Abstract
The current understanding of lactate extends from its origins as a byproduct of glycolysis to its role in tumor metabolism, as identified by studies on the Warburg effect. The lactate shuttle hypothesis suggests that lactate plays an important role as a bridging signaling molecule that coordinates signaling among different cells, organs and tissues. Lactylation is a posttranslational modification initially reported by Professor Yingming Zhao’s research group in 2019. Subsequent studies confirmed that lactylation is a vital component of lactate function and is involved in tumor proliferation, neural excitation, inflammation and other biological processes. An indispensable substance for various physiological cellular functions, lactate plays a regulatory role in different aspects of energy metabolism and signal transduction. Therefore, a comprehensive review and summary of lactate is presented to clarify the role of lactate in disease and to provide a reference and direction for future research. This review offers a systematic overview of lactate homeostasis and its roles in physiological and pathological processes, as well as a comprehensive overview of the effects of lactylation in various diseases, particularly inflammation and cancer.
Similar content being viewed by others
Introduction
Since its discovery in 1780, lactate has been often wrongly assumed to be a metabolic waste product under hypoxic conditions with multiple harmful effects and to be associated with low oxygen conditions1. The lactate shuttle hypothesis describes the roles of lactate in the delivery of oxidative and gluconeogenic substrates and cellular signaling2. Brooks’ research demonstrated how lactate is formed and utilized under completely aerobic conditions3. Evidence for lactate as a significant modulator of the coordination of systemic metabolism has grown immensely4,5. No longer considered a waste product of anaerobic metabolism, lactate is increasingly being explored as a signaling molecule. Lactate has been shown to signal through its specific receptor G protein-coupled receptor 81 (GPR81)6 or to be transported into cells by monocarboxylate transporters (MCTs)7.
In the 1920s, Otto Warburg observed for the first time that tumors consume more glucose than surrounding normal tissue, leading him to propose the phenomenon of aerobic glycolysis, wherein glucose can be fermented to produce lactate instead of carbon dioxide, even in the presence of oxygen; this phenomenon is now known as the Warburg effect. The most immediate consequences of aerobic glycolysis are increased intracellular and extracellular lactate concentrations8. Warburg’s original thesis delineates the process by which irreversible mitochondrial damage leads to aerobic glycolysis in tumor cells9. In contrast, cell metabolic reprogramming leading to the inhibition of mitochondrial oxidative phosphorylation (OXPHOS) is the primary factor underlying aerobic glycolysis in tumor cells10,11. The occurrence of aerobic glycolysis can be attributed to the increased metabolic demand for ATP in proliferative cells, such as tumor cells12. Therefore, glycolysis is highly vigorous in proliferative cells, ensuring higher intracellular and extracellular concentrations of lactate than those found in cells at the resting state. Notably, lactate accumulation in the tissue microenvironment is characteristic of inflammatory diseases and cancer13. Nevertheless, overwhelming evidence suggests the occurrence of the Warburg effect in many nontumor cells and in a wide range of noncancerous diseases, such as pulmonary hypertension, pulmonary fibrosis, heart failure, atherosclerosis, and polycystic kidney disease14,15. Compelling evidence indicates how aerobic glycolysis produces lactate under stressful conditions, such as trauma, infection, myocardial infarction, and heart failure. Lactate is always an inevitable end product of glycolysis, regardless of oxygen availability.
A study published in 2019 illustrated the important role of lactate in promoting the modification of histone lysine residues16. Similar to other posttranslational modifications (PTMs), such as acetylation, succinylation, and malonylation, lactylation leads to transcriptional regulation. Epigenetic modifications that drive metabolic regulation play significant roles in inflammation and cancer17,18,19,20. Hence, this review summarizes the biological functions of lactate and lactylation in regulating immune homeostasis and promoting tumor growth and emphasizes the importance of more comprehensive study of the other functions of lactate and lactylation. Therefore, in this unique review, the important roles of histone acetyltransferases (HATs) and histone deacetylases (HDACs) in the regulation of lactylation are elaborated. In summary, we present a comprehensive description of the important findings in the areas of lactate transport and signaling and other functions of lactate/lactylation in several pathophysiological processes and in specific diseases.
Lactic acid homeostasis
Lactic acid production and clearance
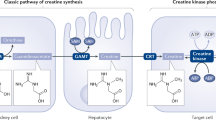
The production of lactate as fuel increases when the demand for oxygen and ATP exceeds the cellular supply, such as during strenuous exercise and infection21,22. Lactate is a classical byproduct of glucose metabolism, and the main lactate production pathway depends on glycolysis (Fig. 1). The glycolysis pathway is activated to compensate for a lack of ATP production when hypoxia inhibits the tricarboxylic acid (TCA) cycle. Specifically, glucose in the cytoplasm is converted to pyruvate through a series of classic catalytic reactions; pyruvate does not enter mitochondria for oxidation but is directly reduced to lactate in a process dependent on lactate dehydrogenase (LDH)23. The accumulation of lactate in the human body is more dangerous than the accumulation of other molecular fuels, and a rise in serum lactate can lead to lactic acidosis24; therefore, lactate needs to be rapidly metabolically removed from tissues and circulation. Irreversible lactate removal is achieved by pyruvate dehydrogenase (PDH)25 (Fig. 1), which catalyzes the formation of pyruvate, which enters the TCA cycle in the form of acetyl-CoA26. Upon entry into the TCA cycle, acetyl-CoA forms a two-carbon unit because in mammals it cannot be converted into a three-carbon unit. Hence, the systemic balance between glycolysis and PDH flux may be a key determinant of lactate levels. PDH is a component of a catalytically active complex that is regulated by the phosphorylation status of the E1α subunit and NADH, which together inhibit PDH activity, resulting in elevated levels of circulating lactate under conditions of impaired mitochondrial activity or respiration27. In addition, lactate accumulation can activate gluconeogenesis in liver and skeletal muscle cells, through which lactate is converted to glucose and released into the blood to drive additional glucose consumption during energy expenditure28.
Lactate metabolism and lactylation in cells. In the cytoplasm, lactate is transported into cells by MCTs and is produced from glycolysis or glutamine decomposition. The catabolism of lactate in cells occurs through two pathways. In one pathway, lactate is oxidized to pyruvate, which enters mitochondria and is metabolized through the tricarboxylic acid cycle. In the other pathway, lactate is converted to glucose through gluconeogenesis. Lactate can be converted into lactyl-CoA and is involved in the lactylation of histones and nonhistone proteins. LDH lactate dehydrogenase; PDH pyruvate dehydrogenase; GLUD glutamate dehydrogenase; HDACs Histone Deacetylases. (Figure was created with Biorender.com.)
In addition to glycolysis, glutamine catabolism is another source of lactate in cancer cells29. Under the regulation of c-Myc, glutamine crosses the cell membrane through the amino acid transporter type 2 (ASCT2) and sodium-coupled neutral amino acid transporter 5 (SN2), enters the cytoplasm, and is converted to glutamate by glutaminase (GLS/GLS2). Then, glutamate is converted to α-ketoglutarate (α-KG) by glutamate dehydrogenase (GLUD) or a group of transaminases, including glutamate-oxaloacetate transaminase (GOT), glutamate-pyruvate transaminase (GPT), and phosphoserine aminotransferase (PSAT); α-KG then enters the TCA cycle. In this cycle, glutamine-derived carbon is converted to oxaloacetate, which is then converted to malate and leaves the mitochondria for subsequent conversion to NADPH and pyruvate by malic enzyme (ME1) in the cytoplasm. NADPH is required for the synthesis of fatty acids and sterols and for antioxidant mechanisms, while pyruvate is a source of lactate. Through this metabolic pathway, glutamine provides the carbon skeleton for lactate production and is a secondary source for lactate production in cancer cells.
MCTs constitute a class of transmembrane lactate transporters in the solute carrier family 16 (SLC16) family7. Among the 14 identified MCTs, MCT1-4 are expressed in a variety of tissues and are involved in catalytic proton coupling and the bidirectional transport of monocarboxylic acid30. MCT1 is an important subtype that was first discovered to be widely distributed in cells and to contribute to basal homeostatic maintenance. Under physiological conditions, synergistic activity of MCT1-4 promotes lactate shuttling between glycolytic and oxidizing cells, a key factor in lactate homeostasis within different tissues. In normal tissues, the high-affinity MCT1 maintains lactate homeostasis because it is responsible for the transfer of lactate according to the transmembrane lactate gradient30. Cells with high intracellular lactate concentrations, such as tumor cells, rely on the low-affinity MCT4 for lactate transport30. The transport process begins with the binding of free protons to MCT, followed by the binding of lactate, which undergoes a conformational change within the transporter and then is expelled on the other side of the membrane. The release of protons follows the release of lactate. When MCT is deprotonated, it undergoes a conformational change, restoring its initial structure in anticipation of the next transfer. Abnormal expression or inactivation of MCT1 has been associated with a variety of diseases, including symptomatic deficiency in lactate transport (SDLT), hyperinsulinemic hypoglycemia familial 7 (HHF7), and monocarboxylate transporter 1 deficiency (MCT1D). More importantly, high expression of MCT1, MCT2, and MCT4 is closely related to the development of cancer. Lactate shuttle mediated by MCT1 and other subtypes establishes intracellular connections and is involved in the synergistic metabolism between glycolytic tumor cells and oxidative tumor cells, thereby promoting tumor occurrence and development.
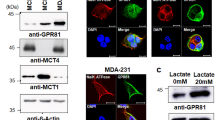
A class of G protein-coupled receptors (GPRs) on the cell membrane interact with endogenous ligands to function as intermediate metabolites in hydroxycarboxylic acid generation during cellular energy metabolism. Among these GPRs, the lactate receptor GPR81 (Fig. 2) is highly expressed in adipose tissue, the kidney, skeletal muscle, the central nervous system, the heart and other organs and tissues6. Studies have found that GPR81 mediates biological processes such as lactate-induced energy metabolism, lipodieresis, neuronal protection, and inflammatory regulation31,32,33,34.
