominal or chest and back pain, which requires imaging examination, such as ultrasound or computed tomography (CT), for diagnosis.
Routine abdominal ultrasound imaging is the gold standard for AAA diagnosis and monitoring in asymptomatic patients, and its diagnostic accuracy is close to 100%.36 Ultrasound has the advantage of a harmless, noninvasive, affordable portable scanner that can be widely used for screening.
CT is the imaging standard for AAA because it can detect aneurysms complicated by other vessels and provide a basis for the planning of surgical intervention.37 CT is also performed with 3D imaging and CT angiography, which provide additional anatomical details, so a CT examination is needed after an aneurysm is found to further determine subsequent therapeutic interventions.
Magnetic resonance imaging (MRI) allows observation of some of the structures adjacent to the aneurysm. MRI combined with magnetic resonance angiography (MRA) enables clear observation of other vessels. MRA uses nonnephrotoxic contrast agents (such as gadolinium). MRA is less harmful than conventional angiography, which uses nephrotoxic contrast agents and has also been used in the further evaluation of aneurysms. In addition to these traditional imaging techniques, functional imaging is also used to assess the pathophysiological pathways involved in aneurysms.
Positron emission tomography (PET) is a clinical imaging method for metabolic and molecular imaging. It uses fluorodeoxyglucose F18 (18F-FDG) as a tracer to identify areas of increased glycolysis, such as some inflammatory sites and tumors.38 Multiple studies have shown that the uptake of 18F-FDG in AAA is associated with inflammation and phagocyte infiltration, proteolytic activity of MMPs, and cellular and molecular signal transduction prior to rupture.39 However, 18F-FDG is nonspecific in the uptake of AAA, and AAA imaging with PET is still a challenge. Nonspecificity of symptoms and imaging dependence of diagnosis challenge the early detection of AA. New molecular targets found in metabolic and immune pathways may help with early screening and process monitoring of AA.
Mechanism
Vascular smooth muscle cells
Vascular smooth muscle cells (VSMCs) are the major component of the vessel wall and perform many functions while maintaining the vascular structure. The various changes in VSMCs are an important cause of AA formation.
VSMC phenotypic switch
During the formation of the embryonic vascular system, smooth muscle precursor cells are recruited into the vascular network composed of endothelial cells, which are further influenced by various cytokines, such as platelet-derived growth factor-BB (PDGF-BB) and transforming growth factor beta (TGF-β), and then differentiate into mature VSMCs. The ascending and descending aortas are of different origins.40 The VSMCs of the ascending aorta develop from the second heart field and the cardiac neural crest, and VSMCs of the descending aorta develop from mesodermal lineages. This difference leads to the distinction between TAA and AAA.41
VSMCs are highly plastic and can switch between two phenotypes. The contractile type has a prominent VSMCs phenotype, and the synthetic type has dedifferentiated properties. Contractile VSMCs are spindle-shaped and express high levels of contractile proteins such as α-smooth muscle actin (α-SMA), SM myosin heavy chain (SMMHC), smooth muscle 22α (SM22α), and calponin (CNN).42 The contractile phenotype is essential for the aortic wall and maintains aortic strength. In contrast, in the case of inflammation and injury, VSMCs switch to a synthetic phenotype with a strong proliferation and migration capacity, as well as a higher secretion of fibrosis-related proteins and inflammation-related proteins. The expression of osteopontin in the synthetic phenotype is increased, while the expression of contractile proteins is decreased.43,44 This process is regarded as a phenotypic switch. In addition to AA, many cardiovascular diseases, such as atherosclerosis and hypertension, are associated with VSMC phenotypic switching.
There are many factors that regulate the phenotypic switching of VSMCs. One of the most well-studied is TGF-β. There are three isoforms of TGF-β, which all have effects on cell differentiation, proliferation, and apoptosis.45 Stimulation of VSMCs with TGF-β upregulates the mRNA and protein expression of α-SMA, SMMHC, and CNN while reducing proliferation.46 The mainstream view is that TGF-β is a protective factor for AAA. However, it has also been suggested that excessive inhibition of VSMC proliferation may weaken the structure of the aortic wall and lead to AA dilatation.47 There is a significant upregulation of TGF-β in individuals with AA.48 Therefore, the role of TGF-β is still controversial.
There are two common TGF-β pathways, both of which play an important role in AA. TGF-β has two receptors, TGF-β receptor (TGFBR)-1 and -2, which are downstream of decapentaplegic protein (SMAD)-dependent or non-SMAD-dependent pathways after binding to TGF-β. SMAD2 or SMAD3 is activated and phosphorylated by the TGF-β-TGF-βR complex, leading to SMAD4 nuclear ectopic, which in turn affects the transcription of downstream contractile proteins.49 In the non-SMAD-dependent pathway, TGF-β initiates the Ras Homolog Family Member A (RhoA) and mitogen-activated protein kinase (MAPK) cascades, the latter including extracellular signal-regulated kinase (ERK), Jun N-terminal kinase (JNK) and p38. TGF-β activates and phosphorylates them separately, which in turn affects the transcription of downstream contractile proteins.50,51 It has also been shown that TGF-β inhibits vascular chronic inflammation and attenuates AAA development by suppressing the activity of inflammation-related signaling pathways such as signal transducer and activator of transcription 3 (STAT3) and NF-κB.52
In Marfan’s syndrome, which is frequently associated with the development of AA, mutations in fibronectin 1 (FBN1) occur. FBN1 restricts TGF-β signaling. Hence, it is suggested that mutated FBN1 fails to bind to TGF-β, leading to TGF-β overexpression, which in turn promotes the development of AA.53 However, a different view has been proposed because in the mouse model of Marfan’s syndrome, an increase in TGF-β levels in the aorta was not found. Instead, physiological SMC TGF-β signaling protects against the AA associated with Marfan’s syndrome.54 Related studies are continuing. In addition, in Loeys‒Dietz syndrome with TGF-β receptor mutations, AA or AD has also been observed.55,56
Because of the important role of TGF-β, its related treatment is also under investigation. It has been shown that the neutralizing therapy of TGF-β (i.e., exogenous injection of TGF-β antibodies) can aggravate the progression of AA and promote AA rupture.57 It has also been demonstrated that specific blockade of TGF-β in VSMCs causes aortic wall thinning.58 Growth differentiation factor 11 (GDF11), a member of the TGF-β,59 cyclophilin A60 and vitamin B61 groups, can affect the formation and progression of AA through TGF-β-related pathways.
miRNAs are another common factor affecting VSMC phenotypic switching. Micro-RNAs are RNA strands of 19–24 nucleotides that regulate gene expression and function to repress gene expression by degrading messenger RNAs or mimicking small interfering RNAs (siRNAs) to inhibit translation. The decrease in miR-23b in AA leads to the upregulation of the transcription factor forkhead box O4, which in turn promotes VSMC switching to the synthetic phenotype and thus exacerbates the disease.62 The miR-143/145 cluster could inhibit the transcription of proteins such as Kruppel-like factor 4 (KLF4) and ETS Like-1 protein, which in turn switches VSMCs to a contractile phenotype.63,64
Other factors include mitochondrial protein and KLF4, all of which can affect the development of AA by regulating the phenotypic switch of VSMCs.65,66 However, the effects of all proteins are compounded and may affect VSMC phenotypic switching along with apoptosis and degradation of the ECM. Therefore, all the factors should be considered comprehensively.
VSMC death
In AA, a decrease in the number of VSMCs can be observed, which could further lead to a decrease in the ECM and weakening of the aortic wall.67 VSMCs are mainly apoptotic in AA. With research progress, other types of cell death, such as ferroptosis and pyroptosis, have been gradually identified.
The apoptosis of VSMCs can be found in the aortas of AA patients and AA model mice.68 Multiple factors can promote apoptosis of VSMCs. When macrophages infiltrate the aortic wall during AA, they express a large number of inflammatory factors, such as interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein-1 (MCP-1). Many of them promote VSMC apoptosis.69 Biomacromolecules such as oxidized low-density lipoprotein could also promote apoptosis. The TGF-β could inhibit apoptosis and play an AA protective role. In addition, endoplasmic reticulum (ER) stress occurs in VSMCs due to cytokine stimulation and mechanical stretch stimulation, which in turn cause programmed cell death.70,71 Therefore, targeting the apoptosis of VSMCs is one of the strategies to treat AA. Transcription factor EB (TFEB) can inhibit apoptosis but is downregulated in the tissues of AA patients. 2-Hydroxypropyl-β-cyclodextrin (HPβCD) can activate TFEB and inhibit AA in a mouse model and is a potential clinical therapeutic agent.72
Pyroptosis is an acute cell death mediated by the caspase family. Exogenous factors lead to the formation of inflammasomes, which in turn lead to the formation of pores on the cell membrane by the gasdermin (GSDM) family. Finally, cytokines such as IL-1β and IL-18 are released.73,74 In human and mouse AA tissues, the NOD-like receptor thermal protein domain-associated protein 3 (NLRP3)-Caspase-1 system is activated, indicating that pyroptosis may occur in VSMCs and Nlrp3-deficient mice exhibit a lower incidence of AA.75,76 However, the pyroptosis pathway is not the only pathway downstream of the inflammasome, and supporting evidence of VSMC-specific knockout animals is lacking. Therefore, the role of VSMCs in pyroptosis remains to be investigated.
Ferroptosis is a kind of programmed cell death dependent on iron. System Xc− and glutathione peroxidase 4 (GPX4) inhibition are the main causes of ferroptosis. System Xc− is a cystine/glutamate antiporter system that transfers glutamate out the cell and imports cystine to participate in the generation of glutathione (GSH).77,78 In addition, abnormal transferrin and iron regulatory protein 2 (IRP2) increase intracellular iron and disrupt iron homeostasis, which directly leads to reactive oxygen species (ROS) production.79 Ultimately, abnormal ROS accumulation leads to death accompanied by inactivation of essential enzymes and DNA damage.80 There are few studies related to ferroptosis in AA. It has been reported that the iron level, ferroptosis-related molecules transferrin receptor (TFR), heme oxygenase 1 (HOMX1), ferritin and the lipid peroxidation product 4-hydroxynonenal are increased in AD. The histone methyltransferase inhibitor BRD4770 has a protective effect against ferroptosis in VSMCs, which in turn attenuates AD in mice.81 Cigarette smoke extract could trigger AA by inducing ferroptosis in VSMCs and upregulating other cytokines, such as IL-6, MMP-2/9, and TNF-α, suggesting that VSMCs may promote the development of AA through ferroptosis.82
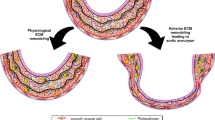
ECM changes
VSMCs are involved in the synthesis and processing of ECM. The ECM contains proteoglycans, glycoproteins, laminin, collagen, and elastic fibers that maintain the strength and elasticity of the vessel wall.83,84 In AA, ECM degrades and leads to the fragmentation and dilatation of the vessel wall.85 One of the main proteins involved in degradation is MMPs, and other proteases, such as a disintegrin and metalloproteinases (ADAMs), are also involved in this process.
Among the MMP family, MMP-2/9 is the most studied matrix metalloproteinase. High levels of MMP-2/9 expression can be observed in AA tissue. MMP-2 and MMP-9 are barely expressed in normal aortic tissue and are more highly expressed in early AAA than in late or ruptured AAA, suggesting that MMP-2 may be more critical for the early formation of AA.86 With inflammatory stimulation, the content of MMP-9 in aortic tissue is upregulated.87 Additionally, as the content of MMP-9 in aortic tissue increases, the content of protein hydrolases is also upregulated, which exacerbates the risk of AA rupture.88
The activity of MMPs is regulated by tissue inhibitor of matrix metalloproteinases (TIMPs). Four members of the TIMP family (TIMPs-1/2/3/4) can inhibit all MMPs and a large number of ADAMs through a covalent bond between their N-terminus and protease catalytic domains.89 AA has been observed in TIMP1 knockout mice90 and a significant decrease in TIMP-2 in the aortic wall of AAA patients.91 In a mouse model, aneurysms in TIMP-3 knockout mice were more prone to rupture, leading to death.92
VSMCs autophagy
Autophagy is a lysosome-mediated process that removes damaged proteins and organelles. Autophagy is regulated by highly conserved autophagy-related genes (ATGs). Impaired autophagy may lead to ER stress, ROS damage and cell death. Autophagy plays an important role in the physiological processes of VSMCs associated with aging and atherosclerosis.93 Autophagy has been found to be associated with VSMC phenotypic switching.94 In the aortic tissue of TAA patients, lower levels of autophagy could be observed.95 After VSMC-specific knockdown of the autophagy-related gene Atg5, an increased incidence of AA in mice with increased ER stress levels and upregulated inflammation was observed, and these phenomena were also verified in human AA tissues.96
The above section describes the role and mechanism of VSMCs in AA, such as phenotype switching, cell death, autophagy, and ECM regulation. As an important component of the aorta, VSMCs could receive more attention as a target for AA prevention and treatment.
Endothelial cells
Endothelial cells (ECs) are in the inner layer of the aorta and play a major role in maintaining aortic homeostasis. An increasing number of studies have shown that endothelial cell dysfunction is an important factor for AA.
Oxidative stress
In response to external stimulation, such as altered blood flow and inflammatory factors, ECs may undergo oxidative stress, which in turn leads to the accumulation of ROS. Endothelial nitric oxide synthases (eNOS) can produce nitric oxide (NO); in turn, NO promotes vasodilation. In addition to endothelial NOS (NOS3), there are also neuronal-derived NOS (nNOS, also called NOS1) and inducible NOS (iNOS, also called NOS2).97 However, in the absence of tetrahydrobiopterin (HB4) and the key enzymes GTP cyclohydroxylase 1 (GCH1) and dihydrofolate reductase (DHFR), uncoupling occurs, and superoxide O2− is produced.98,99 O2− has been found to be significantly increased in human AAA tissue. O2-exacerbates vascular oxidative stress levels, causing a remodeling of the ECM and apoptosis of VSMCs, which leads to exacerbation of AA.100 It has also been noted that O2– produced by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase can interact with NO to generate ONOOO-, an ion with strong oxidative properties that can cause further uncoupling of eNOS, which in turn leads to more severe oxidative stress.101 Adding HB4 or HB4-producing folic acid resulted in the remission of AA in a Marfan syndrome mouse model.102
In addition to eNOS, iNOS may also cause superoxide production in aortic tissue. When ROS are present, they induce upregulation of iNOS levels and produce excess NO to bind to superoxide O2-; the resulting ONOOO- amplifies the oxidative stress effect.101 It has also been found that excessive NO could be produced after activation of iNOS and may promote atherogenesis.103
Another important source of ROS in ECs is NADPH oxidases (NOXs). NOX is a complex containing several regulatory subunits, e.g., p40phox, p47phox, p67phox, rac1 and cytochrome b558 (containing a catalytic Nox subunit and a p22phox subunit). ECs mainly express NOX2 and NOX4. In stimulations with angiotensin (Ang) II and high glucose, endothelial NOX2 is activated, and ROS are generated, leading to endothelial dysfunction and vascular injury.104 It has been reported that mice overexpressing NOX2 produce more severe AD. ECs overexpressing NOX2 could increase ERK1/2 phosphorylation in VSMCs by secreting cyclophilin A (CypA), which in turn upregulates oxidative stress and inflammation in VSMCs.105 By using inhibitors to block the phosphorylated p47phox interaction with p22phox in NOX2, it is possible to attenuate AA progression in mice by inhibiting oxidative stress.106
Biomechanical stress
Biomechanical stress is an important factor affecting vascular homeostasis. Blood flow exerts a variety of forces on the aortic wall, including circumferential and longitudinal stresses as well as shear stress, which is divided into unidirectional laminar shear stress (LSS) and oscillatory shear stress (OSS). ECs are very sensitive to biomechanical stress and are stimulated in areas of stenosis and branching, where wall shear stress increases.107,108 This is why aneurysms often occur in the region of the aortic arch and infrarenal aorta.109 Shear stress activates the PKC pathway,110 JNK pathway,111 etc., in ECs through the mechanosensory complex platelet and endothelial cell adhesion molecule 1 (PECAM-1) on the cell membrane surface.112 The downstream pathways are activated, and one of the important effects is the level of eNOS. Shear stress promotes the synthesis of eNOS in ECs through factors such as scavenger receptor class B type 1 (SR-B1)113 and c-Src-tyrosine kinase.114 Then, eNOS promotes the production of NO. NO promotes EC diastole, which in turn reduces the damage caused by shear stress to the vessel wall. In mice with eNOS knockout, an exacerbation of AA was observed.115
Blood flow tension could also alter the protease expression and activity of ECs. OSS can promote MMP activity in the aortic wall while decreasing the level of TIMP-3.116,117 Additionally, high expression of cathepsin was detected in ECs of AA patients, suggesting that ECs can promote the progression of AA by degrading ECM.118 In addition, vascular endothelial growth factor (VEGF) and cell adhesion molecules vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) have been found to be upregulated in high shear stress, leading to gaps between ECs while increasing the adhesion of inflammatory cells to ECs and VSMCs.113,119,120
Intraluminal thrombus
Almost 75% of AAAs have intraluminal thrombus (ILT) formation,121 which has been mentioned in several articles and reviews.122,123,124 ILTs are usually accompanied by destruction of the adjacent endothelium and contain a large number of macrophages, neutrophils, erythrocytes and platelets, as well as a large number of proteases, such as MMPs.125,126,127 There are two opposing views on the role of ILT in AA in recent years. Some researchers believe that ILT creates a harmful environment that affects oxygen transport in the aortic wall and aggravates inflammation.128,129 However, some articles have also found that ILT may decrease aortic wall stress and play a buffering role from a biomechanical perspective.130,131,132 Researchers are now considering that both conditions occur simultaneously in ILT. Even though ILT alleviates aortic wall stress, insufficient oxygen transport and increased levels of inflammation produce thinning and degradation of the aortic wall in this region, which in turn promotes AA rupture. Overall, the effect of ILT on AA remains pathological rather than protective.127,133 Therefore, anticoagulant treatment could reduce ILT assessment and demonstration, but whether it can reduce the possibility of AAA rupture requires further study.134 Because of the property of ILT to promote AA rupture, several studies have shown that ILT measurement by CT or three-dimensional contrast-enhanced ultrasound can predict the risk of AAA rupture and could be used for clinical purposes.135 Similarly, data about AA, such as ILT, demonstrate that vessel radius and blood flow rate can be mined by deep learning and artificial intelligence techniques for AAA growth prediction, which can assist in diagnosis and treatment in the clinic.136
Immune cells and inflammatory factors
The immune response plays an important role in the course of AA. Immune cells not only secrete inflammatory factors that lead to apoptosis of aortic wall cells and VSMC phenotype switching but also secrete proteases that induce ECM degradation and lead to aortic dilation and rupture. In AA tissue, neutrophils, macrophages, natural killer (NK) and natural killer T (NKT) cells, T and B cells are infiltrated.137,138 Together, these cells contribute to the level of inflammation in the aorta from multiple perspectives.
Adaptive immunity
There is a large infiltration of immune cells in AA tissue, including T cells with B cells. T cells are mainly CD4 + T cells, which are the most abundant immune cells infiltrating AA.139 When CD4 + T cells are not present, the development of AA is significantly inhibited.140 Under different stimuli, CD4 + T cells can differentiate into different T cells, such as Th1 cells, Th2 cells, Th17 cells, regulatory T (Treg) cells and T follicular helper (Tfh) cells.141 All these T cells are involved in the formation of AA, and increased levels of TH1, TH17 and TH22 have been reported in vascular tissues of AA patients.142 There is an upregulation of Th17-, Th1-, Th9-, Th22- and Tfh-specific (TF) cells in the blood of AA patients and a downregulation of Th2 cells and Treg cells.143,144
The differentiation of TH1 cells depends on the stimulation of IL-12, which in turn secretes interferon-γ (IFN-γ) to promote the activation and recruitment of macrophages. Inhibition of IL-12 levels with antibodies could inhibit macrophages and thus AAA progression.145 TH17 cells are induced by stimulation of IL-23, IL-1, and IL-6 and promote macrophage activity by secreting IL-17.146 After knockdown of IL-17 in mice, the progression of AA was inhibited.147 Additionally, inhibition of AA was observed after inhibition of the pathway that activates TH17 cell differentiation.148
Th2 cells are considered anti-inflammatory in the current view. IL-4 stimulates their differentiation. Th2 cells secrete IL-4, IL-5, IL-10 and IL-13 through the STAT6 and GATA-3 pathways. IL-4 and IL-10 limit the cytotoxic potential of macrophages and reduce the expression of MMPs.149 Treg cells are specific CD4 + T cells that regulate other T-cell subsets and thus inhibit proinflammatory effects.150 Both IL-2 and TGF-β can stimulate Treg cells, which in turn increases the secretion of IL-10 and TGF-β, suppressing macrophage inflammation levels and clearing immune T cells.151 The process may also inhibit VSMC apoptosis by secreting trefoil factor 1 (TFF1), which in turn inhibits disease progression in AA.152 Downregulation of Treg cell levels was observed in both AA patients and AA mouse models.153,154
B cells play an important role in adaptive immunity by secreting antibodies. B cells mainly include B1, B2 and regulatory B cells.155 Although B cells account for only 4% to 5% of infiltrating cells in the AA vascular wall, B cells still play an important role. In mice lacking B cells and in mice antagonizing B cells with rituximab mimetic, reduced aortic immune cell infiltration and inflammation were observed, along with a reduction in AA symptoms.156,157 B2 cells are the predominant subtype of B cells. B-cell-activating factor (BAFF) can activate B-cell differentiation into the B2 type, and the use of BAFF antagonists can protect mice from AA disease.158 Since B cells mainly secrete antibodies, accumulation of these immunoglobulins in mouse AA tissues may induce secretion of IL-6 and MMP-9, damage the aortic wall, and aggravate AA disease.159 It has also been reported that anti-β2GPI IgG secreted by B cells may promote AA by causing hyperhomocysteinaemia.160
Neutrophils
Neutrophils are the most abundant cells in the human immune system and are the immune cells that respond fastest.161 In traditional studies, neutrophils accomplish the immune response through phagocytosis and degranulation. Neutrophils contain a large number of antimicrobial proteins, such as lysozyme, lactoferrin and cathepsin. These proteins are released in the pathogen or directly outside the cell after phagocytosis in neutrophils. Neutrophils are the main source of MMPs in AA.162 Neutrophil elastase (NE) released from neutrophils can exacerbate AAA by activating MMPs and inactivating its inhibitor TIMP. The use of neutrophil antibodies to induce neutropenia can effectively suppress AAA.163 In an elastase-induced AAA mouse model, ECs release the family with sequence similarity 3, member D (FAM3D), which promotes the recruitment of peripheral blood neutrophils to the abdominal aorta by activating the downstream protein kinase C (PKC), ERK, and p38MAPK pathways through the activation of membrane attack complex-1 (Mac-1) and formyl peptide receptor 2 (FPR2) in neutrophils, thereby inducing the development of AAA.164
A novel type of neutrophil killing called neutrophil extracellular traps (NETs) has been identified in the past two decades. NETs are neutrophils that enter a death program after stimulation by factors such as IL-1β, where the nuclear membrane ruptures and the nuclear contents are released into the cytoplasm. Finally, the plasma membrane ruptures, and the granule protein-modified chromatin is released outside the cell.165 These complexes contain DNA and histones with a variety of proteases, such as NE, cathepsin G, and myeloperoxidase. The involvement of NETs is crucial in the formation of AA, which can aggravate the development of AAA by degrading and weakening the vascular wall. In addition, NETs can activate NLRP3 in macrophages, which further release IL-1β and IL-18 and upregulate inflammation levels in the aorta.166,167 Citrullinated histone H3, a marker of NETs, is significantly upregulated in the plasma and arterial tissue of patients with AA and decreases after surgical repair of AA.168 NETs are formed in the first 2–3 days of AA formation in mice. Inhibition of NET production by drugs or promotion of NET degradation can effectively inhibit AAA development.169,170 It has also been shown that oxidized low-density lipoprotein (oxLDL) promotes the release of NETs, which in turn exacerbates AAA, while high-density lipoprotein (HDL) has little effect on the release of NETs.171
Macrophages
Circulating macrophages are the main source of macrophages in aortic tissue, and a small number of macrophages reside in the tissue during development.172,173,174 When local vascular tissue is stimulated, chemokines and inflammatory factors are released, and monocytes in the circulating blood are recruited into the vessel wall. When there are more monocytes in the circulating blood, the chance of AA development rises.175 Traditionally, monocytes are thought to be differentiated into two types of macrophages, M1 or M2, both of which are upregulated in AA tissues.176,177 M1 cells are known as proinflammatory macrophages and can be activated by cytokines such as lipopolysaccharide (LPS) or IFN-γ, which in turn secrete cytokines such as IL-6, TNF-α, MCP-1 that upregulate inflammation, as well as MMPs that breakdown ECM and weaken the aortic wall. M2-type macrophages are called anti-inflammatory macrophages. They can secrete TGF-β or IL-10 to exert anti-inflammatory effects and can repair the ECM.178,179 A high M1/M2 ratio can be observed in the aortas of AAA mice.180 Notably, Boytard et al. found lower levels of M2 cells and higher levels of M1 cells in the extravascular membrane at the AA site, but the opposite was found in the study of Dutertre et al.181,182. This may occur because M1- and M2-type cells are constantly transformed over the course of the disease. It is currently believed that in the early phase of AA, the M1/M2 ratio is high, which in turn produces vascular destruction. In the later phase of the disease, with hemodynamic changes and secretion of factors such as miRNA, macrophages polarize toward M2 to repair the vessels.183,184 This also reflects the phenomenon of interconversion of two roles of macrophages to maintain tissue homeostasis in the disease.174 In our laboratory, we have discovered that macrophages upregulate circulating levels of succinate, which is generated through the p38α-cAMP-response element-binding protein (CREB)-oxoglutarate dehydrogenase (OGDH) axis. Thereby, ROS were promoted, and the macrophages were converted to M1, which prompted AAD progression.185
Cytokines
Cytokines play an important role in AA. Cytokines are mainly secreted by immune cells and affect the level of inflammation in vascular tissue and the degradation of ECM. In a mouse model of AA, cytokines change significantly.186 We review several cytokines that are important in AA disease.