Lactate is involved in the regulation of cellular physiological and pathological processes. In addition to the intracellular production of lactate, lactate can enter target cells through intercellular shuttling involving nonchannel pathways or MCT1. As a signaling molecule or metabolic substrate, lactate is involved in glucose metabolism, fatty acid synthesis, redox homeostasis, and the PTM of proteins. Meanwhile, as a GPR81 ligand, lactate stimulates the GPR81 signaling pathway. Lactate has been shown to regulate muscle contraction, wound healing, memory formation, and tumor development. MCT monocarboxylate transporter; OXPHOS oxidative phosphorylation; GLUT glucose transporter. (Figure was partly created with SMART – Servier Medical ART)
Roles of lactic acid in biological processes
Energy regulation
Organisms can obtain energy from glucose through OXPHOS and glycolysis, both of which begin when glucose is broken down into two pyruvate molecules. Pyruvate enters mitochondria to participate in the TCA cycle during OXPHOS but is directly reduced to lactate during glycolysis. In the presence of oxygen, the production of each molecule of glucose through OXPHOS in the mitochondrial electron transport chain (ETC) leads to the rapid release of 36 or 38 molecules of ATP, but in the absence of oxygen, electron transfer cannot occur in mitochondria, and therefore, glycolysis becomes the only available pathway for glucose production35. The traditional view suggests that glucose consumption involves many more processes than lactate consumption and that lactate is a minor byproduct of rapid energy production under anaerobic conditions that is valuable only as a substrate for glucose production in the context of gluconeogenesis36 (Fig. 1). However, advances in research have led lactate to be reconsidered; it is no longer labeled a waste product but rather is recognized as a participant in glucose metabolism. Glucose has been determined to be the primary energy source in the brain, and circulating lactate is a supplementary source of glucose that satisfies excitatory brain activities when blood glucose levels are insufficient37. A study in 1988 reported that in the absence of glucose, lactate supported synaptic transmission in brain slices38. Other studies have shown that lactate can directly support neuronal activity: when lactate shuttling in hypothalamic ependymal-glial cells was inhibited, the energy balance in proopiomelanocortin (POMC) neurons was destroyed. Therefore, lactate, not glucose, is required to maintain POMC neuron activity39, which supports the energy balance in the whole body40. Lactate is largely a promoter of the TCA cycle. The concentration of lactate in circulation is higher than that of other energy substances; in mice, the level of circulating lactate was found to be 1.1-fold higher than that of glucose, and in fasting mice, the difference increased to 2.5-fold. A quantitative analysis showed that lactate has direct functions in organs, except the brain, of fasting mice, whereas glucose mainly contributes indirectly to TCA cycle metabolism. Lactate is the main fuel for the TCA cycle and is crucial for energy generation41, and studies have shown that lactate contributes more than glucose to the TCA cycle in lung and pancreatic cancer. Lactate also regulates energy based on the accumulation of both exogenous and endogenous lactate, which inhibits glycolysis through a product feedback pathway42. In addition, lactate has been found to activate Mg2+ release from the endoplasmic reticulum (ER) in a variety of cells, and this Mg2+ transport mechanism is linked to the main metabolic feedback loop and mitochondrial bioenergetic function to promote mitochondrial Mg2+ production4 (Fig. 2).
Redox buffer
As an important metabolic substrate, lactate is an intercellular and inter-tissue redox signaling molecule that provides energy for oxidative metabolism in many tissues and helps maintain redox homeostasis and tissue and whole-organism integrity. Energy metabolism is based on continuous redox reactions43. Oxidation leads to the release of electrons that are accepted by oxidized nicotinamide adenine dinucleotides (NAD+ or NADP+), which are then reduced to NADH or NADPH44; these reduced coenzymes release electrons during reoxidation through mitochondrial respiration or lactate fermentation, thus maintaining redox homeostasis in cells. In general, disruptions in homeostasis are detrimental to the body, regardless of whether the change leads to excessive oxidation or reduction. For example, when the NAD+/NADH (or NADP+/NADPH) ratio increases, cells enter an oxidation state, produce more active substances, undergo accelerating aging, and potentially cause cardiovascular disease; in contrast, in a high-reduction state, cells can accept electrons, their capacity to prevent oxidative stress is greatly reduced, and glycolysis is inhibited. Cytoplasmic LDH and mitochondrial ETC complex I are the main drivers of NADH oxidation to NAD+. First, the production and removal of lactate, as a metabolic intermediate, maintain electron flux through a specific process in which NADH is oxidized to NAD+ and H+, accompanied by LDH-mediated catalysis of lactate to pyruvate45. High concentrations of lactate have been shown to increase the NADH/NAD+ ratio, leading to the inhibition of GAPDH and PGDH activity and, subsequently, glycolysis and mitochondrial respiration46,47. Second, as a regulator of mitochondrial oxidative respiration, lactate controls the redox balance. When the demand for NAD+ in support of oxidation exceeds the rate of ATP turnover in cells undergoing active aerobic glycolysis, NAD+ is regenerated under conditions of limited mitochondrial respiration. At this point, cells tend to undergo glycolysis, which can lead to an increased NAD+/NADH ratio, and in response, PDH activation increases pyruvate oxidation, which can attenuate lactate accumulation and reduce the NAD+/NADH ratio48. When the mitochondrial ETC is dysfunctional, the intracellular NADH/NAD+ ratio is increased, and the lactate/pyruvate (L/P) ratio is increased in a reactive manner. When lactate oxidase (LOX) and catalase (CAT) irreversibly convert extracellular lactate into pyruvate in a timely manner, the intracellular NADH/NAD+ ratio tends to normalize, and ATP production increases49. Reactive oxygen species (ROS) are produced by the mitochondrial ETC50. Mitochondrial stress leads to increased ROS production, driving cells into a high oxidation state51. Data have confirmed that when lactate is actively oxidized, many ROS are produced in mitochondria, and excess ROS may lead to oxidative damage that can severely and irreversibly harm cells if not neutralized in a timely fashion52,53. A recent study found that the increased intake of lactate by neurons promoted ROS production, enhanced mitochondrial energy metabolism, and produced an oxidative state in neurons. Oxidative stress impairs ATP synthesis in mitochondria, resulting in higher ROS production; this vicious cycle ultimately leads to axon degeneration in the peripheral nervous system54. In addition, the intercellular transport of lactate is critical for the maintenance of the redox state. The inhibition of MCT1 and MCT4 activity and the continued lactate efflux result in intracellular acidification that inhibits LDH activity, leading to a greater loss in NAD+ regeneration capacity, ATP depletion, and ultimately cell death55. In conclusion, lactate is a redox buffer that contributes greatly to the oxidation state. However, when the ratio of oxidizing coenzyme to reducing coenzyme is unbalanced, lactate responds by regulating other forms of energy metabolism to stabilize the redox state of cells.
Regulator of fatty acid metabolism
Fatty acid anabolism is essential for cell membrane structure and function, energy storage, and signal transduction56. Lactate has been reported to accumulate at high concentrations, ranging from the physiological concentration of 1.5–3 to 10–40 mM, in inflammatory environments57. Lactate accumulation is known to promote fatty acid synthesis in cells, and lactate can replenish the intracellular pool of acetyl-CoA, which is necessary for fatty acid synthesis. Specifically, lactate has been reported to increase the activation of acetyl-CoA carboxylase (ACC), a key enzyme that regulates fatty acid synthesis, and to enhance the anabolism of fatty acids. Interestingly, Interestingly, lactate can induce CD4+ T cells to upregulate the expression of the lactate transporter SLC5A12, which mediates the uptake of lactate by CD4+ T cells, forming a positive feedback loop to increase the synthesis of fatty acids58. The lactate produced by glial cells can be transported into neurons, where it promotes adipogenesis by mediating ROS production59. A study on rat muscle showed that lactate underwent glyceroneogenesis, a seemingly unconventional way to promote fatty acid synthesis60. The fatty acid catabolism pathway involves β-oxidation, which releases a large amount of energy. Despite reports that prolonged exposure to lactate increased oleic acid oxidation61, the current understanding suggests that lactate inhibits fatty acid catabolism. A study of metabolic responses to exercise showed that the accumulation of circulating lactate during exercise was inversely related to fat oxidation62. Similarly, a study of post-acute sequelae of COVID-19 (PASC) found that patients with PASC presented with significant β-oxidation disorders and lactate accumulation in the blood during exercise63. Although the mechanism by which lactate regulates β-oxidation is currently unclear, it seems to be related to lactate signaling during exercise-induced mitochondrial adaptation. Some studies have shown that lactate production can be stimulated by treatment with high levels of lipids64, but further study is needed to determine whether a lactate feedback loop increases the inhibitory effect on fatty acid oxidation. Clearly, the effect of lactate on fatty acid oxidation is not unilateral. In the inflammatory stress response, acetyl-CoA produced by fatty acid oxidation can promote glycolysis through nonenzymatic acetylation, which can promote lactate formation65.
Lactate shuttle
The lactate shuttle theory mainly describes intracellular and intercellular lactate shuttling, summarizing the entire process of lactate transmembrane migration66. As mentioned above, the complete removal of lactate through oxidation is achieved through the conversion to pyruvate by LDH, but the cellular location of this reaction remains unclear. The initiation of lactate oxidation to pyruvate is generally considered a cytoplasmic reaction, but to date, no evidence strongly supports this assumption. In fact, numerous studies have reported that contractions in skeletal muscle and beating heart muscle exponentially increase the lactate/pyruvate (L/P) ratio67. Considering this finding and a dynamic LDH model, Brooks proposed that lactate is shuttled within cells68. According to this theory, the lactate level increases more rapidly than the pyruvate level during exercise. Moreover, the oxidation of lactate to pyruvate does not seem to occur in the cytoplasm, where lactate is generated. In addition, lactate can enter mitochondria, where it undergoes direct oxidation without first being converted into pyruvate in the cytoplasm68. To reinforce this theory, Brooks proposed the mitochondrial lactate oxidation complex (mLOC) model, which involves MCT, its membrane partner basigin (BSG or CD147), LDH, and cytochrome oxidase (COX). According to this theory, the mLOC is located in the outer mitochondrial membrane and can oxidize lactate to pyruvate. However, this theory and model are controversial because no lactate transporter in this context has been identified and there is no evidence that LDH is activated in this context. In fact, one experimental study showed that mitochondria isolated from rat skeletal muscle failed to oxidize lactate and that LDH activity in mitochondria constituted only 0.7% of total cellular LDH activity69. However, another study found that MCT1, MCT2, and LDH colocalized with COX, a mitochondrial marker, in mouse cortical, hippocampal and thalamic neurons70. In another study, mitochondria isolated from the heart, skeletal muscle, and liver of rats were incubated with lactate and showed the ability to oxidize lactate71. Colocalization of MCT1, CD147, and LDH in the mitochondrial intima of L6 muscle cells provided evidence for the mLOC72. Hashimoto et al. summarized the following six points in support of the intracellular lactate shuttle theory. One, LDH was detected in mitochondria of the myocardium, liver and skeletal muscle of rats and humans by electron microscopy and laser scanning confocal microscopy. Two, the results of these analyses showed that mitochondria in the skeletal muscle and myocardium of rats and humans colocalized with MCT1. Three, LDH and MCT1, along with its molecular chaperone CD147, have been found in mitochondria isolated from rat and human skeletal muscle, myocardium, liver and kidney. Four, physiological experimental data showed that mitochondria oxidize lactate more quickly than pyruvate. Five, mitochondria in the myocardium and skeletal muscle cells of humans and other mammals can oxidize lactate, as indicated by isotope tracing and nuclear magnetic resonance imaging (MRI). Six, the results of mitochondrial proteomic studies support the lactate shuttle hypothesis71. The intracellular lactate shuttle theory subverts conventional thinking, to a certain extent, because it updates the theory of inherent lactate oxidation in cells. Furthermore, the lactate shuttle theory suggests that lactate production during exercise is an adaptive response by cell signaling molecules, explaining the mechanism through which training enhances lactate removal through oxidation.