Interleukin (IL)-6
IL-6 is an important inflammatory factor involved in many proinflammatory processes in the body.187 IL-6 levels are significantly increased in vascular tissue as well as in circulating blood in AA patients, and circulating blood IL-6 levels may be proportional to the severity of the aneurysm.188,189,190 Animal experiments have demonstrated that IL-6 concentrations in vascular tissues in Ang-II-induced AA mice increase over time.191 The development of AA disease in mice could be inhibited after administration of monoclonal antibodies against the IL-6 receptor.192 Mechanistically, there are two signaling pathways for IL-6, namely, classical and trans-signaling. Classical signaling refers to IL-6 binding to the IL-6 receptor (IL-6R) on cells, and trans-signaling refers to IL-6 activation after binding to free soluble IL-6R, followed by binding to gp130 on a variety of cells.193 Subsequent activation of the STAT3 pathway promotes the differentiation of monocytes into macrophages, overactivation of VSMCs, and recruitment of macrophages, among other pathological changes in AA.194,195 The use of bazedoxifene can inhibit the IL-6/GP130/STAT3 signaling pathway and thus the progression of AA.196 However, it has also been shown that prophylactic IL-6 inhibition has little effect on the formation of AA.197
Interleukin (IL)-1β
IL-1β is an important factor in the regulation of infectious and aseptic inflammatory responses and is mainly produced by macrophages. Pro-IL-1β, the IL-1β precursor, is not biologically active but requires cleavage to IL-1β by caspase-1 to be active.198 Elevated levels of IL-1β have been observed in both the tissue and plasma of patients with AA disease.199,200 In mouse models, knockdown of IL-1β with IL-1R inhibits AA formation.201 In terms of therapeutic prospects, both the downregulation of IL-1β by antibodies and the inhibition of IL-1R with the antagonist anakinra inhibit the progression of AA.199,202 IL-1β plays a central role in mediating the inflammatory response and mediates the activation of secondary inflammatory factors such as IL-6.203 Mechanistically, IL-1β promotes the upregulation of EC adhesion factors, thereby recruiting immune cells.204,205 Meanwhile, IL-1β can degrade ECM and weaken aortic wall strength by promoting MMP-2/9 expression.206 Mechanistically, IL-1β stimulates the phosphorylation of the SMAD4 pathway in VSMCs, which in turn regulates the phenotypic switch of VSMCs.207 Meanwhile, IL-1β activates the NF-κB pathway and increases the levels of MMP-2/9 and IL-6.208
Tumor necrosis factor-α (TNF-α)
TNF-α is a potent proinflammatory cytokine that has been shown to be upregulated in patients with AA.209 A study of single nucleotide polymorphisms (SNPs) in healthy populations and AAA patients found that the TNF-α-238G/A gene was associated with an increased risk of AA development.210 In elastase-induced AA mice, the number of TNF-α-secreting macrophages was significantly increased.145 Animal experiments have demonstrated that TNF-α promotes the inflammatory response during aneurysm progression. Mechanistically, TNF-α deficiency inhibits macrophage polarization toward the M1 type. TNF-α deficiency also attenuates MMP-2 and MMP-9 expression by blocking macrophage activation.211,212 In AAA patients and in Ang-II-induced AA mice, TNF-α upregulated galectin-1 (Gal-1) in VSMCs and fibroblasts and subsequently induced MMP-9 expression.213 The use of klistatin inhibited TNF-α-induced oxidative stress and apoptosis, which in turn inhibited the development of AAA.214,215
Monocyte chemoattractant protein-1 (MCP-1)
MCP-1, also known as C-C motif chemokine ligand 2 (CCL2), is an important chemokine that rapidly promotes the adhesion of migrating macrophages to E-selectin expressed by ECs.216 In both mouse models and patients with AA, the expression of Mcp-1 in aneurysmal tissue was significantly elevated.217 Elevated levels of Mcp-1 in serum exacerbate AA in the atherosclerosis mouse model, and the process can be reversed by knockdown of its receptor C-C chemokine receptor type 2 (CCR2).218 In vitro experiments showed that Mcp-1 could upregulate the expression of Fas ligand (FasL) on the surface of macrophages, promote the cytotoxic effect of macrophages and cause autophagy in VSMCs.219 In VSMCs, Mcp-1 can induce the expression and activity of MMP-9 by activating the ERK1/2, p38MAPK pathway. It was found that hypoxia upregulates MCP-1 in VSMCs, in addition to causing macrophage chemotaxis and upregulating IL-6 expression in THP-1 cells, which in turn leads to apoptosis via STAT1 in VSMCs.220 On the other hand, Mcp-1 can also promote aneurysm repair.221 Slow-release Mcp-1 promoted carotid aneurysm growth and healing in mice in a dose-dependent manner. In this process, Mcp-1 upregulates the expression of inflammatory proteins such as macrophage inflammatory protein-1a/2 (MIP-1α, MIP-2) in macrophages and promotes the migration and aggregation of fibroblasts, macrophages, ECs, and VSMCs. Blocking MIP inhibits the repair function of Mcp-1, and the protein-coated cycle of Mcp-1 has been suggested as a possible treatment for AA.222
NOD-like receptor thermal protein domain-associated protein 3 (NLRP3)
NLRP3 is an innate immunity receptor that recognizes multiple stimuli from pathogens and injured or dead cells and is involved in the pathogenesis of aseptic inflammatory diseases.223 In response to stimuli such as pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), NLRP3 activates and leads to the assembly of the NLRP3 inflammasome, resulting in the activation of caspase-1, which in turn causes the secretion of IL-1β and IL-18.224 Activated caspase-1 also induces an inflammatory cell death process called pyroptosis by cleaving gasdermin D (GSDMD). The NLRP3 inflammasome consists of the sensor NLRP3, the adaptor apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), and the effector enzyme pro-caspase-1. NLRP3 is highly expressed in innate immune cells such as macrophages and neutrophils but also in nonimmune cells such as ECs, cardiomyocytes, fibroblasts, and epithelial cells, which in turn affect the pathological progression of cardiovascular diseases, including atherosclerosis, aneurysms, and vascular injury.225,226
Levels of NLRP3 inflammasome effector mediators are elevated in both clinical AAA patients and AAA animal models. As mentioned previously, IL-1β levels were elevated in the plasma of AAA patients. The mRNA and protein levels of IL-18 were also increased in the aorta of AAA patients compared with nonaneurysmal controls.227 In aneurysmal tissue, an earlier study by Schonbeck et al. showed elevated levels of caspase-1 in AAA patients. AA patients had elevated levels of NLRP3 mRNA in the aorta.228 It was also shown that high levels of plasma IL-1β were observed in individuals who were homozygous for the common C allele of NLRP3 rs35829419, suggesting that genovariation of the NLRP3 inflammasome may play an important role in the progression of AAA.229,230 Furthermore, direct knockdown of the NLRP3 inflammasome component was also able to significantly reduce the incidence and severity of AAA while decreasing the inflammatory response, including IL-1β secretion, MMP activation, and elastic lamina degradation.76
Recently, it was found that the NLRP3 inflammasome can also affect AAA through other pathways in addition to involvement in inflammatory factor secretion. Activated caspase-1 is able to directly bind and cleave contractile proteins of VSMCs in the aortic wall, such as tropomyosin and myosin heavy chain. Knockdown of NLRP3 and caspase-1 significantly reduced the degradation of these proteins and attenuated the formation of AA and AD in the Ang-II model fed a high-fat diet.75 In macrophages, caspase-1 activates MMP-9 by directly cleaving its n-terminal inhibitory domain. In wild-type mice fed a high-fat and high-cholesterol diet, treatment with the NLRP3 inhibitor MCC950 prevented aortic dilatation and dissection in different segments of the thoracic and abdominal aorta.231
Reactive oxygen species (ROS)
ROS and oxidative stress play an important role in the formation of AA, and a rise in oxidative stress has been observed in both patients and mice.232 ROS include small reactive ions and molecules such as superoxide (O2-) and hydrogen peroxide (H2O2). Low levels of ROS act as signaling molecules and are essential for maintaining normal vascular function. Uncontrolled overproduction of ROS exacerbates oxidative stress, leading to vascular cell injury, such as proliferation and migration of VSMCs, recruitment of inflammatory cells and activation of MMPs.233,234 In addition to the role in ECs described above, ROS promote the progression of AA in both macrophages and VSMCs. It has been reported that normal levels of H2O2 generated via NOX4 promote the differentiation of embryonic stem cells into VSMCs.235 In addition, in VSMC-specific overexpression of catalase in AA mouse models, a reduction in VSMC apoptosis and an attenuation of AA could be observed.236
The production of ROS is dependent on NOX, uncoupled eNOS (also known as NOS3), mitochondria and xanthine oxidase (XO).237 The main function of NOX is to produce ROS, so NOX is the most critical enzyme affecting ROS in AA.238 We have mentioned the role of NOX2 and NOX4 above. NOX1 and NOX2 only produce O2-, NOX4 only produces N2O2, while NOX5 produces both products.239 p22phox, p47phox, NOX2, NOX5 and p22phox expression levels are increased in AA patients.100,101 AA was suppressed after NOX1 knockdown or application of the NOX inhibitor apocynin, accompanied by a decrease in MMP-2/9 levels.240,241 Superoxide production and eNOS uncoupling activity were significantly reduced in NOX mutant mice and thus caused the suppression of AA.242
In addition to NOX, MPO is also involved in the production of ROS in AA. MPO causes H2O2 to react with Cl- to form HOCl-, an ion that reacts with various biomolecules, such as proteins, lipids, and nucleic acids, and causes oxidative damage.243 Since neutrophils are involved in the early infiltration of AA formation and neutrophils are the main source of myeloperoxidase production in the body, myeloperoxidase also plays an early role in influencing the level of oxidative stress.163 Both the knockdown of MPO and supplementation with taurine to inhibit MPO activity inhibit AA formation.244
The antioxidant system also plays a key role. In circulating neutrophils of AA patients, lower levels of catalase are detected.245 Tamoxifen inhibits AA formation by upregulating the level of catalase in the aorta.246 Superoxide dismutase (SOD) also scavenges ROS. Different results have been reported in tissues from AA patients, with both increases and decreases.247,248 This may be due to the different selection of control samples. An increase in SOD mRNA levels in the aorta of AA-molded rats has been observed in animal models.249
ROS are also of interest as therapeutic targets for AA. The administration of the antioxidant vitamin E reverses the formation of AA.250 Other natural antioxidants, such as polyphenols/flavonoids, have been shown to have antioxidant mitigating effects in cardiovascular diseases, and their effectiveness in treating AA is promising.251,252 In addition, drugs such as statins and irbesartan have also shown effects in scavenging ROS, and clinical studies are continuing.253,254
In this section, we review the possible mechanisms underlying the pathogenesis of AA. These include the VSMC phenotype switch, apoptosis, autophagy and effects on ECM; oxidative stress; the role of shear stress and ILT formation in endothelial cells; the release of various cytokines, such as IL-1/6, brought by immune cells; the release of granzyme and the formation of NETs in neutrophils; the proinflammatory phenotype switch of macrophages leading to increased levels of inflammation; and the involvement of ROS leading to aortic cell injury (Fig. 2). As research on AA mechanisms continues, we hope to examine different cells, such as fibroblasts, on AA and find more pathways as potential targets for AA therapy.
Clinical cohort study
Multiple clinical cohort studies of aneurysms help us understand the natural history of aneurysms and their relationships, such as sex, age, aneurysm size, aneurysm location, and risk of aneurysm rupture. We briefly summarize some of the information related to the clinical cohort of patients with aneurysms (Table 1).
Research on intracranial aneurysm
Greving et al.255 conducted a systematic review and pooled analysis of individual data from 8382 participants in six prospective cohort studies of subarachnoid hemorrhage to investigate predictors of aneurysm rupture. By developing a new risk scoring system for aneurysm rupture (Phase), researchers are better able to predict patients’ risk of aneurysm rupture. They observed that the average 1-year risk of aneurysm rupture was 1.4% and the average 5-year risk was 3.4%. Six factors, including age, hypertension, history of subarachnoid hemorrhage, aneurysm size, aneurysm location and geographic area, were associated with prognosis. Sex, smoking and multiple aneurysms during aneurysm detection were not helpful in predicting aneurysm rupture.256 The risk prediction of aneurysm rupture factors provides a good starting point for doctors and patients to discuss the pros and cons of treatment options.
Korja et al.257 followed 118 patients (61 women) diagnosed with unruptured intracranial aneurysms between 1956 and 1978 until death or the onset of subarachnoid hemorrhage (SAH). The median age of patients in this cohort at diagnosis of unruptured intracranial aneurysm was 43.5 years (range 22.6 to 60.7 years). The median size of unruptured intracranial aneurysms (UIAs) at diagnosis was 4 mm (range: 225 mm). The risk factors analyzed for rupture included sex, age, smoking, systolic blood pressure, diagnosed hypertension, UIA size, and number of UIAs. Subarachnoid hemorrhage occurred in 34 of 118 patients (29%). The annual rupture rate per patient was 1.6%. Female sex, smoking and an aneurysm diameter of 7 mm are risk factors for lifetime SAH.258 The risk value for aneurysmal subarachnoid hemorrhage ranges from 0% to 100%, and the annual rupture rate ranges from 0% to 6.5%, depending on the risk factors. Among 96 UIA patients with aneurysms less than 7 mm in diameter, 24 (25%) developed aneurysmal SAH during follow-up.259 This study is the first to determine the natural lifetime course of unruptured intracranial aneurysms (UIAs) and identify patients at high and low risk of rupture.
Mocco et al.260 analyzed the morphological features associated with aneurysm rupture by using a case‒control design in the international database of unruptured intracranial aneurysm studies. The cohort included 57 patients with ruptured aneurysms and 198 patients with unruptured intracranial aneurysms. Consistent with previous studies, larger aneurysm size261,262,263 and aneurysm location264,265,266 are important predictors of aneurysm rupture. In addition, vertical height (P = 0.008) and size ratio (the ratio of maximum diameter to mother vessel diameter; univariate analysis (P = 0.01)) were also predictors of aneurysm rupture. However, the length-diameter ratio, ascus, multilobe, aneurysm angle, cervical diameter, parent vessel diameter and calculated aneurysm volume had no statistical significance in predicting aneurysm rupture. In the multivariate analysis, vertical height was the only significant predictive factor (chi square 7.1, P = 0.008). Since this study is only a sample of the ISUIA cohort, its generalizability is limited. The entire ISUIA database needs to be reanalyzed to obtain more reliable results.
Goertz et al.267 analyzed 252 cases of ruptured and unruptured aneurysms treated with microsurgical clips from 2010 to 2018, including 148 cases of ruptured aneurysms and 104 cases of unruptured aneurysms, with an incidence of cerebral infarction of 17.1%. Further analysis found that both ruptured and unruptured aneurysms were independent risk factors for surgery-related infarction. The incidence and risk factors for cerebral infarction associated with microsurgical clipping of intracranial aneurysms have been discussed in a number of studies.262,268,269,270,271,272 Aneurysms with complex shapes are more likely to have infarction (P = 0.084). Similarly, the infarct rate of irregular neck aneurysms (37.5%) was significantly higher than that of normal neck aneurysms (10.1%, P < 0.001). In addition, irregular aneurysms of the neck were associated with higher rates of intraoperative rupture (P = 0.003) and temporary maternal artery occlusion (P = 0.037). In multifactorial analyses, irregular neck morphology was identified as an independent risk factor for infarction (P < 0.001), but there was no significant correlation between aneurysm shape and infarction (P = 0.966). The results of this study provide information on risk factors for cerebral infarction associated with microsurgical resection, which may be of assistance to neurosurgeons in their analysis and decision-making.
Laura et al.273 included 312 patients with growing aneurysms in the triple-S prediction model based on three independent rupture predictors (size, location, and shape) for the absolute risk of rupture of long intracranial aneurysms and found that the 1-year risk of rupture ranged from 2.1% to 10.6%. Consistent with previous studies, they also found that growing aneurysms had a higher risk of rupture than nongrowing aneurysms on subsequent imaging.257,274,275,276,277 The absolute risk of rupture within 1 year after growth testing was 4.3%. This study has implications for clinical practice. Once aneurysm growth is detected, preventive treatment of endovascular or neurosurgical aneurysms should be considered.
Wojciech et al.278 analyzed 147 patients who chose microsurgical clipping for intracranial aneurysms over a five-year period to determine the independent predictors of surgically derived complications (residual aneurysm and cerebral ischemia) and intraoperative aneurysm rupture. They found that an increase in aneurysm volume with a cutoff of 9 mm (P = 0.009; odds ratio [OR]: 0.644) and irregular dome shape (P = 0.003; OR: 4.242) were independently associated with cerebral ischemia and residual aneurysm in 13.6% and 17.3% of the cohort, respectively. Intraoperative rupture occurred in 27% of patients and was associated with patient age (P = 0.002; OR: 1.073), and the aneurysm volume of these patients continued to increase; the cutoff value was 7 mm (P = 0.003; Or: 1.205). The results of this study suggest that the risk of aneurysm rupture during surgery increases with age.
Most clinical cohort studies on intracranial aneurysms have focused on the risk assessment of different factors and aneurysm rupture. In summary, age, hypertension, unruptured aneurysm size, aneurysm location and aneurysm shape are consistently considered risk factors for aneurysm rupture. However, some analyses of some factors in different cohort studies also reached different conclusions. For example, Greving et al.239 excluded sex from the prediction model and believed that it had only limited predictive value. In contrast, in the clinical cohort of the Korja241 study, it was found that women with UIA sizes of more than 7 mm had a higher risk of life-long SAH. If women smoke and the UIA size is more than 7 mm, they will have an extremely high risk of rupture. The reason for the opposite conclusion may be that the sample size of the latter population was relatively small, and the heterogeneity of different populations was not considered in the prediction of disease progression, including gene differences, different living habits, and the use of drugs. In addition, sex is not valuable as an independent predictor in Greving’s study, while in Korja’s study, gender, smoking status and UIA size were comprehensively considered, so their interaction effect may be a reliable predictor. This suggests that when conducting clinical cohort analysis, we should try to collect patient information to exclude the influence of irrelevant variables.
The analysis of different clinical cohorts has its own limitations. In the study by Laura et al.,257 for example, patients with aneurysm growth detected by imaging may choose to receive prophylactic aneurysm treatment, which results in the selection of samples in the cohort favoring patients with a lower risk of rupture. It is also inevitable that researchers may miss some patients who were not diagnosed with SAH because of sudden death due to rupture or who went to another hospital after being diagnosed. These uncontrollable factors may affect the cohort analysis and should not be considered as a natural history study of aneurysm rupture risk after aneurysm growth. Furthermore, in some studies, it was not determined whether the patients had had subarachnoid hemorrhage in the past. Such patients have a relatively high risk of subarachnoid hemorrhage again, which may lead to overestimating the calculated risk.
Other clinical cohort studies
To study the clinical manifestations of the patients with thoracic aortic aneurysm and dissection in Taiwan and genetic features, Duan et al.279 recruited 107 patients, including known aneurysm or dissection in 57 cases, martensite characteristics in 36 cases, members of the family of suspected aortic aneurysm or dissection in 11 cases, 3 cases of ectopic lens, and 73 cases (68.2%) diagnosed as aneurysm or dissection. Clinical manifestations and gene sequencing (NGS) were performed in all patients. Skin distention was the only phenotype significantly associated with AA or dissection (adjusted p = 0.007) of all clinical manifestations. In addition, 46 patients (43.0%) in this clinical cohort had pathogenic genes/variants, including the most common FBN1, followed by TGFBR1, TGFBR2, and FBN2. Patients with positive gene findings had higher rates of dissection than those without aortic aneurysms. This finding is very similar to the observation of Wolford et al.280 that patients with pathogenic variants had significantly earlier dissection than those without variants. In conclusion, this study suggests that skin dilatation may be a simple and convenient screening condition for patients with thoracic aortic aneurysm and dissection. Multigene NGS detection can not only help with early diagnosis but also suggest that diagnosed patients with aortic aneurysm may be at risk of dissection.
Constance et al.281 selected 415 patients with aortic and peripheral aneurysms from the thesis-Express Biobank (a biobank consisting of surgically treated aortic and peripheral aneurysms) to study the association between genetic susceptibility to aneurysm variation and clinical phenotype. The mean age was 69 ± 8.1 years, the majority (85%) were male, and 349 (84%) had been treated. The clinical phenotypes they focused on included three clinical features: aneurysm sac diameter, artery type, and aneurysm-related symptoms. Using GWAS effect estimates from previous studies, a best-fitting polygenic risk score (PRS) model was developed for each clinical phenotype. The best fitted PRS (including 272 variants, PT = 0.01015) showed a significant association with aneurysm diameter (R2 = 0.019, P = 0.001). There was no evidence that polygenes were associated with clinical symptoms or arterial type. In addition, 10 genome-wide significant risk variants for AAA were separately tested, and no association with any clinical phenotype was observed. The models used were adjusted for confounding factors and data normalization. If the sample size can be increased, the potential causal role of susceptibility variation in the initiation and progression of aneurysm disease can be further confirmed.
Owing to the rarity of primary infection of aneurysms in the abdominal aorta and iliac arteries, the optimal treatment strategy remains unclear because of the unknown pathologic mechanism, even though the disease is potentially life-threatening. Hosaka et al.282 examined the medical records of Japanese patients who underwent surgical treatment for primary infection of aortic or common iliac artery (CIA) aneurysms from 2011 to 2017. A total of 862 patients were selected from the National Clinical Database (NCD) in Japan, of which approximately 30.2% were found to be infected. The cumulative overall survival rates were 94.0, 89.7%, 82.6, 74.9, and 68.5% at 30 days, 90 days, 1 year, 3 years and 5 years after the operation, respectively. Age, preoperative shock, and hypoalbuminemia were independently associated with early- and late mortality. Once-in-a-lifetime patient replacement (EVAR) is associated with more persistent or recurrent aneurysm-related infections than open repair (P < 0.001). Propensity score matching analysis showed no significant difference between EVAR and in situ graft replacement in 3-year all-cause mortality and aortic-related mortality (P = 0.093 and P = 0.472, respectively). The study could help treat this rare disease by collecting data from a large number of patients, although it may not accurately reflect the current reality of the disease.
Clinical cohort studies of other types of aneurysms include abdominal aortic aneurysms, thoracic aortic aneurysms, and peripheral aneurysms. In these cohort analyses, in addition to the study of rupture risk factors, the analysis of auxiliary diagnosis and treatment for early clinical manifestations, the construction of a multigene risk score model and the exploration of primary aneurysm infection266 were also included. Limited by the number of samples and population selection, although these studies may have errors or conclusions that may not be suitable for all populations, they can still help clinical disease judgment.
In summary, an important link in the study of disease phenotypes is the construction of a clinical cohort. Medical evidence is needed for the diagnostic criteria, traceability, and prognostic research of chronic diseases.283 Therefore, it is important and meaningful to select more representative samples for clinical cohort studies. Research design schemes applied to different continents, races, and countries tend to draw different conclusions because the disease is influenced by external factors, their mutual influence and multiple gene regulation. If the sample is too small or comes from the same region, conclusions are often not universal. When collecting information from patients and healthy people, strict and uniform standards should be adopted, and quality management should be implemented during the study. Privacy protection and secure storage of the collected data should be considered. Clinical cohort studies can also integrate multiple studies. On the one hand, large samples of clinical data can be obtained, and on the other hand, the time and financial resources spent on data collection can be saved. At present, researchers have proposed that the combination of longitudinal cohort studies and multiomics analysis can greatly improve the effectiveness of disease research, target search and marker discovery. In addition, with the development of big data and the use of internet technology to build a network platform for clinical cohort studies, sharing data also promotes the development of clinical cohort studies.
Therapy
At present, there is no effective drug for the prevention or treatment of AA. Although in the past few decades, studies have found that angiotensin II converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs) and β-blockers may have some effect on the growth of AAA.284 However, long-term clinical trials have shown no significant effect compared with placebo. Another statin used to target aortic inflammation is also gradually proving limited efficacy. Therefore, in clinical practice, the most effective treatment for patients with AA is still open surgical repair or endovascular aortic aneurysm repair (EVAR). Generally, when the diameter of the aneurysm is greater than 5.5 cm, surgery is required. However, due to the small diameter of the original abdominal aorta in women, treatment is often considered when it is greater than 5 cm.285 Open surgical treatment is performed by opening the aneurysm and placing an artificial blood vessel in it and fixing it in the normal vessel wall. In addition to midline abdominal incision to expose AAA, some chirurgeons make incisions outside the peritoneum from the left side, but whether the latter would reduce the incidence of postoperative intestinal obstruction, pulmonary complications, cardiovascular complications and fluid transfer needs further research.286 Moreover, because patients with aneurysm are at high risk of cardiovascular disease, the cardiac function of patients should be evaluated before surgery. Some studies have found that the perioperative mortality of AAA surgery is significantly related to the preoperative cardiac function of patients. For some patients with poor cardiac function, the mortality rate will be significantly increased.
EVAR is performed by directing blood flow through placement of a covered stent, during which the aneurysm remains intact. The stent was fixed in a segment of the normal aorta below the renal aorta and extended into the normal segment of the iliac artery.287 Compared with open surgical repair, EVAR is equivalent to intermediate to low grade surgery and has a lower perioperative mortality. However at the same time, the operation also has more stringent requirements for patients. First, because the stent needs to be fixed below the renal aorta, it must be ensured that there is at least 1.5 cm of normal aorta below the renal aorta as the anchoring area, and the diameter of the tumor should be within 28 mm. Second, because the stent passes through the external iliac and femoral arteries, the arteries there are large enough in diameter to accommodate the stent. For this reason, the proportion of women giving up EVAR due to the small diameter of external iliac artery is higher than that of men.288 Although the procedure is associated with lower mortality, the patient’s heart and other organ function should be evaluated before the procedure. For patients with AAA, the choice of surgical methods should be judged according to their own actual conditions,37 and clinicians should carefully consider taking more effective treatment methods on the premise of ensuring safety.
As a research hotspot in recent years, nanoparticles (NPs) have also been applied to the treatment of AA. The cell adhesion molecule α(v)β integrin, a marker of neovascularization, is a highly expressed receptor and can be used as a target of arginine-glycine-aspartic acid (RGD) peptides.289 Based on this, Kitagawa et al. used recombinant human ferritin with RGD (RGD-HFN) and encapsulated superparamagnetic iron oxide nanoparticles (SPION) to target AA lesions.290 Camardo et al. previously demonstrated that cathepsin K is overexpressed in aneurysm tissue and can be used as a potential target, so they combined matrix regenerated PEG-PLGA nanoparticles with antibodies to cathepsin K for targeted therapy of aortic aneurysms.291 MMP inhibitors have been widely studied as a potential treatment for AAA. However, due to low efficacy at low doses and high toxicity at high doses, an appropriate delivery system is urgently needed. The Nosoudi group delivered MMP inhibitors to AAA sites based on targeted nanoparticles to mitigate side effects in patients.292
Some researchers have turned to cell therapy. Wang’s team applied human mesenchymal stem cells (MSCs) in the mouse local elastase AAA model. They found that MSCs enhanced the suppressive function of Tregs and the number of Tregs in the aortic wall, thereby inhibiting the overall growth of AAA.284 Based on this, they also conducted a phase I Aortic aneuRysm Repression with mEsenchymal STem cells (ARREST) trial in patients, and the preliminary clinical results supported their findings.
In summary, the treatment of AA is in its infancy currently, and a large number of drugs and targeted therapies are still in clinical trials stage. There are also many drugs that have demonstrated possible therapeutic effects in mouse models that do not work in clinical cohorts. Based on current research, the treatment of AA is still surgery-based. Therefore, exploring the potential targets of AA is the main research direction of researchers in future.
Animal model
The establishment of good animal models is helpful to elucidate the complex pathogenesis, which is used to develop new therapeutic methods or improve endovascular and surgical procedures. The same is true for aneurysm research. AA is regarded as an epidemic life-threatening disease. The first animal aneurysm models were published in the 1960s, and many other methods and models have been developed since then and have been variously upgraded and improved.293,294,295
Perhaps the most significant changes seen in human aneurysm tissue are degradation of extracellular elastic fibers and inflammatory infiltration. One of the first attempts to construct hemangiomas was to breakdown elastic fibers using protease. Anidjar et al. demonstrated the possibility of developing a rat aortic aneurysm model using porcine pancreatic elastase (PPE).296 Carsten et al. studied several batches of elastase and confirmed the need for inflammatory infiltration to activate macrophages, eventually causing the necessary extracellular matrix degradation in the rats, mimicking aneurysmal development in patients.297 The model building by Anidjar became the basis for future PPE model modifications. Periadventitial application of elastase in mice may cause similar changes and lead to the development of AAA.298 In addition, the calcium chloride model (CaCl2) is also commonly used to induce the occurrence of aneurysms and was first used in the carotid arteries of rabbits.299 The main symptoms induced by CaCl2 are the infiltration of macrophages into the middle and outer membranes and increased MMP-2/9 activity.300 The other most important animal model was Ang-II in apolipoprotein E (ApoE)-deficient mice, which was found to cause aneurysm development.301 This phenotype is associated with high rates of hyperlipidemia, vascular wall remodeling, inflammatory infiltration, and thrombosis in clinical aneurysms for male patients.302 In addition, animal models combining multiple drugs or physical methods have also been established.303
To study the etiology of aneurysms and new embolization materials, reliable aneurysm models must be established. At present, most models are focused on AAs. There are many kinds of animal models of AAA, which are relatively perfect.304 However, there are few animal models of TAA. In the establishment of animal models of AAA, some methods also lead to the formation of TAA, but the incidence of TAA is lower than that of AAA. Generally, AA animal models can be divided into two types, namely, nondissecting AA animal models and dissecting AA animal models. Among them, dissection AA is formed during the expansion of the aortic wall when the aortic intima opens up a gap, causing blood to enter the aortic middle layer and resulting in dissection thrombosis.
There are many common non-AA animal models, including the elastase-induced model, CaCl2-induced model and acellular aortic transplantation model. The animal models of dissected AA included Ang-II-induced animal models, aldosterone receptor agonist plus high salt-induced animal models and gene-edited aneurysm animal models. Models constructed by gene editing were mainly used for thoracic aortic aneurysms, including mutations of the FBN1 gene and mutations of the TGFBR1 and TGFBR2 genes.305
Elastase induction model
Elastase is a proteolytic enzyme that can hydrolyze a variety of proteins, including insoluble elastin, gelatin, fibrin, hemoglobin, and albumin.306 In addition, elastase also has lipase and lipoprotein hydrolase activities and hydrolyzes peptide bonds with aliphatic nonpolar amino acids as carboxyl groups. Because of its strong hydrolytic activity, elastase can degrade the elastic tissue in the connective tissue of the arterial canal, resulting in damage to the arterial canal and then inducing the formation of an aneurysm model.307
Elastase was first used in Wistar rats in 1990 by Anidjar et al.296. By pressurizing PPE into the aortic segment for two hours, elastase would penetrate the inner layer, damage elastic fibers and induce arterial dilation. Nchimi et al. described in detail the two-stage process of constructing rat aneurysms using PPE.308 In the first stage, PPE directly damaged the elastic layer, resulting in the loss of elastic retraction force. The second stage is the appearance of white blood cells during thrombosis. Usually, within one week, aneurysms constructed by this model can reach three to four times the diameter of the original aorta.309 However, in some cases, aneurysm rupture is less common because of the large amount of elastic fiber degradation. Moreover, simple elastin may not induce successful aneurysms due to the presence of pollutants in the preparation.310
In addition to rats, Pyo et al.311 used mice to study the different roles of MMP-9 and MMP-12 in the destruction of medial elastic fibers after initial aortic injury caused by PPE use. Molacek et al.294 compared different aortic aneurysm induction techniques using pigs as experimental models and found that intravascular infusion of PPE caused aortic dilation with inflammation and destruction of the elastic lamina.
Calcium chloride (CaCl2)-induced model
Gertz et al.299 surgically cut the carotid sheath of male rabbits and treated the surface of the outer membrane of the common carotid artery with 0.5 mol/L CaCl2 calcium chloride solution and found progressive focal aneurysm dilation, endometrial fibromyosis, calcium deposition, and rupture of middle elastic tissue. The media tissue was disturbed and infiltrated by inflammatory cells. Chiou et al.312 successfully induced aneurysms in mice by using CaCl2 in 2001. Aortic aneurysm formation was observed in C57BL/6 mice treated with 0.68 M CaCl2 for 10 min and three weeks later. Histological examination also showed aortic dilation with VSMC depletion, elastin degradation, and lymphocyte and macrophage infiltration. High concentrations of proinflammatory cytokines and MMPs were also detected in the dilated aorta.313,314 At present, the specific mechanism by which CaCl2 induces aneurysms is not clear, but it is known that calcium ions have a high affinity for elastin. When treated with CaCl2, cells will transport soluble ionized calcium into the cell, and VSMC alkaline phosphatase will further convert it into calcium phosphate (CaPO4) and precipitate it on the elastin network. Elastic fibers are damaged.68
Acellular aortic transplantation model
Allaire et al.315 found that arterial homografts using sodium dodecyl sulfate (SDS) treatment can prevent expansion and reduce inflammatory infiltration, keep the inside of the outer membrane of elastin, and process allogeneic grafts with a uniform distribution of inflammatory intimal thickening. Their elastic protein fiber content is higher than that of untreated allograft grafts. The researchers took guinea pigs’ inferior renal aortas, treated them with SDS solution and decellularized them. Decellularization resulted in the loss of smooth muscle cells from the graft, while elastin and collagen networks remained intact. The aorta was then orthotopically transplanted into Lewis rats. The acellular aorta thus became the target of an immune response, leading to degradation of the extracellular matrix and progressive arterial dilation, forming a piriform aneurysm. Aneurysms and collagen-rich intracavitary thrombi began to form within 3–4 weeks of implantation.
This aneurysm model can be used to observe the role of ECM immune-driven proteolysis and adaptive immunity. In addition, this model can also be used to study the protective effect of homologous VSMCs and endothelial cells on the development of abdominal aortic aneurysm, as well as the ability of VSMCs to inhibit MMPs.315 The model can also be used to study the effects of serine and MMPs and their inhibitors.
Ang-II-induced model
Earlier studies have found that AAA occasionally occurred in C57BL/6 mice after subcutaneous infusion of Ang-II. Daugherty et al. first reported in 1999 that subcutaneous infusion of Ang-II with a slow-release pump at a dose of 0.5 or 1 μg/min/kg for 28 days induced AAAs in ApoE-/- mice.316 Since then, hundreds of studies have used this model, which is the most commonly used modeling method for chemically induced AAA at present. Ang-II infusion mimics AAA inflammation and promotes suprarenal aortic dilatation, atherosclerosis in hypercholesterolemic mice, macrophage accumulation, and thrombosis.