The concept of intercellular lactate shuttling was proposed and systematically explained in 19852. The theory suggests that at the beginning of exercise, lactate is rapidly produced and accumulates in muscle cells; then, some of this lactate enters tissues, where it is internalized and oxidized by adjacent cells, whereas the remaining lactate enters the blood circulatory system and is delivered to the heart, liver, and kidney, where it is a substrate for oxidative energy production and gluconeogenesis. Brooks and his team explained the important roles of lactate identified during the formation and validation of the lactate shuttle theory. They not only confirmed that aerobic oxidation is the main pathway of lactate removal during and after exercise but also emphasized the function of lactate as an energy source and a substrate for gluconeogenesis. More importantly, this theory recognizes a new biologically significant role of lactate by detailing how it regulates oxidation and intercellular signal communication73,74,75. With advances in research, lactate shuttling between skeletal muscle and the heart has been increasingly reported and verified to provide energy to the heart76. Lactate shuttling has also been identified between cardiomyocytes and fibroblasts. In a coculture system, the production of lactate increased in fibroblasts, and MCT1, which induces the influx of lactate, migrated to the myocardial membrane77. This study provided direct evidence that lactate is a paracrine signaling molecule. Another study showed that in the brain, lactate produced by astrocytes entered neurons, subsequently participated in energy metabolism, and was converted into pyruvate and acetyl-CoA to regulate fatty acid synthesis59. Defective lactate shuttling from glial cells to neurons led to dysregulated brain metabolism, causing degeneration similar to that in Alzheimer’s disease (AD)78. In line with these findings, disruption of lactate shuttling has been shown to negatively affect motor function and destabilize motor units79. In the kidney, lactate is produced by the proximal tubule and consumed by the distal tubule, and the lactate shuttle allows lactate to fulfill its function as a fuel through transfer from the proximal to the distal nephron80. In summary, the lactate shuttle theory is applicable to a variety of practical areas, such as sports nutrition and hydration, acidosis, the treatment of traumatic brain injury, the maintenance of blood glucose, the reduction of inflammation, cardiac support after heart failure, and myocardial infarction, and the enhancement of cognition, whereas dysregulation of the lactate shuttle disrupts metabolic flexibility and supports tumorigenesis81.
Lactate shuttling between different cell populations in the tumor microenvironment (TME) is a new phenomenon in the field of tumor biology. Lactate shuttling occurs in many physiological and pathological conditions, where in lactate is exported by one cell type and imported by another cell type. The well-known Cori cycle involves lactate shuttling between skeletal muscle and the liver. Because lactate is an energy-rich metabolite that can be used as a precursor for gluconeogenesis and ATP synthesis, it is especially important for this metabolite to shuttle through the TME, which contains hypoxic and normoxic cell populations. In essence, glycolysis is dependent on oxygen as cancer cells cannot oxidize lactatea, and glucose is known to decrease oxygen uptake; therefore, mitochondrial respiration is used for ATP synthesis. As the understanding of tumor cell heterogeneity based on oxygen availability has increased, there has been a paradigm shift in our current understanding of cancer related to the Warburg effect. Because tumors grow faster than blood vessels can form, cancer cells close to blood vessels receive oxygen and are therefore normoxic, while those farther from blood vessels lack sufficient oxygen supply and are hypoxic. Specifically, hypoxic tumor cells utilize LDH-A to produce lactate, which is exported from the cell to be absorbed by normoxic tumor cells, which convert it to pyruvate through LDH-B to produce ATP. According to the functional characteristics of MCT1 and MCT4 and the differential regulation of hypoxia-related genes, MCT4 mediates the release of lactate by hypoxic tumor cells, while MCT1 mediates the uptake of lactic acid by normoxic tumor cells, highlighting the mutual relationship and metabolic symbiosis between cancer cells in different parts of a single tumor. This metabolic symbiosis occurs between different types of cancer cells within the tumor and between normoxic cancer cells and tumor-associated stromal cells.
Lactylation modification
Yingming Zhao at the University of Chicago used high-performance liquid chromatography (HPLC)–tandem mass spectrometry (MS/MS) to detect core histone proteins in human MCF-7 cells. They found that the mass shift on the lysine residues of three proteolytic peptides was the same as that caused by the addition of a lactyl group to the lysine ε-amino group16. This study demonstrated for the first time the presence of histone lysine lactylation (Kla) and indicated that Kla is a new type of epigenetic modification that occurs after the translation of lactate-derived proteins. Surprisingly, many studies have shown the accumulation of histone Kla on gene promoters in cells stimulated by hypoxia, interferon (IFN)-γ, lipopolysaccharide (LPS), or bacterial attack to produce lactate16,82, thereby directly regulating gene expression16 (Fig. 1).
To date, research on Kla has focused on both histone and nonhistone aspects. Histone Kla has unique time dynamics compared to those of histone acetylation. Histone Kla is significantly increased on the promoters of M2-like genes in the later stage of stimulated M1 macrophage polarization, suggesting that histone Kla probably acts as a lactate clock to promote the switch from an inflammatory phenotype to a steady-state phenotype in macrophages. This switch occurs in the later stages of inflammation, which may be related to wound healing. B-cell adapter for PI3K (BCAP), a signal adapter for Toll-like receptors (TLRs), has been shown to play a vital role as an internal cell switch in promoting macrophage gene expression that supports the transition from a proinflammatory state to a reparative state83,84. Ricardo et al. revealed that mice with macrophage-specific BCAP deletion had decreased Arg1 and Klf4 expression, failed to recover from dextran sodium sulfate-induced colitis, and eventually died82. Other studies found that BCAP deficiency also resulted in defective aerobic glycolysis and reduced lactate production, causing decreased histone Kla. The addition of exogenous sodium lactate (NaLa) to bone marrow-derived macrophages (BMDMs) lacking BCAP promoted histone Kla and recovered the decreases in ARG1 and Klf4 expression mediated by BACP deficiency82. These observations led to the conclusion that BCAP is an upstream adapter that connects TLR signals with the optimal aerobic glycolysis in macrophages, resulting in the lactate production necessary for proper histone Kla to promote the rescue of gene expression. This phenomenon thereby promotes the transformation of macrophages from a proinflammatory phenotype to a reparative phenotype. Another recent study found that lactate produced by probiotic Saccharomyces cerevisiae effectively inhibited BMDM activation and thus attenuated ulcerative colitis, as lactate increased H3K9 acetylation and H3K18 lactylation in BMDMs. Regarding, the specific mechanism for this therapeutic effect, the genes or proteins regulated by H3K18 lactylation may be involved in the inhibition of M1 macrophage polarization and the NLRP3 inflammasome85. In addition, increased lactate levels in human alveolar macrophages led to an increase in histone Kla86. Increased levels of histone Kla were observed in a mouse model of bleomycin-induced pulmonary fibrosis and in human pulmonary fibrosis. Results from CHIP assays using lactate-treated BMDMs confirmed increased histone Kla in the promoter regions of the ARG1, PDGFA, THBS1, and VEGFA genes, leading to their significant upregulation. It is obvious from these observations that lactate activates gene expression by inducing histone Kla of the promoters of profibrotic mediators.
AD was recently shown to be associated with histone lactylation87. Studies showed increased lactylation of H4K12 in AD mice, and this histone modification was enriched at the promoter of glycolysis-related genes and activated transcription, thereby increasing glycolytic activity. Finally, activation of the positive feedback cycle of glycolysis-H4K12La-PKM2 was shown to exacerbate microglial dysfunction in AD87.
Histone Kla has been proven to promote tumor development (Fig. 3). Yu et al. found that increased histone Kla was associated with the poor prognosis of patients with ocular melanoma88. Inhibition of histone Kla in ocular melanoma cells confirmed the significant positive correlation of ocular melanoma with intracellular histone Kla. The underlying mechanism leading to the occurrence of melanoma involves activating YTHDF2 expression by increasing histone Kla of the promoter. YTHDF2, a reader of m6A, plays a vital role in promoting tumorigenesis in ocular melanoma. Although some studies support the notion that histone Kla is essential for tumor growth, others state that histone Kla cannot transform normal cells into cancerous cells, indicating a role of histone Kla in promoting the growth of developing tumors rather than driving initial tumorigenesis. Tumor cells interact with extracellular matrix (ECM) components to form a complex TME. Hypoxia and deficiencies in blood-derived nutrients are the main characteristics of the TME, and tumor cells adjust their metabolism to survive in this unfavorable environment89. A subsequent study on non-small cell lung cancer (NSCLC) confirmed that histone Kla downregulated the gene expression of the glycolysis-related enzymes hexokinase (HK)-1 and pyruvate kinase (PKM) and upregulated that of the TCA cycle-related enzymes succinate dehydrogenase (SDH) and isocitrate dehydrogenase (IDH)90. These results reveal that lactate-mediated changes in metabolic gene expression via histone Kla induce glucose uptake by tumor cells; therefore, lactate plays an essential role in the metabolic disorders of NSCLC. Similarly, the study by Zhao et al. verified that ARG1 gene expression was positively correlated with histone Kla in TAMs isolated from B16F10 melanoma and LLC1 lung tumor tissues16. In addition, histone Kla has been detected in various other tumor cell lines, such as HeLa cells, MCF-7 cells, and HepG2 cells (Table 1); these data support further investigation into the mechanisms by which histone Kla is regulated in these cells. In summary, lactate in the TME induces immunosuppression and promotes immune evasion in tumors, thereby helping maintain tumor survival and growth. Moreover, histone Kla fulfills the goals of lactate-mediated signaling to support tumor growth. Additionally, histone Kla regulates the transcription of tumor-related genes such as YTHDF2, which promotes tumor growth, metastasis, and invasion by encouraging the degradation of downstream tumor suppressors. Histone Kla induces the expression of TCA cycle-related enzymes such as SDH, which promotes tumor cell metabolism and growth. The genetic and phenotypic heterogeneity of tumor cells is a major obstacle to cancer treatment. Random genetic changes create highly chaotic and unpredictable intratumoral heterogeneity. Hence, the regulation of histone Kla could be a powerful target for adjuvant treatment to influence the epigenetic landscape of tumors.