Compared with transgenic mice with congenital dyslipidemia, C57BL/6 mice have a much lower incidence of Ang-II-induced AA. Studies have shown that AAA can be induced in only ~10–20% of C57BL/6 mice after Ang-II infusion. This limits the application of the Ang-II model. To augment Ang-II-induced AAA in C57BL/6 mice, one approach is to increase plasma cholesterol by inducing a PCSK9 gain-of-function mutation in mice on a C57BL/6 background.317 Another popular modification318 is to use Ang-II in combination with β-amino-propanitrile (BAPN). Male C57BL/6 mice were subcutaneously infused with Ang-II with a sustained release pump for 4 weeks at a dose of 1 μg/kg/min, and BAPN was added to drinking water at a dose of ~1 g/kg/d. This approach leads to a high incidence of aortic rupture.
Aldosterone receptor agonist induced by high salt intake
Deoxycorticosterone acetate is a precursor of aldosterone and a ligand of glucose and melanocortin receptors. Liu et al.319 induced AA models by administering deoxycorticosterone acetate (DOCA) and high salt to 10-month-old male C57BL/6 mice over a three-week period. AAs induced using this model were similar to human AAs in terms of elastin degradation, inflammatory cell infiltration, VSMC degeneration and apoptosis, and oxidative stress. Wu et al. found that p-selectin glycoprotein ligand-1 (PSGL-1) promoted the formation of DOCA salt-induced aneurysms in mice, and PSGL-1 knockdown significantly reduced the aneurysm formation rate and mortality in mice. Further studies found that PSGL-1 knockdown inhibited the adhesion of white blood cells and ECs and then inhibited the infiltration of inflammatory cells and the expression of inflammatory factors,320 indicating the important role of the inflammatory response in salt-induced aneurysms.
Gene editing
Aneurysm models constructed based on gene editing are mainly animal models of TAA because genetic factors are important factors for the occurrence and development of TAA. Under the condition of specific gene mutation, the pathogenesis of TAA is mainly attributed to the degradation of ECM, contractile dysfunction of VSMCs and dysfunction of the TGFβ signaling pathway.321 More than 20 gene mutations have been associated with the occurrence of TAA and dissection.
FBN1 encodes fibrin, an ECM glycoprotein, whose mutation was first identified in Marfan syndrome. Approximately 75% of patients with the disease have TAA and dissections, so FBN1 was first identified as the gene responsible for TAAs. Pereira et al. constructed mice with TAA and dissection by knockout of the FBN1 gene, but this method was not suitable for model construction because the born mice died of cardiovascular complications 10~14 days later.322 However, by mutating cysteine 1039 on FNB1 protein into glycine (C1039G), heterozygous mutant mice with normal life spans were created, and lesions in the middle vascular layer of mice gradually appeared two months after birth. This model was also gradually applied to the study.
Mutations in TGFBR1 and TGFBR2, the receptors for TGF-β, cause Leudiz syndrome. Leuditz syndrome is similar to Marfan syndrome in terms of the phenotype of cardiovascular disease, but in the former, it has a faster rate of aneurysm expansion. Direct knockout of the TGFBR1 and TGFBR2 genes in mice or specific knockout of the TGFBR1 and TGFBR2 genes in VSMCs can lead to embryo death.323 Li et al.324 used tamoxifen to induce specific knockout of TGFBR2 in VSMCs after mouse birth, which led to TAA. In addition, the mice with Tgfbr1M318R/+ and Tgfbr2G357W/+ heterozygous mutations also developed TAA and dissection, and death began to occur two months after birth.
Although there are a variety of ways to build an aneurysm model, each has certain limitations and advantages. For example, if there are pollutants in the elastin used in the elastase-induced model, the aneurysm will not be induced. The aldosterone receptor agonist plus high salt-induced model requires 10-month-old elderly mice. In Ang-II-induced models, aneurysms mainly occur in the superior renal aorta or thoracic aorta, while the most commonly affected site in human patients is the inferior renal aorta. Animal models of aneurysms constructed by gene editing technology often require a long time of hybridization and matching, which is relatively difficult to obtain. Therefore, the selection of the model should be based on the actual experimental requirements and conditions. After construction, the models were evaluated by morphometry, histology, immunohistochemistry, Western blotting, and quantitative real-time PCR. For example, a relative dilatation rate of the abdominal aorta above 1.5-fold change was defined as AAA. Morphological assessment was performed using a grading scale. Elastin degradation in the aorta was scored in a double-blinded manner by staining sections of the abdominal aorta with hematoxylin eosin and Elastin Van Gieson (EVG). Western blotting and ELISA can be used to detect related proteins, and real-time PCR can be used to analyze mRNA differential expression. High-resolution ultrasound provides a detailed morphological assessment that can accurately define the size of AAA, the presence of intramural hematoma, and luminal thrombus, as well as assess biomechanical properties of the vessel wall, such as perimeter strain or pulse wave velocity.325
It is a conventional and most commonly used method to construct aneurysm models using rodents such as mice and rats. However, due to the large differences between the structures of rodents and the human body, it is difficult to directly translate the corresponding mechanism studies into clinical practice. More animal models that can relatively accurately simulate the occurrence and development of human aneurysms are needed, which will require larger animals or improvements to the existing rodent model construction methods to better provide a basis for disease research (Fig. 3).
Conclusions
Throughout the history of aneurysm research, from the initial definition of aneurysm to the exploration of the pathological characteristics and etiology of aneurysm to the in-depth molecular mechanisms of the aneurysm formation process, researchers have progressively developed their understanding of aortic aneurysms. However, how to set up a standard protocol to screen aneurysms earlier, how to treat the progression of the pathologies, and how to prevent aneurysms from rupturing remain to be resolved.
To prevent AA, we believe that researchers should enhance the combination of clinical medicine and basic medicine and conduct multiomics assays in patient samples of different clinical typologies. By combining deep learning and artificial intelligence, researchers could find the expression profiles of genes, proteins, metabolism and other characteristic substances in AA patients of different ethnicities. Therefore, we could perform routine aortic diameter monitoring in populations with susceptibility gene mutations or high levels of expression of pathogenic proteins or metabolites, and better assays of these targets could be developed to monitor AA in the form of kits or rapid tests. Inspired by the features of abnormally expressed pathogenic proteins in AA tissues, targeted molecular probe techniques should be developed to help quantify the aortic diameter and rupture risk of AA via noninvasive imaging. Moreover, AA-related science education is needed for high-incidence populations such as older men with smoking habits.
To treat AA, researchers should find drug treatment targets, such as proteins and metabolites that participate in VSMC phenotypic switching or inflammation progression, by multiomics assays in AA patients. Moreover, the development of smart drug delivery systems provides a good alternative to highly efficiently inhibit AA progression. For researchers, finding animal models that are faster, cheaper, and more closely resemble the state of AA in human patients is needed. Overall, more studies are needed to further explore the definitive mechanisms related to AA in the coming decades.
References
Coats, J. & Auld, A. G. Preliminary communication on the pathology of aneurysms, with special reference to atheroma as a cause: read in the section of pathology at the Annual Meeting of the British Medical Association Held at Newcastle-on-Tyne, August, 1893. BMJ 2, 456–460 (1893).
Stehbens, W. E. History of aneurysms. Med. Hist. 2, 274–280 (1958).
Lasheras, J. C. The biomechanics of arterial aneurysms. Annu. Rev. Fluid Mech. 39, 293–319 (2007).
Malek, A. M., Alper, S. L. & Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282, 2035–2042 (1999).
Fox, J. A. & Hugh, A. E. Localization of atheroma: a theory based on boundary layer separation. Br. Heart J. 28, 388–399 (1966).
Zhang, L., Issa Bhaloo, S., Chen, T., Zhou, B. & Xu, Q. Role of resident stem cells in vessel formation and arteriosclerosis. Circ. Res. 122, 1608–1624 (2018).
Chou, E., Pirruccello, J. P., Ellinor, P. T. & Lindsay, M. E. Genetics and mechanisms of thoracic aortic disease. Nat. Rev. Cardiol. https://doi.org/10.1038/s41569-022-00763-0 (2022).
Li, Y. et al. Genetic lineage tracing of nonmyocyte population by dual recombinases. Circulation 138, 793–805 (2018).
Golledge, J. & Eagle, K. A. Acute aortic dissection. Lancet 372, 55–66 (2008).
Johnston, K. W. et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 13, 452–458 (1991).
Sakalihasan, N., Limet, R. & Defawe, O. D. Abdominal aortic aneurysm. Lancet 365, 1577–1589 (2005).
Reed, D., Reed, C., Stemmermann, G. & Hayashi, T. Are aortic aneurysms caused by atherosclerosis? Circulation 85, 205–211 (1992).
Xu, C., Zarins, C. K. & Glagov, S. Aneurysmal and occlusive atherosclerosis of the human abdominal aorta. J. Vasc. Surg. 33, 91–96 (2001).
Sadaghianloo, N. et al. Blunt abdominal aortic trauma in paediatric patients. Injury 45, 183–191 (2014).
Launay, D. & Hachulla, É. Les aortites inflammatoires. La Presse Méd. 33, 1334–1340 (2004).
Judge, D. P. & Dietz, H. C. Marfan’s syndrome. Lancet 366, 1965–1976 (2005).
Vardulaki, K. A. et al. Quantifying the risks of hypertension, age, sex and smoking in patients with abdominal aortic aneurysm. Br. J. Surg. 87, 195–200 (2000).
Lederle, F. A. et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm detection and management veterans affairs cooperative study investigators. Arch. Intern. Med. 160, 1425–1430 (2000).
Kent, K. C. et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J. Vasc. Surg. 52, 539–548 (2010).
Wilmink, T. B. M., Quick, C. R. G. & Day, N. E. The association between cigarette smoking and abdominal aortic aneurysms. J. Vasc. Surg. 30, 1099–1105 (1999).
Forsdahl, S. H., Singh, K., Solberg, S. & Jacobsen, B. K. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994–2001. Circulation 119, 2202–2208 (2009).
Knuutinen, A. et al. Smoking affects collagen synthesis and extracellular matrix turnover in human skin. Br. J. Dermatol. 146, 588–594 (2002).
Brown, L. C. & Powell, J. T. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann. Surg. 230, 289–296 (1999). discussion 296-287.
Shiraya, S. et al. Hypertension accelerated experimental abdominal aortic aneurysm through upregulation of nuclear factor kappaB and Ets. Hypertension 48, 628–636 (2006).
Wanhainen, A. et al. Risk factors associated with abdominal aortic aneurysm: a population-based study with historical and current data. J. Vasc. Surg. 41, 390–396 (2005).
Cornuz, J., Sidoti Pinto, C., Tevaearai, H. & Egger, M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur. J. Public Health 14, 343–349 (2004).
Iribarren, C. et al. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the Kaiser multiphasic health checkup cohort study. Ann. Epidemiol. 17, 669–678 (2007).
Pleumeekers, H. J. et al. Aneurysms of the abdominal aorta in older adults. The Rotterdam Study. Am. J. Epidemiol. 142, 1291–1299 (1995).
Golledge, J., Muller, J., Daugherty, A. & Norman, P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler. Thromb. Vasc. Biol. 26, 2605–2613 (2006).
Ning, X. et al. Diabetes, its duration, and the long-term risk of abdominal aortic aneurysm: The Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 313, 137–143 (2020).
Raffort, J. et al. Diabetes and aortic aneurysm: current state of the art. Cardiovasc. Res. 114, 1702–1713 (2018).
Fleming, C., Whitlock, E. P., Beil, T. L. & Lederle, F. A. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 142, 203–211 (2005).
Fielding, J. W., Black, J., Ashton, F., Slaney, G. & Campbell, D. J. Diagnosis and management of 528 abdominal aortic aneurysms. Br. Med. J. (Clin. Res Ed.) 283, 355–359 (1981).
Smidfelt, K., Nordanstig, J., Davidsson, A., Torngren, K. & Langenskiold, M. Misdiagnosis of ruptured abdominal aortic aneurysms is common and is associated with increased mortality. J. Vasc. Surg. 73, 476–483.e473 (2021).
Nordon, I. M., Hinchliffe, R. J., Loftus, I. M. & Thompson, M. M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 8, 92–102 (2011).
Wilmink, A. B., Forshaw, M., Quick, C. R., Hubbard, C. S. & Day, N. E. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J. Med. Screen. 9, 125–127 (2002).
Chaikof, E. L. et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 67, 2–77.e72 (2018).
Rudd, J. H. The role of 18F-FDG PET in aortic dissection. J. Nucl. Med. 51, 667–668 (2010).
Kato, K. et al. Uptake of 18F-FDG in acute aortic dissection: a determinant of unfavorable outcome. J. Nucl. Med. 51, 674–681 (2010).
Wang, G., Jacquet, L., Karamariti, E. & Xu, Q. Origin and differentiation of vascular smooth muscle cells. J. Physiol. 593, 3013–3030 (2015).
Sawada, H., Rateri, D. L., Moorleghen, J. J., Majesky, M. W. & Daugherty, A. Smooth muscle cells derived from second heart field and cardiac neural crest reside in spatially distinct domains in the media of the ascending aorta-brief report. Arterioscler. Thromb. Vasc. Biol. 37, 1722–1726 (2017).
Alexander, M. R. & Owens, G. K. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu. Rev. Physiol. 74, 13–40 (2012).
Ross, R. The pathogenesis of atherosclerosis. Mech. Ageing Dev. 9, 435–440 (1979).
Owens, G. K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 75, 487–517 (1995).
Gadue, P., Huber, T. L., Paddison, P. J. & Keller, G. M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl Acad. Sci. USA 103, 16806–16811 (2006).
Hautmann, M. B., Madsen, C. S. & Owens, G. K. A transforming growth factor beta (TGFbeta) control element drives TGFbeta-induced stimulation of smooth muscle alpha-actin gene expression in concert with two CArG elements. J. Biol. Chem. 272, 10948–10956 (1997).
Fukui, D. et al. Overexpression of transforming growth factor beta1 in smooth muscle cells of human abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 25, 540–545 (2003).
Doyle, A. J. et al. Differential expression of Hedgehog/Notch and transforming growth factor-beta in human abdominal aortic aneurysms. J. Vasc. Surg. 62, 464–470 (2015).
Kang, J. S., Liu, C. & Derynck, R. New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol. 19, 385–394 (2009).
Akhurst, R. J. & Hata, A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev. Drug Discov. 11, 790–811 (2012).
Moustakas, A. & Heldin, C. H. Non-Smad TGF-beta signals. J. Cell Sci. 118, 3573–3584 (2005).
Gao, P. et al. Transforming growth factor beta1 suppresses proinflammatory gene program independent of its regulation on vascular smooth muscle differentiation and autophagy. Cell Signal 50, 160–170 (2018).
Benke, K. et al. The role of transforming growth factor-beta in Marfan syndrome. Cardiol. J. 20, 227–234 (2013).
Wei, H. et al. Aortopathy in a mouse model of marfan syndrome is not mediated by altered transforming growth factor beta signaling. J. Am. Heart Assoc. 6, e004968 (2017).
Van Laer, L., Dietz, H. & Loeys, B. Loeys-Dietz syndrome. Adv. Exp. Med. Biol. 802, 95–105 (2014).