Mechanism by which lactylation promotes tumorigenesis. Lactylation leads to tumor immunosuppression by maintaining TAM homeostasis. Lactylation directly promotes the expression of the oncogene YTHDF2 in tumor cells. Lactylation maintains the metabolic homeostasis of tumor cells. Lactylation may lead to tumor immunosuppression and immune escape by inhibiting the function of various immune cells in the TME. HK-1 hexokinase-1; G6PD glucose-6-phosphate dehydrogenase; PKM pyruvate kinase; SDH succinate dehydrogenase; IDH isocitrate dehydrogenase; TCA cycle tricarboxylic acid cycle
Kla has been found to also occur on nonhistone proteins. In terms of acute inflammation, abnormally elevated levels of lactate in the blood of sepsis patients can be taken up by macrophages, thereby increasing the Kla of intracellular HMGB1 protein91. This research also proved that lactate can mediate the transfer of HMGB1 from the nucleus to the cytoplasm through GPR81 and MCTs and can cause an increase in HMGB1 modification by lactylation. Meanwhile, lactylated HMGB1 is secreted and released through the exosome pathway, whereby it damages endothelial integrity and increases vascular permeability, leading to endothelial barrier dysfunction and promoting the development of sepsis. A recent study revealed that lactate accumulated in the TME regulated the N6-methyladenosine (m6A) modification of tumor-infiltrating myeloid cells (TIMs) mediated by the RNA methyltransferase METTL3 through Kla to promote the immunosuppressive function of TIMs and mediate tumor immune escape92. Moreover, lactate promoted METTL3 transcription in TIMs through histone Kla. In addition, Kla can occur in the zinc finger domain (ZFD) of METTL3, which functions as the target recognition domain (TRD), thereby enhancing METTL3 binding and catalysis of the m6A modification of target RNA. Systemic lupus erythematosus (SLE) is an autoimmune inflammatory connective tissue disease. Defects in red blood cell (RBC) development have been reported as a possible trigger of SLE. During RBC maturation, the regulatory metabolic switch responsible for activating the ubiquitin proteasome system (UPS) is mediated by hypoxia-inducible factor (HIF). However, the UPS undergoes Kla in SLE93, which impacts UPS activation mediated by the metabolic switch, resulting in an inability to clear mitochondria by autophagy and a consequent increase in these organelles in mature RBCs. Once abnormal RBCs are taken up by macrophages, the mitochondrial DNA in the RBCs stimulates the powerful inflammatory cGAS/STING pathway, which promotes the production of type I IFN and causes SLE93. Recently, a study explained how lactate levels in the brain are regulated by systemic changes in brain cells, neural excitement, and behavior-related stimuli, thereby leading to Kla94. Murine brain neurons stimulated with electrical convulsions were excited, accompanied by an increase in Kla. In the social frustration stress model of depression with elevated brain lactate levels, the increase in brain neuron excitability also increased Kla. Studies have reported reduced social behavior and increased anxiety-like behavior in mice. It is hypothesized that stress-induced meta-excitation may lead to Kla, which affects emotion-related behaviors in such cases. The report mentioned above was from a pioneering study that led to the discovery of the potential role of Kla in neuronal activity.
Classical acyltransferases such as p300/CREB binding protein (CBP) are known to catalyze various acylation modifications, including the acetylation of transcription factors, histones, and other nuclear proteins, thereby regulating gene expression95,96,97. In vitro cell-free experiments showed that p300 may catalyze the chemical Kla reaction, with a strong dependence on the p53 pathway. p300/CBP has been confirmed by several studies to regulate histone lactylation in macrophages and induced pluripotent stem cells (iPSCs)16,86,91,98,99. In addition, there are reports that p300 can serve as a “writer” of lactylation on the YTHDF2 promoter in ocular melanoma cells88. Another major study that led to the discovery of histone Kla demonstrated that the addition and removal of this modification were enzymatic processes driven by HDACs. A detailed analysis of the mechanism of delactylation was carried out in vitro using 18 recombinant HDACs and core histones as substrates; the results revealed that HDAC1-3 and SIRT1-3 reduced histone (including H3K18 and H4K5) Kla and that HDAC3 was the most potent eraser of Kla99.
Consistent with many other PTMs, Kla is theoretically regulated by adding and removing lactyl groups from histone proteins100,101. However, the currently understood biochemical process of lactylation may rely on the function of two metabolic mechanisms. Among the components of these mechanisms, lactyl-CoA is closely associated with enzymatic lactylation, and lactyl-glutathione (LGSH) participates in nonenzymatic lactylation (Fig. 1)102. Studies have shown that methylglyoxal (MGO) is a byproduct of glucose metabolism; in glycolysis, MGO is generated to produce triose phosphate and glyceraldehyde triphosphate through spontaneous nonenzymatic dephosphorylation103. Under physiological conditions, glyoxalase (GLO) activity maintains a low level of MGO. GLO1 promotes the synthesis of glutathione (GSH) and MGO to produce D-LGSH, and GLO2 hydrolyzes LGSH to produce GSH and D-lactate104. A previous study found an increase in Kla accompanied by a significant increase in LGSH in GLO2-knockout cell lines102. This study further revealed that proteins that underwent Kla were enriched in carbon metabolism and glycolytic pathways. Taken together, these data indicate that glycolysis-related proteins undergo nonenzymatic Kla regulated by glucose metabolites.
Lactate and allosteric binding
Lactate is commonly produced as three isomers, D-lactate, L-lactate, and racemic DL-lactate, because of carbon atom asymmetry. L-Lactate is the main form in the human body, and LDH-A reduces pyruvate to lactate. Studies showed that excessive lactate accumulation in the cytoplasm of HepG2 hepatoma cells exposed LDH-A to an acidic environment that induced an allosteric transformation resulting in reduced activity105.
In contrast, D-lactate is a primary metabolite in gut bacteria106, and only approximately 1–5% of L-lactate content is derived from pyruvate metabolism107. Generally, D-LDH is not present in mammals, and D-lactate is metabolized by D-α-hydroxy acid dehydrogenase, which is active in only a very narrow pH range and induces very slow catalysis. Therefore, most scholars believe that D-lactate content in humans under normal conditions is too low to activate enzymes related to catabolism108. However, recent studies have suggested that D-LDH is expressed in human and mammalian mitochondria109. Moreover, D-lactate has been shown to be metabolized more readily than initially thought, as confirmed by studies on the half-life of D-lactate in plasma and its excretion in urine after infusion or oral administration110,111. D-Lactate may be involved in the transport of metabolic substrates in vivo. Studies have reported the identification of three new D-lactate/H+ cotransporters, D-lactate/pyruvate reverse transporters, and D-lactate/malate reverse transporters that transport D-pyruvate from the cytoplasm to the mitochondrial membrane. Hence, D-lactate/malate reverse transporters are suspected to localize to the mitochondrial intima where D-pyruvate is transported after mitochondrial D-LDH-mediated oxidation, and malic acid is transported in the reverse direction to the cytoplasm108.
Roles of lactic acid in pathophysiological processes
Inflammatory responses
Inflammatory response involves a variety of acute and chronic diseases in almost all organs112,113,114,115,116. In addition to participating in inflammatory injury and immune energy metabolism, accumulated lactate triggers the activation of a series of cellular signaling pathways that regulate inflammatory progression and tumor immune tolerance. Notably, these regulatory effects are not related to the ability of lactate to acidify the cellular environment. The occurrence of acute inflammation is generally considered a host defense mechanism, but the unrestrained activation of acute inflammation will lead to tissue necrosis and prolonged disease. Recent studies have confirmed that lactate has an inhibitory effect on acute inflammation (Fig. 4).
NF-κB Signaling regulates genes involved in numerous biological processes such as innate and acquired immunity, inflammation, stress response, B cell formation, and lymphoid organ formation117,118. In the classical pathway, NF-κB/Rel binds to and is inhibited by IκB119. Proinflammatory factors, LPS, growth factors, and antigen receptors activate the IKK complex (including IKKβ, IKKα, and NEMO), and the latter phosphorylates the IκB protein, leading to ubiquitination and lysosomal degradation of IκB, resulting in the release of NF-κB. Activated NF-κB is further phosphorylated and transferred into nuclear-induced target gene expression. TLRs Pathway also plays a significant role in inflammation. TLRs signal transduction from the intracellular TIR domain of the receptor (Toll/IL-1 receptor) When stimulated by ligands, MyD88 binds the IRAK(IL-1 receptor-associated kinase) to TLRs through the interaction of the two molecular death domains120. IRAK-1 is phosphorylated and activated, which then binds to TRAF6, resulting in the activation of JNK and NF-KB121. Lactate was shown to inhibit the production of inflammatory cytokines and the degranulation of mast cells in vitro122, delay the LPS-induced upregulation of monocyte inflammatory genes, and reduce nuclear NF-κB accumulation. Moreover, lactate was shown to significantly reduce the production of TNF-α and IL-6 and the activation and nuclear translocation of NF-κB and YAP through the GPR81 pathway in LPS-stimulated macrophages123,124. Lactate can inhibit TLR-mediated activation of mononuclear macrophages, delay the phosphorylation of Akt and the degradation of IκBα, and inhibit the secretion of cytokines TNF-α, IL-23, and chemokine CCL2, CCL7123.
Macrophages undergo polarization into different phenotypes based on local microenvironmental stimuli. As mentioned earlier, the activation of glycolytic pathways is accompanied by the activation of hypoxia and inflammation, which increase the production and release of lactate125. Macrophages must react quickly to synthesize and release a burst of proinflammatory factors to fight pathogenic microorganisms and simultaneously recruit more immune cells to the inflammation site to cope with bacterial infections. Such phenomena result in the M1 polarization of macrophages. During this process, macrophages secrete various proinflammatory cytokines (e.g., TNFα, IFNγ, IL-12, etc.), shifting the metabolic pattern toward aerobic glycolysis126,127,128. In contrast, M2-polarized macrophages express and release more anti-inflammatory factors (e.g., ARG1, TGFβ, etc.), which are generally involved in tissue repair and wound healing129, and in the late stage of inflammation, macrophages were often observed to have an M2-like immunophenotype which played a key role in the pathogenesis of immune system dysfunctions130. High concentration of lactate infiltration has a great impact on the polarization and cell function of monocyte-macrophages. First of all, lactate can inhibit the key glycolysis enzyme PFK-1 and promote the decomposition of active PFK-1 into less active dimer, thus reducing the glycolysis flux of monocytes131 and affect the immune function and further differentiation of monocytes. Secondly, lactate can be used as a signaling molecule to induce polarization of M2-like macrophages132. Selleri et al. reported that lactate secreted by human mesenchymal stromal cells induced the differentiation of monocytes into M2 macrophages in a dose-dependent manner133.
Mast cells are unique tissue-resident immune cells of the myeloid lineage that have long been implicated in the pathogenesis of allergic and autoimmune disorders134. Lactate was found to target MAS-associated G protein-coupled receptor X2 (MRGPRX2) expressed by mast cells to inhibit both the early (calcium mobilization and degranulation) and late (chemokine/cytokine release) phases of mast cell activation in asthma135. In addition, lactate has been shown to rely on MCT1 to inhibit the production and degranulation of inflammatory cytokines in IgE-mediated mast cells, thereby limiting mast cell-mediated inflammation122.
Studies in mouse models of colitis showed that activation of the lactate receptor GPR81 reduced inflammation136, In a DSS-induced mouse model of colitis, MCTs-mediated increased uptake of lactate inhibits overactivation of inflammasome NLRP3 and its downstream caspase-1 pathways in macrophages85. In septic acute kidney injury, the lactate-activated PD-1/PD-L1 pathway induced immunosuppression by evoking lymphocyte apoptosis137,138. In addition, lactate inhibits the activation of inflammasomes that cause liver and pancreatic damage through the GPR81 pathway139. In sepsis, accumulated lactate can inhibit the activation of NF-KB pathway, the production of inflammatory factors, the glycolysis process and ATP production through MCT1 in mast cell, and participate in the inflammatory stage to the secondary immunosuppression stage of sepsis140. Thus, local administration of lactate can be one of the potential treatments for acute inflammatory diseases.
Chronic inflammation is characterized by greater infiltration of T lymphocytes and macrophages and, in contrast to acute inflammation, is enhanced by lactate141. Lactate has been reported to inhibit the migration of T cells and thus retain T cells at the site of inflammation, thereby prolonging chronic inflammation by increasing the production of inflammatory cytokines and decreasing cell lysis142,143. In mouse arthritis models, lactate activated the expression of its transporter SLC5A12, which mediates the entry of lactate into CD4+ T cells, and promoted IL-17 production through the PKM2/STAT3 signaling pathway. Conversely, blocking SLC5A12 was shown to decrease disease severity58. Additionally, studies have shown that LDH-A is overexpressed in all CD8+ T-cell subsets in the context of rheumatoid arthritis, and LDH-A inhibition can alleviate the inflammatory and destructive effects of CD8+ T cells in the development of autoimmune diseases144; to some extent, these findings may provide indirect evidence that lactic acid promotes chronic inflammation. Enhanced glycolysis causes the localized accumulation of lactate in the fibrotic lung, which promotes the profibrotic activity of alveolar macrophages145. Studies have also found that lactate is an effective stimulant of the profibrotic phenotype of macrophages146.
The TME is hypoxic, and both hypoxia and high lactate concentrations may be key drivers of the recruitment and polarization of tumor-associated macrophages (TAMs)147. In recent years, tumor immunity has attracted increasing attention. Immunosuppression plays an important role in tumor growth and invasion. Lactate plays a crucial role in regulating the functions of macrophages and lymphocytes in the process of immune suppression.
Hypoxic tissues secrete high levels of the chemokines, HIF1/2 and endothelin-2, which attract macrophages to hypoxic areas to affect the local immune response. Continuous tumor antigen stimulation and immune activation prompt a state of exhaustion or remodeling in the immune effector cells in the TME, rendering these cells unable to perform their normal functions. TAMs integrate hypoxia and lactate levels into activation of the MAPK signaling cascade to promote malignant and tumorigenic factors, such as the expression of arginase 1 (ARG1) and mannose receptor type C1 (MRC1)148,149. M1-like macrophages in the TME inhibit tumor cell growth. M1 macrophage polarization is positively correlated with a favorable clinical prognosis in many types of cancer, while M2-polarized macrophages promote tumor occurrence and development150,151,152,153,154. Macrophages in an acidic TME tend to have an M2 phenotype: tumor-derived lactate induces M2 macrophage polarization by activating the ERK/STAT3 signaling pathway. Inhibition of ERK/STAT3 signaling is known to hinder tumor growth and angiogenesis by restraining lactate-mediated M2 macrophage polarization155. Previous studies have shown that the treatment of BMDMs with lactate extracted from tumor cells drives an M2-like phenotype characteristic of TAMs156. In summary, lactate in the TME induces immunosuppression and promotes tumor immune evasion, which helps maintain tumor growth and survival.
Inflammatory factors in the cellular microenvironment activate the immune response, thereby promoting glycolysis and increasing the production and release of lactate157. The response to lactate by different cells is highly variable. For example, immune cells in cancer respond to lactate in the opposite way as those in chronic inflammatory diseases, although both sets of cells ultimately promote the disease. Moreover, tumor-derived lactate has been reported to inhibit the immune response to the tumor itself and to promote the expression of anti-inflammatory genes, thus creating a conducive microenvironment for tumor growth158,159. Although the significant effect of lactate on cellular function is well established, its precise contributions remain to be further explored13.
It is not difficult to find interesting effects of lactate signaling. As an immune system modulator, lactate seems to have contradictory potential proinflammatory and anti-inflammatory functions. For example, lactate signaling inhibited the LPS-induced expression of a series of cytokines and chemokines, as measured in cell culture medium160. In macrophages and monocytes, lactate inhibited glycolysis and various specific receptor signaling cascades123. Lactate can also regulate and control gene expression through PTMs; therefore, lactate not only inhibits inflammatory macrophage (M1) function but also enhances the regulation of anti-inflammatory M2 polarization, helping reduce macrophage-mediated inflammation and restore homeostasis through an intrinsic regulatory feedback pathway. In contrast, lactate has been shown to enhance the macrophage secretion of IL-6, matrix metalloproteinase 1 (MMP1), and IL-1β and to increase NF-κB activity through MCTs161. In addition, lactate appears to play an inconsistent role in T cells. Lactate was shown to inhibit the proliferation, degranulation, and active cytolysis of CD8+ T cells and to release inflammatory mediators of CD8+ T cells162. However, in the CD4+ T-cell lineage, lactate can increase the release of IL-2143, inhibit the inflammatory function of regulatory T cells (Tregs), and promote the differentiation of T-helper 17 (Th17) cells163. There are three possible explanations for the different regulatory effects of lactate in immune cells. First, the microenvironment of immune cells differs from that of other somatic cells; for example, granulocyte macrophage colony-stimulating factor (GM-CSF) can induce an increase in M1 and regulatory M2 mediators in monocytes in the presence of lactate, and this induction is consistent with the phenotype acquired by TAMs164. Second, the expression of lactate transporters in effector T cells results in different functions of lactate; for example, the differential effects in CD8+ and CD4+ T cells are mediated by the selective expression of MCT-1 and SCL5A12, respectively143. Third, the concentration of lactate and its effect on culture medium acidity are related to different effects; for example, 12.5 mM lactate reduced the pH of the culture medium to 6.7 and inhibited cytokine production140,165, and adjusting the pH to 7.4 reversed the inhibitory effect of lactate in dendritic cells166. Needless to say, lactate can indeed be used as a research target for clinical diagnosis and treatment of inflammatory diseases. At present, the determination of serum lactate is of great significance for clinical monitoring of the course of diseases such as acute peritonitis, acute pancreatitis, sepsis, and septic shock. At the same time, clinical trials have confirmed that serum lactate concentration can be used to guide the medication of clinical patients, as the goal of treatment167,168, prognosis forecast169,170, and predict the prognosis171,172,173,174. Experiments have revealed that lactate can prevent cardiac dysfunction in rats with sepsis, improve microcirculation, and reduce inflammation175.
Memory formation and neuroprotection
At rest, the brain is considered a net lactate producer176,177. The lactate produced by the cells in the brain is released to the extracellular fluid gap through the membrane, and then the blood vessels or lymphatic system within the gap is led into the blood, thus entering the systemic circulation. When the blood lactate level rises, the brain transforms into a net organ of lactate uptake, and blood lactate is transported into the center by MCT on the blood-brain barrier. The uptake of lactate by brain cells increases, and the brain can remove up to 11% of the body lactate in exercise state176.
Under different physiological and pathophysiological conditions, lactate exerts different effects at the molecular and organ levels in the brain to influence behaviors, such as facilitating learning and memory and enabling the regulation of emotions. Lactate and MCT-mediated lactate transport have been shown to be important for brain energy metabolism178. Pyruvate could not rescue memory impairment caused by MCT2 downregulation in neurons, but an increase in endogenous lactate levels increased learning-induced mRNA translation and Arc/Arg3.1 expression, suggesting that lactate plays a key role as fuel for the neuronal responses required for long-term memory179. N-Methyl-D-aspartate receptors (NMDARs) are glutamate receptors and typical mediators of synaptic plasticity. Lactate enhances the NMDAR-dependent inward current flow and calcium influx induced by glutamate and glycine, thereby activating NMDARs and downstream ERK1/2 signaling and increasing the expression of c-Fos and Zif268, which are involved in neuronal plasticity and activity maintenance180,181. The abovementioned evidence for lactate shuttling between astrocytes and neurons suggests that lactate homeostasis plays a coordinating role in long-term memory formation. Disrupted gene expression of the lactate transporter MCT1 in brain endothelial cells eliminated lactate transport and impaired hippocampal neurogenesis and cognitive function182. Lactate injections prevented the destruction of memory retention caused by damage to the hippocampus, but injections of glucose at the same concentration did not have the same effect183. Studies have shown that the lactate content in the cerebral cortex and hippocampus decreases during memory impairment in mouse models of AD184. Coincidentally, exercise-generated lactate enters the hippocampus through the lactate shuttle and increases the expression of brain-derived neurotrophic factor (BDNF) by activating SIRT1185, and increases in BDNF expression improve cognition by facilitating learning and memory formation. Moreover, lactate signaling between astrocytes and neurons is disrupted in AD, amyotrophic lateral sclerosis (ALS), and schizophrenia180.