Inamoto, S. et al. TGFBR2 mutations alter smooth muscle cell phenotype and predispose to thoracic aortic aneurysms and dissections. Cardiovasc. Res. 88, 520–529 (2010).
Wang, Y. et al. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Invest. 120, 422–432 (2010).
Angelov, S. N. et al. TGF-beta (transforming growth factor-beta) signaling protects the thoracic and abdominal aorta from angiotensin II-induced pathology by distinct mechanisms. Arterioscler. Thromb. Vasc. Biol. 37, 2102–2113 (2017).
Ren, K. et al. GDF11 prevents the formation of thoracic aortic dissection in mice: Promotion of contractile transition of aortic SMCs. J. Cell Mol. Med. 25, 4623–4636 (2021).
Zhou, G. et al. Cyclophilin A contributes to aortopathy induced by postnatal loss of smooth muscle TGFBR1. FASEB J. 33, 11396–11410 (2019).
Huang, T. H., Chang, H. H., Guo, Y. R., Chang, W. C. & Chen, Y. F. Vitamin B mitigates thoracic aortic dilation in marfan syndrome mice by restoring the canonical TGF-beta pathway. Int. J. Mol. Sci. 22, 11737 (2021).
Si, X. et al. MicroRNA-23b prevents aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching via FoxO4 suppression. Life Sci. 288, 119092 (2022).
Garvey, S. M., Sinden, D. S., Schoppee Bortz, P. D. & Wamhoff, B. R. Cyclosporine up-regulates Kruppel-like factor-4 (KLF4) in vascular smooth muscle cells and drives phenotypic modulation in vivo. J. Pharm. Exp. Ther. 333, 34–42 (2010).
Elia, L. et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 16, 1590–1598 (2009).
Paredes, F., Williams, H. C., Quintana, R. A. & San Martin, A. Mitochondrial protein poldip2 (polymerase delta interacting protein 2) controls vascular smooth muscle differentiated phenotype by O-Linked GlcNAc (N-acetylglucosamine) transferase-dependent inhibition of a ubiquitin proteasome system. Circ. Res. 126, 41–56 (2020).
Alencar, G. F. et al. Stem cell pluripotency genes Klf4 and Oct4 regulate complex SMC phenotypic changes critical in late-stage atherosclerotic lesion pathogenesis. Circulation 142, 2045–2059 (2020).
Quintana, R. A. & Taylor, W. R. Cellular mechanisms of aortic aneurysm formation. Circ. Res. 124, 607–618 (2019).
Yamanouchi, D. et al. Accelerated aneurysmal dilation associated with apoptosis and inflammation in a newly developed calcium phosphate rodent abdominal aortic aneurysm model. J. Vasc. Surg. 56, 455–461 (2012).
Hsieh, C. Oxidized low density lipoprotein induces apoptosis via generation of reactive oxygen species in vascular smooth muscle cells. Cardiovasc. Res. 49, 135–145 (2001).
Jia, L. X. et al. Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J. Pathol. 236, 373–383 (2015).
Jia, L.-X. et al. ER stress dependent microparticles derived from smooth muscle cells promote endothelial dysfunction during thoracic aortic aneurysm and dissection. Clin. Sci. 131, 1287–1299 (2017).
Lu, H. et al. Cyclodextrin prevents abdominal aortic aneurysm via activation of vascular smooth muscle cell transcription factor EB. Circulation 142, 483–498 (2020).
Broz, P., Pelegrin, P. & Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020).
Vande Walle, L. & Lamkanfi, M. Pyroptosis. Curr. Biol. 26, R568–R572 (2016).
Wu, D. et al. NLRP3 (nucleotide oligomerization domain-like receptor family, pyrin domain containing 3)-caspase-1 inflammasome degrades contractile proteins: implications for aortic biomechanical dysfunction and aneurysm and dissection formation. Arterioscler. Thromb. Vasc. Biol. 37, 694–706 (2017).
Usui, F. et al. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 35, 127–136 (2015).
Cao, J. Y. & Dixon, S. J. Mechanisms of ferroptosis. Cell Mol. Life Sci. 73, 2195–2209 (2016).
Ghasemitarei, M., Yusupov, M., Razzokov, J., Shokri, B. & Bogaerts, A. Transport of cystine across xC(-) antiporter. Arch. Biochem. Biophys. 664, 117–126 (2019).
Bogdan, A. R., Miyazawa, M., Hashimoto, K. & Tsuji, Y. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem. Sci. 41, 274–286 (2016).
Dixon, S. J. & Stockwell, B. R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10, 9–17 (2014).
Chen, Y. et al. BRD4770 functions as a novel ferroptosis inhibitor to protect against aortic dissection. Pharm. Res. 177, 106122 (2022).
Sampilvanjil, A. et al. Cigarette smoke extract induces ferroptosis in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 318, H508–H518 (2020).
Wagenseil, J. E. & Mecham, R. P. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 89, 957–989 (2009).
Jana, S., Hu, M., Shen, M. & Kassiri, Z. Extracellular matrix, regional heterogeneity of the aorta, and aortic aneurysm. Exp. Mol. Med. 51, 1–15 (2019).
Gurung, R., Choong, A. M., Woo, C. C., Foo, R. & Sorokin, V. Genetic and epigenetic mechanisms underlying vascular smooth muscle cell phenotypic modulation in abdominal aortic aneurysm. Int. J. Mol. Sci. 21, 6334 (2020).
Airhart, N. et al. Smooth muscle cells from abdominal aortic aneurysms are unique and can independently and synergistically degrade insoluble elastin. J. Vasc. Surg. 60, 1033–1041 (2014). discussion 1041-1032.
Nagase, H., Visse, R. & Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 69, 562–573 (2006).
Koole, D. et al. Intraluminal abdominal aortic aneurysm thrombus is associated with disruption of wall integrity. J. Vasc. Surg. 57, 77–83 (2013).
Chute, M., Jana, S. & Kassiri, Z. Disintegrin and metalloproteinases (ADAMs and ADAM-TSs), the emerging family of proteases in heart physiology and pathology. Curr. Opin. Physiol. 1, 34–45 (2018).
Silence, J., Collen, D. & Lijnen, H. R. Reduced atherosclerotic plaque but enhanced aneurysm formation in mice with inactivation of the tissue inhibitor of metalloproteinase-1 (TIMP-1) gene. Circ. Res. 90, 897–903 (2002).
Defawe, O. TIMP-2 and PAI-1 mRNA levels are lower in aneurysmal as compared to athero-occlusive abdominal aortas. Cardiovasc. Res. 60, 205–213 (2003).
Di Gregoli, K. et al. MicroRNA-181b controls atherosclerosis and aneurysms through regulation of TIMP-3 and elastin. Circ. Res. 120, 49–65 (2017).
De Meyer, G. R. et al. Autophagy in vascular disease. Circ. Res. 116, 468–479 (2015).
Song, T. F. et al. LncRNA MALAT1 regulates smooth muscle cell phenotype switch via activation of autophagy. Oncotarget 9, 4411–4426 (2018).
Irace, F. G. et al. Role of oxidative stress and autophagy in thoracic aortic aneurysms. JACC Basic Transl. Sci. 6, 719–730 (2021).
Clement, M. et al. Vascular smooth muscle cell plasticity and autophagy in dissecting aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 39, 1149–1159 (2019).
Forstermann, U. & Sessa, W. C. Nitric oxide synthases: regulation and function. Eur. Heart J. 33, 829–837 (2012). 837a-837d.
Crabtree, M. J., Hale, A. B. & Channon, K. M. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic. Biol. Med. 50, 1639–1646 (2011).
Balligand, J. L., Feron, O. & Dessy, C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 89, 481–534 (2009).
Miller, F. J. Jr. et al. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler. Thromb. Vasc. Biol. 22, 560–565 (2002).
Guzik, B. et al. Mechanisms of oxidative stress in human aortic aneurysms-association with clinical risk factors for atherosclerosis and disease severity. Int. J. Cardiol. 168, 2389–2396 (2013).
Huang, K., Wang, Y., Siu, K. L., Zhang, Y. & Cai, H. Targeting feed-forward signaling of TGFbeta/NOX4/DHFR/eNOS uncoupling/TGFbeta axis with anti-TGFbeta and folic acid attenuates formation of aortic aneurysms: novel mechanisms and therapeutics. Redox Biol. 38, 101757 (2021).
Oller, J. et al. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat. Med. 23, 200–212 (2017).
Cassuto, J. et al. Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow-mediated dilation in coronary arterioles of diabetic patients. Diabetes 63, 1381–1393 (2014).
Fan, L. M. et al. Endothelial cell-specific reactive oxygen species production increases susceptibility to aortic dissection. Circulation 129, 2661–2672 (2014).
Fan, L. M., Liu, F., Du, J., Geng, L. & Li, J. M. Inhibition of endothelial Nox2 activation by LMH001 protects mice from angiotensin II-induced vascular oxidative stress, hypertension and aortic aneurysm. Redox Biol. 51, 102269 (2022).
Kwak, B. R. et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur. Heart J. 35, 3013–3020,3020a-3020d (2014).
Back, M., Gasser, T. C., Michel, J. B. & Caligiuri, G. Biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc. Res. 99, 232–241 (2013).
Platt, M. O. & Shockey, W. A. Endothelial cells and cathepsins: Biochemical and biomechanical regulation. Biochimie 122, 314–323 (2016).
Magid, R. & Davies, P. F. Endothelial protein kinase C isoform identity and differential activity of PKCzeta in an athero-susceptible region of porcine aorta. Circ. Res. 97, 443–449 (2005).
Chaudhury, H. et al. c-Jun N-terminal kinase primes endothelial cells at atheroprone sites for apoptosis. Arterioscler. Thromb. Vasc. Biol. 30, 546–553 (2010).
Chatzizisis, Y. S. et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 49, 2379–2393 (2007).
Zhang, Y. et al. Shear stress regulates endothelial cell function through SRB1-eNOS signaling pathway. Cardiovasc. Ther. 34, 308–313 (2016).
Davis, M. E., Cai, H., Drummond, G. R. & Harrison, D. G. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ. Res. 89, 1073–1080 (2001).
Kuhlencordt, P. J. et al. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 104, 448–454 (2001).
Heath, J. M. et al. Mechanosensitive microRNA-181b regulates aortic valve endothelial matrix degradation by targeting TIMP3. Cardiovasc. Eng. Technol. 9, 141–150 (2018).
Shen, Y. H. et al. Aortic aneurysms and dissections series. Arterioscler. Thromb. Vasc. Biol. 40, e37–e46 (2020).
Lohoefer, F. et al. Histopathological analysis of cellular localization of cathepsins in abdominal aortic aneurysm wall. Int. J. Exp. Pathol. 93, 252–258 (2012).
Sunderland, K., Jiang, J. & Zhao, F. Disturbed flow’s impact on cellular changes indicative of vascular aneurysm initiation, expansion, and rupture: A pathological and methodological review. J. Cell Physiol. 237, 278–300 (2022).
Chiu, J. J. & Chien, S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91, 327–387 (2011).
Harter, L. P., Gross, B. H., Callen, P. W. & Barth, R. A. Ultrasonic evaluation of abdominal aortic thrombus. J. Ultrasound Med. 1, 315–318 (1982).
DeRoo, E. et al. Endothelial dysfunction in the pathogenesis of abdominal aortic aneurysm. Biomolecules 12, 509 (2022).
Speelman, L. et al. The mechanical role of thrombus on the growth rate of an abdominal aortic aneurysm. J. Vasc. Surg. 51, 19–26 (2010).
Vorp, D. A. et al. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J. Vasc. Surg. 34, 291–299 (2001).
Houard, X. et al. Mediators of neutrophil recruitment in human abdominal aortic aneurysms. Cardiovasc. Res. 82, 532–541 (2009).
Folkesson, M., Silveira, A., Eriksson, P. & Swedenborg, J. Protease activity in the multi-layered intra-luminal thrombus of abdominal aortic aneurysms. Atherosclerosis 218, 294–299 (2011).
Ma, X., Xia, S., Liu, G. & Song, C. The detrimental role of intraluminal thrombus outweighs protective advantage in abdominal aortic aneurysm pathogenesis: the implications for the anti-platelet therapy. Biomolecules 12, 942 (2022).
Boyd, A. J. Intraluminal thrombus: innocent bystander or factor in abdominal aortic aneurysm pathogenesis? JVS Vasc. Sci. 2, 159–169 (2021).
Lim, C. S., Kiriakidis, S., Sandison, A., Paleolog, E. M. & Davies, A. H. Hypoxia-inducible factor pathway and diseases of the vascular wall. J. Vasc. Surg. 58, 219–230 (2013).
Wang, D. H., Makaroun, M. S., Webster, M. W. & Vorp, D. A. Effect of intraluminal thrombus on wall stress in patient-specific models of abdominal aortic aneurysm. J. Vasc. Surg. 36, 598–604 (2002).
Haller, S. J. et al. Intraluminal thrombus is associated with early rupture of abdominal aortic aneurysm. J. Vasc. Surg. 67, 1051–1058.e1051 (2018).
Bluestein, D. et al. Intraluminal thrombus and risk of rupture in patient specific abdominal aortic aneurysm–FSI modelling. Comput. Methods Biomech. Biomed. Eng. 12, 73–81 (2009).
Throop, A., Bukac, M. & Zakerzadeh, R. Prediction of wall stress and oxygen flow in patient-specific abdominal aortic aneurysms: the role of intraluminal thrombus. Biomech. Model Mechanobiol. 21, 1761–1779 (2022).
Skov, R. A. C. et al. Anticoagulants and reduced thrombus load in abdominal aortic aneurysms assessed with three-dimensional contrast-enhanced ultrasound examination. J. Vasc. Surg. 77, 143–149 (2022).
Liu, H. et al. High-density thrombus and maximum transverse diameter on multi-spiral computed tomography angiography combine to predict abdominal aortic aneurysm rupture. Front. Cardiovasc. Med. 9, 951264 (2022).
Kim, S. et al. Deep learning on multiphysical features and hemodynamic modeling for abdominal aortic aneurysm growth prediction. IEEE Trans. Med. Imaging https://doi.org/10.1109/tmi.2022.3206142 (2022).
Bobryshev, Y. Vascular-associated lymphoid tissue (VALT) involvement in aortic aneurysm. Atherosclerosis 154, 15–21 (2001).
Chan, W. L. et al. Atherosclerotic abdominal aortic aneurysm and the interaction between autologous human plaque-derived vascular smooth muscle cells, type 1 NKT, and helper T cells. Circ. Res. 96, 675–683 (2005).
Ocana, E. Characterisation of T and B lymphocytes infiltrating abdominal aortic aneurysms. Atherosclerosis 170, 39–48 (2003).
Xiong, W., Zhao, Y., Prall, A., Greiner, T. C. & Baxter, B. T. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J. Immunol. 172, 2607–2612 (2004).
Zhou, L., Chong, M. M. & Littman, D. R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009).
Teo, F. H. et al. Characterization of CD4(+) T cell subsets in patients with abdominal aortic aneurysms. Mediat. Inflamm. 2018, 6967310 (2018).
Wang, H. et al. Circulatory CD4-positive T-lymphocyte imbalance mediated by homocysteine-induced AIM2 and NLRP1 inflammasome upregulation and activation is associated with human abdominal aortic aneurysm. J. Vasc. Res. 57, 276–290 (2020).
Ye, J. et al. Circulating Th1, Th2, Th9, Th17, Th22, and Treg levels in aortic dissection patients. Mediat. Inflamm. 2018, 5697149 (2018).