In conclusion, lactate not only acts as an energy substrate in the brain, but also plays a certain role in the maintenance of long-term memory formation and cognitive function. In addition, lactate can be used as a signal molecule to bind to GPR81 receptor in the brain186 for reducing excitatory injury, which suggests that lactate may be involved in the whole brain metabolism and functional regulation (Table 2).
Wound healing
Wound healing is a dynamic, complex biological process. Generally, wound healing can be divided into four overlapping stages, namely, hemostasis, inflammation, proliferation, and remodeling, which are regulated by various cytokines and growth factors187. Importantly, lactate is metabolized in mass during wound healing and plays an indispensable role because of the high levels of secreted cytokines and growth factors and the neovascularization that occurs upon immune system activation, resulting in increased metabolism and possibly hypoxia188. Thus, lactate, an energy substrate, can meet the high metabolic demands of wound healing. In addition, accumulated lactate reduces the pH of the alkaline environment caused by the reduction in carbon dioxide levels and the high oxygen tension, enabling cells to proliferate and differentiate within the optimized physiological pH range189. Hence, Trabold et al. suggested that lactate can be used as a substitute for oxygen to initiate healing190. Lactate also plays a role in promoting angiogenesis, as supported by the increased vascular endothelial growth factor (VEGF) levels and increased angiogenesis at oxygenated sites after the subcutaneous implantation of a matrix glue containing a lactate-releasing agent in mice191. In addition, some evidence indicates that lactate can stimulate fibroblasts to synthesize collagen in the ECM192,193. Lactate has been shown to stimulate vasogenic stem cells through the redox system194. Liu et al. demonstrated that lactate facilitated the activation of the transcription factor HIF-1α, which can regulate hypoxia195. Previously, Vural et al. found that HIF stimulated VEGF, promoted angiogenesis, and accelerated wound healing196. M2-polarized macrophages mediate wound healing through Akt, ERK1/2, and STAT3 pathway197,198,199,200, and the promoting effect of lactate on the polarization and function of M2 macrophages has been discussed.
It has been shown that lactate can promote wound healing. Preclinical trials have found that the improvement of wound healing in recombinant L.reuteri depends not only on the transformation of CXCL12 in the bacteria, but also on the combined presence of lactate produced by L.Reuteri201,202. Lactate production by L.reuteri alters the local wound environment, lowering pH, inhibiting the enzyme CD 26 that degrades CXCL12, and allowing increased bioavailability of CXCL12203.
Ischemic injury
Tissue ischemia disrupts oxygen and glucose delivery, resulting in metabolic disorders204. Hypoxia directly leads to lactate accumulation, which lowers pH to activate ion transporters205, primarily Na+/H+ exchange (NHE) proteins that regulate intracellular pH by exchanging protons for extracellular sodium ions206. In acute tissue ischemia, NHE1 elevates intracellular Na+ levels, leading to increased Ca2+–Na+ exchange and intracellular calcium overload. During ischemia/reperfusion injury, lactate induced the release of TNF-α, IL-6, and IL-1 in the myocardium and brain, further exacerbating neuronal damage in acute stroke207. Inhibition of the lactate-activated GPR81 signaling pathway plays a protective role in ischemic brain injury208. In addition, evidence suggests that lactate treatment alleviated brain damage and improved behavior in rat models of hypoxic ischemia (HI)209,210, indicating that lactate may play a neuroprotective role in ischemic hypoxic encephalopathy. In a study of acute heart failure (AHF) in zebrafish, lactate acquired as an active component of herbal extracts was found to inhibit AHF, inflammation, and cardiac hypertrophy211. Moreover, increasing lactate levels during ischemia restored the M2-like polarization of macrophages in an MCT1-dependent manner, and an increase in VEGF production by polarized macrophages generated a positive feedback loop that further stimulated angiogenesis and ultimately facilitated postischemia revascularization and the regeneration of damaged muscle132.
As a product of tissue hypoxia, lactate itself can be used as an indicator of tissue hypoperfusion. Clinically, the determination of serum lactate plays a very important role in guiding the treatment of patients with ischemic injury, especially in the treatment of tissue hypoxia and ischemia-reperfusion injury caused by cerebral ischemia and insufficient blood flow in patients with myocardial infarction212,213,214,215. In addition, lactate is considered to be a predictor of major complications after cardiac surgery216,217, the significance of blood lactate measurement in monitoring the progress of cardiac surgery has been clarified.
Tumor growth and metastasis
GPR81 and acidification function
Lactate and lactate-mediated activation of the GPR81 signaling pathway contribute to several facets of tumor progression, including cell proliferation, invasion, angiogenesis, immune tolerance, and immune cell escape from surveillance6. Because of their low perfusion, tumors are usually considered to reside in an isolated metabolic microenvironment, where local nutrient exchange predominates over circulating nutrient exchange. When the nutrient demand required for rapid tumor growth exceeds the energy supply, tumor growth depends on glycolysis, which produces a large amount of lactate. This process is called the Warburg effect, and lactate is considered a metabolic substrate that enables cancer cells to proliferate. Overexpression of TAp73, a key regulator of glycolysis, has been identified as a protector of tumor proliferation by promoting lactate production218. A large number of studies have recognized that lactate is a possible mediator of the loss of p53 signaling, which promotes tumor cell proliferation219. Lactate signals through the cell-surface receptor GPR81 in a process that is independent of MCT, protons, and cellular glucose metabolism but that plays an important role in tumor growth. Studies have shown that GPR81 expression increases in tumor cells in response to autocrine signaling by lactate; that is, the lactic acid produced by tumor cells activates GPR81 on tumor cells and produces an oncogenic phenotype220. In addition, lactate can act in a paracrine manner by activating GPR81 on nontumor cells in the TME to promote tumor growth, and tumor cell-derived lactate can activate GPR81 on dendritic cells and thus prevent tumor-specific antigen presentation to other immune cells221. A more specific study showed that GPR81 activation reduced the intracellular cAMP concentration, thereby decreasing the phosphorylation of the transcriptional activator TAZ in the Hippo pathway, promoting TAZ to enter the nucleus and bind TEAD1 (forming the TaZ-Tead1 complex), leading to the activation of PD-L1 expression to provide an effective means for tumor cells to escape the immune system222. In conclusion, lactate secreted by tumor cells acts on GPR81 of tumor cells or nontumor cells in the TME, ultimately affecting tumor cell function as well as information exchange and interactions between cells, all of which has the potential to affect tumor cell growth and proliferation. The flow of lactate out of tumor cells prevents further acidification of the intracellular environment but acidifies the extracellular environment. The mechanisms by which tumor cells can release H+ include the upregulation of NHE1 (Na+/H+ exchanger) and CAR9 (carbonic anhydrase IX)223,224. NHE1 promotes H+ efflux across the plasma membrane of cancer cells via a naturally occurring, internally oriented Na+ gradient. CAR9 catalyzes the conversion of extracellular water and carbon dioxide to carbonic acid, which is decomposed into bicarbonate and H+; then, sodium bicarbonate is transported into tumor cells through the sodium bicarbonate cotransporter HVCn1, leaving H+ outside. In addition, vacuolar (V)-type proton pumps in the tumor cell plasma membrane225 actively pump H+ out of the cell in a manner driven by direct binding and hydrolysis of ATP. Studies have confirmed that NHE1, HVCn1, and V-type proton pumps can promote the proliferation, migration, and drug resistance of tumor cells225,226,227,228,229,230,231,232,233,234, and silencing NHE1 or SLC4A7 significantly reduced tumor growth in a mouse xenograft model235. In conclusion, NHE1, CAR9, HVCn1, and V-type proton pumps act together on the plasma membrane of tumor cells to form a transmembrane pH gradient based on the inward H+ gradient. This pH regulation is crucial for tumor cell survival and proliferation. Therefore, the data support further investigation of these transporters, which promote cancer cell growth by exporting H+, as potential drug targets.
Tumor angiogenesis
We have previously discussed the role of lactate in promoting angiogenesis during wound healing. To a certain extent, the TME is similar to a trauma-related microenvironment. Lactate in the TME stimulates endothelial cell activation and angiogenesis through HIF-independent and HIF-dependent pathways. In the HIF-independent pathway, lactate is transported into cells via MCT1, wherein it is oxidized to pyruvate to produce NADH, which activates ROS production; as previously established, ROS production stimulates angiogenesis236. Another HIF-independent mechanism that facilitates angiogenesis involves the direct binding of lactate to NDRG3, a downstream regulatory protein in the N-Myc pathway; this binding event prevents NDRG3 degradation237. NDRG3 binds c-Raf to activate RAF-ERK signaling and promote angiogenesis under conditions of low oxygen tension and high lactate concentrations. HIF-1α has been reported to induce VEGF expression. The HIF-dependent pathway is based on the ability of lactate to stabilize HIF-1α under normoxic conditions. HIF-1α is a key regulator of the response to hypoxia238,239 that is continuously synthesized and degraded in the cytoplasm. Under normoxic conditions, proline hydroxylases (PHDs) are the main oxygen sensors in cells, and PHDs can hydroxylate HIF-1α at specific proline residues to promote the subsequent degradation of HIF-1α by the UPS. PHD, however, is inactivated under hypoxic conditions. This inactivation of PHD prevents the proteasomal degradation of HIF-1α, allowing HIF-1α to migrate to the nucleus, where it binds HIF-1β and promotes the transcription of many tumor-promoting genes240. Lactate has been reported to be absorbed by tumor cells and subsequently converted to pyruvate, which directly competes with α-KG to inhibit the activity of PHD, thereby stabilizing HIF-1α levels240. Hence, exogenous lactate stabilizes HIF-1α by acting as a substrate for pyruvate production, and a high lactate concentration in the TME ensures the transcriptional activation of tumor-promoting genes in all tumor cells regardless of oxygen supply.
Tumor invasion
Considerable evidence suggests that basal membrane (BM) remodeling241 and epithelial–mesenchymal transition (EMT)242 are two features of invasive tumors. Cancer-associated fibroblasts (CAFs) synthesize type I collagen, an important promoter of BM remodeling243. There is evidence that lactate promotes the proliferation and migration of CAFs and enhances the synthesis of type I collagen in CAFs244. In CAFs, COX-2 regulates the biosynthesis of type I collagen, and lactate was shown to increase COX-2 expression in a p38 kinase-dependent manner245. MMPs degrade collagen and glycoproteins and are the key collagenases that promote tumor invasion246. Lactate has been reported to promote LPS-stimulated MMP expression through the PLD/MAPK/NF-κB pathway247 and to increase caveolin1-induced MMP expression through the ERK/p90RSK pathway248. In conclusion, this evidence indicates that lactate may be a signaling molecule that increases MMP expression, leading to collagen degradation and the increased invasion of tumor cells. EMT is mainly activated by a cascade of specific factors/cytokines and related signaling pathways, and lactate has been shown to be involved in the TGF-β/Smad and Wnt/β-catenin signaling pathways, among others, that can activate EMT249.
Tumor immunity
Immune cells are an important component of the TME, and lactate is generally considered an immunosuppressive molecule that promotes malignant tumor growth due to the dependence on both H+ and lactate (Table 3). The pH in tumors can be as low as 5.6 but primarily ranges from 6.0 to 7.0250. The accumulation of lactate in the TME entails acidification of the TME, and a lower extracellular pH has been shown to impair almost all aspects of CD8+ and CD4+ T lymphocyte function, including the activation of cytotoxicity, chemotactic motility, and proliferation251. Experiments have shown that252 the accumulation of lactate in the TME entails acidification of the TME, and a lower extracellular pH has been shown to impair almost all aspects of CD8+ and CD4+ T lymphocyte function, including the activation of cytotoxicity, chemotactic motility, and proliferation253,254. During immune activation, the coactivator CD28 binds to the B7 receptor on antigen-presenting cells, stimulates the PI3K/Akt signaling pathway, and allows T cells to accelerate the synthesis of cytokines required for the immune response255,256. Specifically, for T cells, the activation of immune function directly coincides with metabolic reprogramming257; activated T cells and tumor cells both depend on glycolytic metabolism, and both must excrete lactate to avoid intracellular acidification. Due to the change in the lactate concentration gradient across the cell membrane, the accumulation of extracellular lactate from tumors prevents activated T cells from releasing lactate. In essence, high extracellular lactate in the TME leads to the accumulation of endogenous lactate within T cells, which reduces the secretion of proinflammatory cytokines258. However, this phenomenon is unique to activated cytotoxic T cells because in Tregs, the Treg-specific transcription factor FoxP3 inhibits c-Myc signaling to enable a switch to OXPHOS, thus keeping these cells active in the TME158. Alcinotto et al. used the proton pump inhibitor esomeprazole to restore normal pH and reverse the acid-induced impairment of tumor-specific CD8+ T lymphocytes in humans and mice259. Further studies confirmed that inhibition of the acidifier ion channel Ae2 or activation of the alkalization channel HVCn1 significantly improved T-cell function and the antitumor activity of Glypican 3 (GPC3)-specific CAR T cells in vitro260. In conclusion, the inhibition of lactate is a potential strategy for overcoming T-cell dysfunction under acidic pH conditions that could enhance adoptive T-cell transfer-based immunotherapy. Lactate itself has been found to mediate mechanisms of immunosuppression. High lactate production by tumor cells increases the acidity of the TME. The hypoxic TME induces the HIF-1α-mediated upregulation of PD-L1, leading to CD8+ T-cell dysfunction261. Studies have also found that SLC43A2, a methionine transporter in tumor cells, disrupts methionine metabolism in CD8+ T cells and reduces the methylation of T-cell histones, thereby inhibiting T-cell-mediated immunity262. Lactate was found to inhibit nuclear factor of activated T cells (NFAT), leading to the inactivation of tumor-infiltrating CD8+ T cells and NK cells and reduced IFNγ production263. Therefore, some researchers have speculated that lactate could ultimately promote the differentiation of Th1 and Th2 effector cells through signal transduction and metabolic regulation while inhibiting Treg induction and T-cell inactivation264,265. It is worth mentioning that a recent study found that in tumors, the metabolite lactate affected the TME by regulating the lactylation of MOESIN in Tregs and enhancing TGF-β signal transduction, thus promoting a new mechanism of tumorigenesis266. Regarding innate immunity, lactate can increase the expression of VEGF and ARG1 in TAMs, polarizing them toward the immunosuppressive and protumorigenic M2 phenotype156. The ability of lactate to mediate this process has been thought to depend on MCTs and HIF-1α stability. However, the subsequent discovery of histone lactate modification suggests a new mechanism, namely, histones can be modified by lactatelto regulate the polarization state of TAMs through increased expression of ARG1 and other TAM-related genes; thus, TAMs function through multiple mechanisms to disrupt antitumor immune responses, and their presence is considered a negative prognostic marker267. TAMs produce IL-10, an immunosuppressive cytokine268. TAMs also express the checkpoint inhibitor PD-L1, which binds to its receptor PD-1 on activated T lymphocytes and drives cell apoptosis269. In addition, TAMs express CCL22, attract Tregs to increase the production of molecules such as PD-L1, disrupt T-cell metabolism by expressing ARG1, and deprive T cells of L-arginine, a key nutrient for T-cell growth270. Therefore, lactate-driven TAM polarization is a key mechanism of malignant tumor cell immune escape. Lactate and lactylation are also associated with the release of high mobility group box protein 1 (HMGB1), a damage-associated molecular pattern (DAMP), from activated macrophages91,271. Emerging evidence suggests that DAMPs, including HMGB1, play an important role in initiating and sustaining chronic inflammation that impairs antitumor immunity and promotes cancer progression272,273. However, HMGB1 is also thought to have antitumor properties, and interestingly, it inhibits glycolysis in tumor cells, leading to metabolic cell death274. Therefore, it is necessary to clarify the effects of DAMPs such as HMGB1 on tumor metabolism and their dual roles in promoting or inhibiting cancer progression.
Clinical significance
Previous studies have highlighted how lactate creates a conducive microenvironment for tumor cell growth and represents an energy course for tumor cells. Lactate promotes tumor growth in a number of ways. On the one hand, lactate inhibits immune cell effects in the TME. Lactate blocks the activity of MCT1, a lactate transporter in immune cells in the TME275, to prevent the removal of lactate from cells and inhibits the proliferation and survival of immune cells263. Moreover, lactate-mediated signaling activates tumor cell proliferation and drug resistance through GPRs that enhance the expression of PD-L1222. Lactate also prevents dendritic cells in the TME from presenting tumor-specific antigens to immune cells221. On the other hand, lactate can be directly absorbed and metabolized by tumor cells to promote the TCA cycle41,42, and as a signaling factor that regulates the hypoxia response, lactic acid promotes tumor cell growth237. In view of its immunosuppressive role in promoting angiogenic tumor invasion and metastasis, lactate is considered an indicator of high malignancy and poor prognosis in several types of cancer. In lung cancer, elevated systemic lactate is a negative prognostic factor for metastatic NSCLC and is associated with significantly shorter overall survival276. Wei et al. found that serum lactate levels were higher in patients with metastatic colorectal cancer than in patients without systemic disease277. In addition, studies have reported that increased lactate levels are negatively correlated with the percentages of Th1 cells and cytotoxic T lymphocytes (CTLs) in tumors, reflecting the impaired immune capacity in the TME. In head and neck cancer, an increased tumor lactate concentration was identified as a predictor of subsequent lymph node metastasis or distant metastasis278. New techniques, such as nuclear magnetic resonance spectroscopy (MRS) and hyperpolarized (HP) 13C-MRI, are changing the prospects of measuring intracellular lactate concentration and increasing the feasibility of using this concentration as a tumor biomarker279,280,281,282. MRS has been used to show that an elevated lactate level within tumors is a poor prognostic indicator in breast cancer283. In addition, in HER2-positive breast cancer, elevated intratumoral lactate levels are associated with HER2 addiction status and susceptibility to trastuzumab, a HER2 inhibitor. Therefore, lactate can be used as a predictive biomarker for the optimal prescription of HER2-targeted drugs in this patient population284. MRS-based detection of lactate predicted the poor prognosis of patients with diffuse endogenous pontine glioma285. Therefore, the future development of metabolic imaging must consider the different metabolic phenotypes of tumors. Current studies on the application of lactic acid imaging as a biomarker for tumor diagnosis include NCT01881386 (the use of MRS to observe changes in tumor lactic acid levels in vivo in response to treatment), NCT04584827 (the effects of lactic acid levels on mortality and morbidity in patients undergoing intracranial mass surgery under general anesthesia), NCT03129776 (HP 13C-MRI of lactic acid in cervical tumors to identify areas of radiation resistance and to guide radiotherapy), and NCT03531307 (the association of lactic acid levels with the tumor proliferation marker Ki67 in patients with brain tumors).
Therapeutic targets
Studies have confirmed that glycolysis accelerates the metabolic switch associated with increased lactate production in tumor cells as a result of the increased expression of oncogenes, mainly c-Myc and HIF-1α286. The conversion of intracellular pyruvate to lactate is catalyzed by LDH, a heteromeric protein composed of two subunits each of LDH-A and LDH-B that exists in five isomeric forms. LDH-5 (A4) has a higher affinity for pyruvate than for lactate, which is conducive for the conversion of pyruvate to lactate, while LDH-1 (B4) has a higher affinity for lactate than for pyruvate, which is conducive for the conversion of lactate to pyruvate. In general, the induction of LDH-A and silencing of LDH-B leads to increased LDH-5 activity, decreased LDH-1 activity, and an increase in intracellular lactic acid production. The malignant proliferative and invasive potential of tumors has been shown to be dependent on LDH-A activity287, and the activation of HIF-1α and c-Myc and the inhibition of p53 regulate LDH-A gene transcription288. LDH-B is inhibited by epigenetic mechanisms such as DNA methylation289,290,291,292. The priority of glycolysis is to convert pyruvate into lactate. Because LDH-A is increased in cancer and is responsible for generating the main form of lactate, LDH-A is a potential drug target for cancer treatment. In fact, a large number of studies have shown that the inhibition of LDH-A both in vivo and In vitro can block tumor growth and invasion293,294,295,296,297. FX-11, a selective LDH-A inhibitor, has shown antitumor activity in xenograft tumor models298,299 and has potential for development as a cancer treatment. Galloflavin inhibited LDH-A activity300 and thus inhibited lactate production and proliferation in Burkitt lymphoma cells301. Chronic stress-induced epinephrine alters the pH to promote cancer stem cell-like properties in breast cancer cells through lactic acid production involving LDH-A; as vitamin C can inhibit LDH-A, it may be an effective therapeutic in stress-related breast cancer302. LDH-A is considered a safe target for cancer treatment because the loss of LDH-A protein due to an inherited deletion in the LDHA gene results in only mild myopathy303,304. Theoretically, blocking lactate production in tumor cells by inhibiting LDH-A could have some unmanageable adverse effects. For example, the intracellular accumulation of pyruvate and NADH drives ECM remodeling by inducing collagen hydroxylation, which results in increased collagen stability and thereby promotes the metastatic growth of breast cancer cells305. As pyruvate is catalyzed to lactate, a molecule of NADH is oxidized to NAD+; blocking these reactions directly leads to a lack of NAD+ for glycolysis, which slows the reaction rate. In addition, the production and activity of lactic acid play significant roles in the maintenance of cellular and biological functions and immune regulation. Therefore, even though the anticancer potential of LDH-A has been confirmed, LDH-A blockade may have many nontarget effects; these potential challenges must be overcome in the application of LDH-A inhibitors for the treatment of cancer.