Yan, H. et al. Interleukin-12 and -23 blockade mitigates elastase-induced abdominal aortic aneurysm. Sci. Rep. 9, 10447 (2019).
Dale, M. A., Ruhlman, M. K. & Baxter, B. T. Inflammatory cell phenotypes in AAAs: their role and potential as targets for therapy. Arterioscler. Thromb. Vasc. Biol. 35, 1746–1755 (2015).
Sharma, A. K. et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation 126, S38–S45 (2012).
Wei, Z. et al. Inhibiting the Th17/IL-17A-related inflammatory responses with digoxin confers protection against experimental abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 34, 2429–2438 (2014).
Schönbeck, U., Sukhova, G. K., Gerdes, N. & Libby, P. TH2 predominant immune responses prevail in human abdominal aortic aneurysm. Am. J. Pathol. 161, 499–506 (2002).
Barbi, J., Pardoll, D. & Pan, F. Treg functional stability and its responsiveness to the microenvironment. Immunol. Rev. 259, 115–139 (2014).
Ehrenstein, M. R. et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J. Exp. Med. 200, 277–285 (2004).
Li, J. et al. Aorta regulatory T cells with a tissue-specific phenotype and function promote tissue repair through Tff1 in abdominal aortic aneurysms. Adv. Sci. (Weinh.) 9, e2104338 (2022).
Zhou, Y. et al. Regulatory T cells in human and angiotensin II-induced mouse abdominal aortic aneurysms. Cardiovasc. Res. 107, 98–107 (2015).
Yin, M. et al. Deficient CD4+CD25+ T regulatory cell function in patients with abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 30, 1825–1831 (2010).
Wang, Y., Liu, J., Burrows, P. D. & Wang, J. Y. B cell development and maturation. Adv. Exp. Med. Biol. 1254, 1–22 (2020).
Meher, A. K. et al. B2 cells suppress experimental abdominal aortic aneurysms. Am. J. Pathol. 184, 3130–3141 (2014).
Schaheen, B. et al. B-cell depletion promotes aortic infiltration of immunosuppressive cells and is protective of experimental aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 36, 2191–2202 (2016).
Spinosa, M. D. et al. B cell-activating factor antagonism attenuates the growth of experimental abdominal aortic aneurysm. Am. J. Pathol. 191, 2231–2244 (2021).
Furusho, A. et al. Involvement of B cells, immunoglobulins, and syk in the pathogenesis of abdominal aortic aneurysm. J. Am. Heart Assoc. 7, e007750 (2018).
Shao, F. et al. B cell-derived anti-beta 2 glycoprotein I antibody contributes to hyperhomocysteinaemia-aggravated abdominal aortic aneurysm. Cardiovasc. Res. 116, 1897–1909 (2020).
Selders, G. S., Fetz, A. E., Radic, M. Z. & Bowlin, G. L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 4, 55–68 (2017).
Kurihara, T. et al. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation 126, 3070–3080 (2012).
Eliason, J. L. et al. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation 112, 232–240 (2005).
He, L. et al. Deficiency of FAM3D (family with sequence similarity 3, member D), a novel chemokine, attenuates neutrophil recruitment and ameliorates abdominal aortic aneurysm development. Arterioscler. Thromb. Vasc. Biol. 38, 1616–1631 (2018).
Fuchs, T. A. et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241 (2007).
Bonaventura, A., Vecchie, A., Abbate, A. & Montecucco, F. Neutrophil extracellular traps and cardiovascular diseases: an update. Cells 9, 231 (2020).
Plana, E., Oto, J., Medina, P., Fernandez-Pardo, A. & Miralles, M. Novel contributions of neutrophils in the pathogenesis of abdominal aortic aneurysm, the role of neutrophil extracellular traps: a systematic review. Thromb. Res. 194, 200–208 (2020).
Eilenberg, W. et al. Histone citrullination as a novel biomarker and target to inhibit progression of abdominal aortic aneurysms. Transl. Res. 233, 32–46 (2021).
Yan, H. et al. Neutrophil proteases promote experimental abdominal aortic aneurysm via extracellular trap release and plasmacytoid dendritic cell activation. Arterioscler. Thromb. Vasc. Biol. 36, 1660–1669 (2016).
Meher, A. K. et al. Novel role of IL (Interleukin)-1beta in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 38, 843–853 (2018).
Brandau, A. et al. Association of lipoproteins with neutrophil extracellular traps in patients with abdominal aortic aneurysm. Biomedicines 10, 217 (2022).
Miyabe, C. et al. Dectin-2-induced CCL2 production in tissue-resident macrophages ignites cardiac arteritis. J. Clin. Invest. 129, 3610–3624 (2019).
Ginhoux, F. & Guilliams, M. Tissue-resident macrophage ontogeny and homeostasis. Immunity 44, 439–449 (2016).
Raffort, J. et al. Monocytes and macrophages in abdominal aortic aneurysm. Nat. Rev. Cardiol. 14, 457–471 (2017).
Mellak, S. et al. Angiotensin II mobilizes spleen monocytes to promote the development of abdominal aortic aneurysm in Apoe-/- mice. Arterioscler. Thromb. Vasc. Biol. 35, 378–388 (2015).
Lawrence, T. & Natoli, G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761 (2011).
Wu, S. et al. Genome-wide identification of immune-related alternative splicing and splicing regulators involved in abdominal aortic aneurysm. Front. Genet. 13, 816035 (2022).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Lamblin, N. et al. Profile of macrophages in human abdominal aortic aneurysms: a transcriptomic, proteomic, and antibody protein array study. J. Proteome Res. 9, 3720–3729 (2010).
Qin, Z. et al. Angiotensin II-induced TLR4 mediated abdominal aortic aneurysm in apolipoprotein E knockout mice is dependent on STAT3. J. Mol. Cell Cardiol. 87, 160–170 (2015).
Dutertre, C. A. et al. Deciphering the stromal and hematopoietic cell network of the adventitia from non-aneurysmal and aneurysmal human aorta. PLoS ONE 9, e89983 (2014).
Boytard, L. et al. Role of proinflammatory CD68(+) mannose receptor(-) macrophages in peroxiredoxin-1 expression and in abdominal aortic aneurysms in humans. Arterioscler. Thromb. Vasc. Biol. 33, 431–438 (2013).
Chen, X. et al. Galactose-modified nanoparticles for delivery of microRNA to mitigate the progress of abdominal aortic aneurysms via regulating macrophage polarization. Nanomedicine 44, 102564 (2022).
Zhou, J. et al. CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses. J. Exp. Med. 207, 1951–1966 (2010).
Cui, H. et al. Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection. Eur. Heart J. 42, 4373–4385 (2021).
Li, Y. et al. Discovery of crucial cytokines associated with abdominal aortic aneurysm formation by protein array analysis. Exp. Biol. Med (Maywood) 244, 1648–1657 (2019).
Mihara, M., Hashizume, M., Yoshida, H., Suzuki, M. & Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. (Lond.) 122, 143–159 (2012).
Lindeman, J. H. et al. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL-6- and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin. Sci. (Lond.) 114, 687–697 (2008).
Takagi, H. et al. Circulating interleukin-6 levels are associated with abdominal aortic aneurysm presence: a meta-analysis and meta-regression of case-control studies. Ann. Vasc. Surg. 28, 1913–1922 (2014).
Rohde, L. E. et al. Plasma concentrations of interleukin-6 and abdominal aortic diameter among subjects without aortic dilatation. Arterioscler. Thromb. Vasc. Biol. 19, 1695–1699 (1999).
Tieu, B. C. et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J. Clin. Invest. 119, 3637–3651 (2009).
Nishihara, M. et al. The role of IL-6 in pathogenesis of abdominal aortic aneurysm in mice. PLoS ONE 12, e0185923 (2017).
Harrison, S. C. et al. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur. Heart J. 34, 3707–3716 (2013).
Chomarat, P., Banchereau, J., Davoust, J. & Palucka, A. K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 1, 510–514 (2000).
Hou, T., Ray, S. & Brasier, A. R. The functional role of an interleukin 6-inducible CDK9.STAT3 complex in human gamma-fibrinogen gene expression. J. Biol. Chem. 282, 37091–37102 (2007).
Yan, D. et al. Bazedoxifene attenuates abdominal aortic aneurysm formation via downregulation of interleukin-6/glycoprotein 130/signal transducer and activator of transcription 3 signaling pathway in apolipoprotein E-knockout mice. Front. Pharm. 11, 392 (2020).
Kokje, V. B. C. et al. IL-6: A Janus-like factor in abdominal aortic aneurysm disease. Atherosclerosis 251, 139–146 (2016).
Williams, J. W., Huang, L. H. & Randolph, G. J. Cytokine circuits in cardiovascular disease. Immunity 50, 941–954 (2019).
Johnston, W. F. et al. Inhibition of interleukin-1beta decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation 130, S51–S59 (2014).
Wu, X. et al. Sex- and disease-specific inflammasome signatures in circulating blood leukocytes of patients with abdominal aortic aneurysm. Mol. Med. 22, 505–518 (2016).
Johnston, W. F. et al. Genetic and pharmacologic disruption of interleukin-1beta signaling inhibits experimental aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 33, 294–304 (2013).
Isoda, K. et al. Inhibition of interleukin-1 suppresses angiotensin II-induced aortic inflammation and aneurysm formation. Int. J. Cardiol. 270, 221–227 (2018).
Wenjing, F. et al. The role of IL-1beta in aortic aneurysm. Clin. Chim. Acta 504, 7–14 (2020).
Zheng, Y., Gardner, S. E. & Clarke, M. C. Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 31, 2781–2786 (2011).
Libby, P. & Vromman, A. Swell, or not too swell: cytokines regulate arterial aneurysm formation. Immunity 47, 814–816 (2017).
Jiang, Y. F. et al. Local upregulation of interleukin-1 beta in aortic dissecting aneurysm: correlation with matrix metalloproteinase-2, 9 expression and biomechanical decrease. Interact. Cardiovasc. Thorac. Surg. 28, 344–352 (2019).
Zhang, P. et al. Smad4 deficiency in smooth muscle cells initiates the formation of aortic aneurysm. Circ. Res. 118, 388–399 (2016).
Perkins, N. D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 8, 49–62 (2007).
Golledge, A. L., Walker, P., Norman, P. E. & Golledge, J. A systematic review of studies examining inflammation associated cytokines in human abdominal aortic aneurysm samples. Dis. Markers 26, 181–188 (2009).
Jablonska, A. et al. Polymorphisms in the IL-6 and TNF-alpha gene are associated with an increased risk of abdominal aortic aneurysm. Int. J. Cardiol. 329, 192–197 (2021).
Batra, R. et al. IL-1beta (Interleukin-1beta) and TNF-alpha (tumor necrosis factor-alpha) impact abdominal aortic aneurysm formation by differential effects on macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 38, 457–463 (2018).
Xiong, W. et al. Blocking TNF-alpha attenuates aneurysm formation in a murine model. J. Immunol. 183, 2741–2746 (2009).
Chiang, M. T. et al. Gal-1 (Galectin-1) upregulation contributes to abdominal aortic aneurysm progression by enhancing vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 41, 331–345 (2021).
Li, J., Krishna, S. M. & Golledge, J. The potential role of kallistatin in the development of abdominal aortic aneurysm. Int. J. Mol. Sci. 17, 1312 (2016).
Krishna, S. M. et al. Kallistatin limits abdominal aortic aneurysm by attenuating generation of reactive oxygen species and apoptosis. Sci. Rep. 11, 17451 (2021).
Gerszten, R. E. et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398, 718–723 (1999).
Koch, A. E. et al. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am. J. Pathol. 142, 1423–1431 (1993).
de Waard, V. et al. Systemic MCP1/CCR2 blockade and leukocyte specific MCP1/CCR2 inhibition affect aortic aneurysm formation differently. Atherosclerosis 211, 84–89 (2010).
Wang, Q. et al. Monocyte chemoattractant protein-1 (MCP-1) regulates macrophage cytotoxicity in abdominal aortic aneurysm. PLoS ONE 9, e92053 (2014).
Wang, Q., Shu, C., Su, J. & Li, X. A crosstalk triggered by hypoxia and maintained by MCP-1/miR-98/IL-6/p38 regulatory loop between human aortic smooth muscle cells and macrophages leads to aortic smooth muscle cells apoptosis via Stat1 activation. Int. J. Clin. Exp. Pathol. 8, 2670–2679 (2015).
Hoh, B. L. et al. Monocyte chemotactic protein-1 promotes inflammatory vascular repair of murine carotid aneurysms via a macrophage inflammatory protein-1alpha and macrophage inflammatory protein-2-dependent pathway. Circulation 124, 2243–2252 (2011).
Hu, J. et al. Advances in biomaterials and technologies for vascular embolization. Adv. Mater. 31, e1901071 (2019).
Takahashi, M. Cell-specific roles of nlrp3 inflammasome in myocardial infarction. J. Cardiovasc. Pharm. 74, 188–193 (2019).
Takahashi, M. NLRP3 inflammasome as a common denominator of atherosclerosis and abdominal aortic aneurysm. Circ. J. 85, 2129–2136 (2021).
Takahashi, M. NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc. Res. 118, 372–385 (2022).
Abbate, A. et al. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ. Res. 126, 1260–1280 (2020).
Liu, C. L. et al. Adipocytes promote interleukin-18 binding to its receptors during abdominal aortic aneurysm formation in mice. Eur. Heart J. 41, 2456–2468 (2020).
Gonzalez-Hidalgo, C. et al. Differential mRNA expression of inflammasome genes NLRP1 and NLRP3 in abdominal aneurysmal and occlusive aortic disease. Ther. Adv. Cardiovasc. Dis. 12, 123–129 (2018).
Roberts, R. L. et al. Interaction of the inflammasome genes CARD8 and NLRP3 in abdominal aortic aneurysms. Atherosclerosis 218, 123–126 (2011).
Wagner, O. et al. Interleukin-1 gene cluster variants and abdominal aortic aneurysms. Thromb. Haemost. 94, 646–650 (2017).
Ren, P. et al. Targeting the NLRP3 inflammasome with inhibitor MCC950 prevents aortic aneurysms and dissections in mice. J. Am. Heart Assoc. 9, e014044 (2020).
Sawada, H. et al. Aortic iron overload with oxidative stress and inflammation in human and murine abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 35, 1507–1514 (2015).
Konior, A., Schramm, A., Czesnikiewicz-Guzik, M. & Guzik, T. J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal 20, 2794–2814 (2014).
Vara, D. & Pula, G. Reactive oxygen species: physiological roles in the regulation of vascular cells. Curr. Mol. Med. 14, 1103–1125 (2014).
Xiao, Q. et al. Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am. J. Physiol. Cell Physiol. 296, C711–C723 (2009).
Parastatidis, I., Weiss, D., Joseph, G. & Taylor, W. R. Overexpression of catalase in vascular smooth muscle cells prevents the formation of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 33, 2389–2396 (2013).
Cai, H. & Harrison, D. G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 87, 840–844 (2000).
Lassegue, B., San Martin, A. & Griendling, K. K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 110, 1364–1390 (2012).
Helmcke, I., Heumuller, S., Tikkanen, R., Schroder, K. & Brandes, R. P. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid. Redox Signal 11, 1279–1287 (2009).
Gavazzi, G. et al. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension 50, 189–196 (2007).
Xiong, W. et al. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 202, 128–134 (2009).
Siu, K. L. et al. NOX isoforms in the development of abdominal aortic aneurysm. Redox Biol. 11, 118–125 (2017).
Lau, D. & Baldus, S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharm. Ther. 111, 16–26 (2006).
Kim, H. W. et al. Role of myeloperoxidase in abdominal aortic aneurysm formation: mitigation by taurine. Am. J. Physiol. Heart Circ. Physiol. 313, H1168–H1179 (2017).
Ramos-Mozo, P. et al. Proteomic analysis of polymorphonuclear neutrophils identifies catalase as a novel biomarker of abdominal aortic aneurysm: potential implication of oxidative stress in abdominal aortic aneurysm progression. Arterioscler. Thromb. Vasc. Biol. 31, 3011–3019 (2011).
Grigoryants, V. et al. Tamoxifen up-regulates catalase production, inhibits vessel wall neutrophil infiltration, and attenuates development of experimental abdominal aortic aneurysms. J. Vasc. Surg. 41, 108–114 (2005).
Lucas, M. L. et al. Oxidative stress in aortas of patients with advanced occlusive and aneurysmal diseases. Ann. Vasc. Surg. 52, 216–224 (2018).
Dubick, M. A. et al. Antioxidant enzyme activity in human abdominal aortic aneurysmal and occlusive disease. Proc. Soc. Exp. Biol. Med. 220, 39–45 (1999).
Sinha, I. et al. Differential regulation of the superoxide dismutase family in experimental aortic aneurysms and rat aortic explants. J. Surg. Res. 138, 156–162 (2007).
Gavrila, D. et al. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 25, 1671–1677 (2005).
Ponzo, V. et al. Dietary flavonoid intake and cardiovascular risk: a population-based cohort study. J. Transl. Med. 13, 218 (2015).
Dauchet, L., Amouyel, P., Hercberg, S. & Dallongeville, J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J. Nutr. 136, 2588–2593 (2006).
Carrepeiro, M. M. et al. Effect of n-3 fatty acids and statins on oxidative stress in statin-treated hypercholestorelemic and normocholesterolemic women. Atherosclerosis 217, 171–178 (2011).
Chiarelli, F. et al. Effects of irbesartan on intracellular antioxidant enzyme expression and activity in adolescents and young adults with early diabetic angiopathy. Diabetes Care 28, 1690–1697 (2005).
Greving, J. P. et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 13, 59–66 (2014).
Juvela, S., Poussa, K., Lehto, H. & Porras, M. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke 44, 2414–2421 (2013).
Korja, M., Lehto, H. & Juvela, S. Lifelong rupture risk of intracranial aneurysms depends on risk factors: a prospective Finnish cohort study. Stroke 45, 1958–1963 (2014).
Korja, M. et al. Risk factors and their combined effects on the incidence rate of subarachnoid hemorrhage-a population-based cohort study. PLoS ONE 8, e73760 (2013).
Kassell, N. F. & Torner, J. C. Size of intracranial aneurysms. Neurosurgery 12, 291–297 (1983).
Mocco, J. et al. Aneurysm morphology and prediction of rupture: an international study of unruptured intracranial aneurysms analysis. Neurosurgery 82, 491–496 (2018).
International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms-risk of rupture and risks of surgical intervention. N. Engl J. Med. 339, 1725–1733 (1998).
Wiebers, D. O. et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362, 103–110 (2003).
Juvela, S., Porras, M. & Poussa, K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J. Neurosurg. 93, 379–387 (2000).
Weir, B., Disney, L. & Karrison, T. Sizes of ruptured and unruptured aneurysms in relation to their sites and the ages of patients. J. Neurosurg. 96, 64–70 (2002).
Investigators, U. J. et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N. Engl. J. Med. 366, 2474–2482 (2012).
Carter, B. S., Sheth, S., Chang, E., Sethl, M. & Ogilvy, C. S. Epidemiology of the size distribution of intracranial bifurcation aneurysms: smaller size of distal aneurysms and increasing size of unruptured aneurysms with age. Neurosurgery 58, 217–223 (2006). discussion 217-223.
Goertz, L. et al. Impact of aneurysm shape and neck configuration on cerebral infarction during microsurgical clipping of intracranial aneurysms. J. Neurosurg. 132, 1539–1547 (2020).
Goertz, L. et al. Preoperative three-dimensional angiography may reduce ischemic complications during clipping of ruptured intracranial aneurysms. World Neurosurg. 120, e1163–e1170 (2018).
Kunz, M. et al. Interdisciplinary treatment of unruptured intracranial aneurysms: impact of intraprocedural rupture and ischemia in 563 aneurysms. J. Neurol. 260, 1304–1313 (2013).
Li, M. et al. Symptomatic and silent cerebral infarction following surgical clipping of unruptured intracranial aneurysms: incidence, risk factors, and clinical outcome. Neurosurg. Rev. 41, 675–682 (2018).
Pereira, B. J. et al. Assessment of cerebral blood flow with micro-doppler vascular reduces the risk of ischemic stroke during the clipping of intracranial aneurysms. World Neurosurg. 84, 1747–1751 (2015).
Pei-Sen, Y. et al. Predictors of postoperative cerebral ischemia in patients with ruptured anterior communicating artery aneurysms. World Neurosurg. 103, 241–247 (2017).
van der Kamp, L. T. et al. Risk of rupture after intracranial aneurysm growth. JAMA Neurol. 78, 1228–1235 (2021).
Villablanca, J. P. et al. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: growth and rupture incidence and correlation with epidemiologic risk factors. Radiology 269, 258–265 (2013).
Mehan, W. A. Jr. et al. Unruptured intracranial aneurysms conservatively followed with serial CT angiography: could morphology and growth predict rupture? J. Neurointerv. Surg. 6, 761–766 (2014).
Inoue, T., Shimizu, H., Fujimura, M., Saito, A. & Tominaga, T. Annual rupture risk of growing unruptured cerebral aneurysms detected by magnetic resonance angiography. J. Neurosurg. 117, 20–25 (2012).
Chien, A. et al. Unruptured intracranial aneurysm growth trajectory: occurrence and rate of enlargement in 520 longitudinally followed cases. J. Neurosurg. 132, 1077–1087 (2019).
Swiatnicki, W., Szymanski, J., Szymanska, A. & Komunski, P. Predictors of intraoperative aneurysm rupture, aneurysm remnant, and brain ischemia following microsurgical clipping of intracranial aneurysms: single-center, retrospective cohort study. J. Neurol. Surg. A Cent. Eur. Neurosurg. 82, 410–416 (2021).
Duan, D. M. et al. Clinical manifestations and genetic characteristics in the Taiwan thoracic aortic aneurysm and dissection cohort-a prospective cohort study. J. Formos. Med. Assoc. 121, 1093–1101 (2022).
Wolford, B. N. et al. Clinical implications of identifying pathogenic variants in individuals with thoracic aortic dissection. Circ. Genom. Precis. Med. 12, e002476 (2019).
van Laarhoven, C. et al. Polygenic susceptibility of aortic aneurysms associates to the diameter of the aneurysm sac: the aneurysm-express biobank cohort. Sci. Rep. 9, 19844 (2019).
Hosaka, A. et al. Nationwide study of surgery for primary infected abdominal aortic and common iliac artery aneurysms. Br. J. Surg. 108, 286–295 (2021).
Ma, L. L. et al. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil. Med. Res. 7, 7 (2020).
Wang, S. K. & Murphy, M. P. Immune modulation as a treatment for abdominal aortic aneurysms. Circ. Res. 122, 925–927 (2018).
Brewster, D. C. et al. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J. Vasc. Surg. 37, 1106–1117 (2003).
Della Schiava, N. & Lermusiaux, P. There is no more wound complications with transabdominal open abdominal aortic aneurysm repair compared with the retroperitoneal approach. J. Vasc. Surg. 73, 1474–1475 (2021).
Schanzer, A. & Oderich, G. S. Management of abdominal aortic aneurysms. N. Engl. J. Med. 385, 1690–1698 (2021).
Buck, D. B., van Herwaarden, J. A., Schermerhorn, M. L. & Moll, F. L. Endovascular treatment of abdominal aortic aneurysms. Nat. Rev. Cardiol. 11, 112–123 (2014).
Yodsanit, N. et al. Recent progress on nanoparticles for targeted aneurysm treatment and imaging. Biomaterials 265, 120406 (2021).
Kitagawa, T. et al. RGD targeting of human ferritin iron oxide nanoparticles enhances in vivo MRI of vascular inflammation and angiogenesis in experimental carotid disease and abdominal aortic aneurysm. J. Magn. Reson. Imaging 45, 1144–1153 (2017).
Camardo, A., Carney, S. & Ramamurthi, A. Assessing the targeting and fate of cathepsin k antibody-modified nanoparticles in a rat abdominal aortic aneurysm model. Acta Biomater. 112, 225–233 (2020).
Nosoudi, N. et al. Prevention of abdominal aortic aneurysm progression by targeted inhibition of matrix metalloproteinase activity with batimastat-loaded nanoparticles. Circ. Res. 117, e80–e89 (2015).
Morimoto, K. et al. Free-radical scavenger edaravone inhibits both formation and development of abdominal aortic aneurysm in rats. J. Vasc. Surg. 55, 1749–1758 (2012).
Molacek, J. et al. Optimization of the model of abdominal aortic aneurysm-experiment in an animal model. J. Vasc. Res. 46, 1–5 (2009).
Turnbull, I. C. et al. Aortic implantation of mesenchymal stem cells after aneurysm injury in a porcine model. J. Surg. Res. 170, e179–e188 (2011).
Anidjar, S. et al. Elastase-induced experimental aneurysms in rats. Circulation 82, 973–981 (1990).
Houdek, K. Abdominal Aortic Aneurysm-From Basic Research to Clinical Practice (IntechOpen, 2018).
Bhamidipati, C. M. et al. Development of a novel murine model of aortic aneurysms using peri-adventitial elastase. Surgery 152, 238–246 (2012).
Gertz, S. D., Kurgan, A. & Eisenberg, D. Aneurysm of the rabbit common carotid artery induced by periarterial application of calcium chloride in vivo. J. Clin. Invest. 81, 649–656 (1988).
Freestone, T., Turner, R. J., Higman, D. J., Lever, M. J. & Powell, J. T. Influence of hypercholesterolemia and adventitial inflammation on the development of aortic aneurysm in rabbits. Arterioscler Thromb. Vasc. Biol. 17, 10–17 (1997).
Daugherty, A., Manning, M. W. & Cassis, L. A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Invest. 105, 1605–1612 (2000).
Shiraya, S. et al. Inhibition of development of experimental aortic abdominal aneurysm in rat model by atorvastatin through inhibition of macrophage migration. Atherosclerosis 202, 34–40 (2009).
Tanaka, A., Hasegawa, T., Chen, Z., Okita, Y. & Okada, K. A novel rat model of abdominal aortic aneurysm using a combination of intraluminal elastase infusion and extraluminal calcium chloride exposure. J. Vasc. Surg. 50, 1423–1432 (2009).
Golledge, J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 16, 225–242 (2019).
Patelis, N. et al. Animal models in the research of abdominal aortic aneurysms development. Physiol. Res. 66, 899–915 (2017).
Korkmaz, B., Moreau, T. & Gauthier, F. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie 90, 227–242 (2008).
Speicher, J. E. & Traverso, L. W. T1740 pancreatic exocrine function in patients undergoing distal pancreatectomy as assessed by human stool elastase-1. Gastroenterology 134, A-888–A-888 (2008).
Nchimi, A. et al. Multimodality imaging assessment of the deleterious role of the intraluminal thrombus on the growth of abdominal aortic aneurysm in a rat model. Eur. Radiol. 26, 2378–2386 (2016).
Anidjar, S., Dobrin, P. B., Eichorst, M., Graham, G. P. & Chejfec, G. Correlation of inflammatory infiltrate with the enlargement of experimental aortic aneurysms. J. Vasc. Surg. 16, 139–147 (1992).
Carsten, C. G. 3rd et al. Elastase is not sufficient to induce experimental abdominal aortic aneurysms. J. Vasc. Surg. 33, 1255–1262 (2001).
Pyo, R. et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Invest. 105, 1641–1649 (2000).
Chiou, A. C., Chiu, B. & Pearce, W. H. Murine aortic aneurysm produced by periarterial application of calcium chloride. J. Surg. Res. 99, 371–376 (2001).
Longo, G. M. et al. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Invest. 110, 625–632 (2002).
Yoshimura, K. et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat. Med. 11, 1330–1338 (2005).
Allaire, E. et al. Paracrine effect of vascular smooth muscle cells in the prevention of aortic aneurysm formation. J. Vasc. Surg. 36, 1018–1026 (2002).
Daugherty, A. & Cassis, L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor -/- mice. Ann. N. Y Acad. Sci. 892, 108–118 (1999).
Lu, H. et al. Hypercholesterolemia induced by a PCSK9 gain-of-function mutation augments angiotensin ii-induced abdominal aortic aneurysms in C57BL/6 mice-brief report. Arterioscler. Thromb. Vasc. Biol. 36, 1753–1757 (2016).
Cooper, H. A. et al. Targeting mitochondrial fission as a potential therapeutic for abdominal aortic aneurysm. Cardiovasc. Res. 117, 971–982 (2021).
Liu, S. et al. Mineralocorticoid receptor agonists induce mouse aortic aneurysm formation and rupture in the presence of high salt. Arterioscler. Thromb. Vasc. Biol. 33, 1568–1579 (2013).
Wu, X. et al. P-selectin glycoprotein ligand-1 deficiency protects against aortic aneurysm formation induced by DOCA plus salt. Cardiovasc. Drugs Ther. 36, 31–44 (2022).
Guo, D. C. et al. LOX Mutations predispose to thoracic aortic aneurysms and dissections. Circ. Res. 118, 928–934 (2016).
Pereira, L. et al. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat. Genet. 17, 218–222 (1997).
Larsson, J. et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 20, 1663–1673 (2001).
Lindsay, M. E. et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat. Genet. 44, 922–927 (2012).
Golledge, J., Krishna, S. M. & Wang, Y. Mouse models for abdominal aortic aneurysm. Br. J. Pharm. 179, 792–810 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81970425), and by the National High Technology Research and Development Program of China (2020YFA0803700), and by Hangzhou Qianjiang Distinguished Expert Project (Prof. Lemin Zheng).
Author information
Authors and Affiliations
Contributions
All authors have read and approved the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, J., Cao, H., Hu, G. et al. The mechanism and therapy of aortic aneurysms. Sig Transduct Target Ther 8, 55 (2023). https://doi.org/10.1038/s41392-023-01325-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-023-01325-7
This article is cited by
-
LncRNA CARMN inhibits abdominal aortic aneurysm formation and vascular smooth muscle cell phenotypic transformation by interacting with SRF
Cellular and Molecular Life Sciences (2024)
-
Aortic aneurysms: current pathogenesis and therapeutic targets
Experimental & Molecular Medicine (2023)
-
Glucagon-Like Peptide-1 Inhibits the Progression of Abdominal Aortic Aneurysm in Mice: The Earlier, the Better
Cardiovascular Drugs and Therapy (2023)
-
Interaktion zwischen Darmmikrobiom und Aorta – Wirkung kurzkettiger Fettsäuren auf die endotheliale Integrität
Gefässchirurgie (2023)