The regulation of lactate by c-Myc and HIF-1α in tumors can also be achieved by the induction of MCT; c-Myc induces MCT1306,307, and HIF-1α induces MCT1 and MCT4308, the major lactate transporters utilized by cancer cells. Blocking MCT1 and MCT4 function or reducing their density in the plasma membrane are potential strategies for cancer treatment309. The rationale is that if the outlet for lactic acid in cancer cells is blocked or reduced, the resulting intracellular acidification will kill the cell; importantly, selective inhibitors of MCT1/4 have been shown to be effective cancer treatments in preclinical studies310. Syrosingopine is an effective dual inhibitor of MCT1 and MCT4311 that has been proven to sensitize cancer cells to metformin in vivo312, thus enhancing its antitumor activity; these data suggest the potential for syrosingopine as an adjunctive therapy for future clinical anticancer drugs. AZD3965, a dual inhibitor of MCT1 and MCT2, was shown in both in vitro and in vivo preclinical studies to be safe for breast cancer treatment313. A phase I clinical trial of AZD3965 in patients with advanced solid tumors and B-cell lymphoma has been completed (NCT01791595). MCT1 and MCT4 transport to the cell surface after expression depends on the glycoprotein chaperone CD147314. Therefore, CD147 has become a potential drug target to disrupt MCT membrane insertion and function in cancer therapy.
There are several possible antitumor therapies that aim to inhibit lactate production (Table 4): 2-deoxyglucose (2-DG), which can compete with glucose in vivo, reduces lactate production in tumor cells, and inhibits rapid tumor cell growth by reducing glucose utilization315. Phase I clinical trials have been conducted to evaluate the dosage of 2-DG in patients with several types of solid tumors, including prostate cancer and glioblastoma, based on preclinical efficacy316. Breast cancer cells were treated with 2-DG as a metformin adjuvant, and the results indicated a synergistic effect on growth arrest317. In addition, 2-DG enhanced CD8+ memory cell formation and antitumor function, including increasing the homing of lymphocytes to lymph nodes, IFN-γ and TNF-α production, and tumor regression in mice with melanoma318. These results suggest that glucometabolic modulators can exert certain antitumor effects alone or as adjuvants to traditional drugs. Lonidamine, a dechlorinated derivative of indolizazol-3-carboxylic acid, strongly inhibits oxygen consumption, aerobic glycolysis, and lactate transport and accumulation in tumor cells. Because its specific mechanism of action and side effect profiles do not overlap with those of standard antineoplastic drugs, lonidamine combined with standard chemotherapy for the treatment of solid tumors has been widely studied. Lonidamine was confirmed to increase the toxicity of anthracycline-based drugs in human breast cancer cell lines and to enhance cisplatin activity in platinum-sensitive and platinum-resistant ovarian cancer cell lines. The results from phase II-III clinical trials of lonidamine for advanced breast, ovarian and lung cancer are encouraging. Dichloroacetate (DCA) inhibits pyruvate dehydrogenase kinase, increases glucose uptake into mitochondrial oxidation, and decreases lactate production. The results of a phase I clinical trial showed that long-term oral DCA treatment was feasible and well tolerated in patients with relapsed glioblastoma and other tumors that have metastasized to the brain319. The results from another phase II study of DCA in combination with chemoradiotherapy in unresected locally advanced squamous cell carcinoma of the head and neck showed that the addition of DCA to cisplatin-based chemoradiation (CRT) appeared to be safe and had no adverse effect on survival or expected metabolite changes. These data provide support for further studies on combinations of metabolic drugs with CRT320.
Conclusions and perspectives
Two ATP production systems are functional in mammalian cells: glycolysis and OXPHOS. Glycolysis does not require oxygen, and the final product of this pathway is lactate. Less ATP is generated through glycolysis than through OXPHOS. Therefore, in textbooks, glycolysis has been described as a supplementary method of ATP production under anaerobic conditions, and historically, lactate has been considered a waste product that helps maintain temperature and generate heat produced by muscle movement; hence, lactate is known to cause muscle fatigue. However, a “lactate revolution” in the 1970s led researchers to realize that lactate, at the intersection of anaerobic and aerobic carbohydrate metabolism, is a direct and critical component in the cellular energy repository and that the steps through which lactate leads to ATP production are simpler and faster than those involved in the oxidative utilization of glucose. Subsequent studies gradually revealed that lactate is a major renewable carbohydrate fuel in mammals and a recyclable redox buffer that maintains a balanced cellular redox state in tissues throughout the body. Thus, the roles of lactate as a metabolic feedback regulator and unique signaling molecule were considered, and the function of lactylation as a novel PTM of proteins has been explored. Therefore, lactate is recognized to play a significant role in many physiological and pathological processes, including the regulation of energy metabolism, immunity responses, memory formation, wound healing, and tumor development. These processes are regulated by lactate as a signaling molecule or through lactylation, in addition to its role as a metabolic substrate. Currently, lactate is mostly used as an indicator for disease diagnosis, prognosis, and efficacy evaluation in the clinical (Table 2). Lactate has rarely been used as a therapeutic target in clinical practice, and studies focus more on the therapeutic potential of small nucleic acid drugs that target cell function321,322,323. As a plasma biomarker of disease, lactate theoretically has potential to be a molecule that stimulates specific-responsive drugs as same as glucose324. With the exploration of its functions in signal transduction and post-translational modification of proteins, lactate is expected to become a valuable target for the treatment of chronic cardiovascular diseases, nerve injury, inflammatory disease, and tumors.
As a necessary product of glycolysis, lactate has continued to be the subject of study for reasons that include abnormally elevated levels in inflammatory diseases and the TME. Since the discovery of the Warburg effect, glycolysis and lactate production are no longer considered specific to hypoxia, which better explains disease heterogeneity. This recognition has also greatly improved the understanding of inflammatory diseases and cancer. Advances in research have revealed the metabolic regulation of cell function, the adaptability of cells to different environments, and the feedback regulation of metabolism by metabolites. The breakthrough in discovering the epigenetic function of lactate as a substrate of histone Kla seems to represent a fulcrum of metabolism and gene regulation.
PTMs are important epigenetic mechanisms that regulate cell function. With the rapid development of high-resolution MS technology in recent years, various novel acylation pathways have been identified in succession, including formylation, propionylation, butyrylation, crotonylation, 2-hydroxyisobutyrylation, β-hydroxybutyrylation (bhb), succinylation, malonylation, glutarylation, benzoylation, and lactylation. Among the known classical HATs, p300/CBP has been identified as a mediator of the most diverse acyltransferase activities325. Given the functional specificity of acyl modifications, what are the main factors that determine the specificity and selectivity of p300/CBP activity? In other words, how does a nonspecific HAT specifically catalyze lactylation? First, dynamic changes in intracellular acyl-CoA concentrations are clearly involved100. However, since the concentration of acetyl-CoA is higher than that of other acyl-CoAs in most cases, more studies are needed to clarify the regulatory mechanisms. Second, acyltransferase activity differs by organelle, and this preference is an important factor. For example, recently identified Kbhb substrates are more highly concentrated in the nucleus; therefore, acyltransferases in the nucleus seem to interact preferentially with Kbhb326. Another interesting question that has yet to be answered is: what is the relationships among simultaneous different PTMs? Acylation is realized through cellular metabolites that are acyl donors, such as acetyl-CoA, succinyl-CoA and lactyl-CoA, and histone acylation can usually activate transcription and regulate gene expression327. Previous studies have shown that acetylation and succinylation show a preference for lysine residues at different locations in the same protein. However, two or more different PTMs can often be observed at some of the same lysine residues, and different modifications have also been found at certain sites of proteins in the central metabolic pathway328. For example, in Glis1-induced histone modification, p300 has been shown to increase both the acetylation of H3K27 and the lactylation of H3K18 in a second wave of PTMs at pluripotent gene promoters, which activates gene expression and facilitates somatic reprogramming98. Moreover, studies of Kla in rice have found other types of acylation simultaneously with lactylation on the same lysine residues of the same proteins, suggesting the potential for crosstalk between these modifications329. These results indicate coordinated interactions between different PTMs in cells. In fact, it has been proposed that two different types of PTMs target different lysine residues or that PTM machineries compete to modify the same lysine. However, the mechanism through which different acyl modifications coordinate and coregulate cell physiology and biochemistry has not been clarified in vivo or in vitro. In some cases, PTMs can be established in a nonenzymatic manner330. Notably, the enzymatic and nonenzymatic deposition of the same PTM is coordinated. Organelles may play a decisive role in the way in which different modifications are rendered, and nonenzymatic regulation has been proposed as the main mechanism of mitochondrial acetylation331. Interestingly, the regulation of cell function through the same modification may involve a division of labor between enzymatic and nonenzymatic regulators. Nonenzymatic lactylation is enriched through the glycolysis pathway, which can inhibit enzymatic activity and reduce glycolysis metabolites102, while enzyme-regulated lactylation is enriched in inflammatory pathways and can regulate inflammatory homeostasis16. Unfortunately, it remains unknown whether this division of labor is an accurate description or whether the limited number of related studies has led to an inaccurate understanding. In general, the complexity of PTMs is far beyond our current understanding, and researchers’ knowledge of PTMs is currently limited. However, recent studies have provided insight into some aspects of PTMs and suggested worthwhile future directions of exploration in the field of life science.
Kla in tumor tissues generally promotes tumor immune evasion by inducing the anti-inflammatory phenotype of TAMs. Moreover, it may directly regulate oncogene expression to promote tumor growth, metastasis, and invasion. Lactylation also regulates gene expression in macrophages, maintains immune homeostasis, and plays a vital role in inflammation, repair, and wound healing. Therefore, lactylation holds great potential as a therapeutic target in inflammation, cancer, and systemic diseases caused by immunosuppression. Furthermore, lactylation also plays a potential role in regulating neuronal excitation. However, the specific underlying mechanism leading to such neuronal excitation remains unexplored. The function and specific mechanisms of lactylation and its regulatory enzymes in exercise, lipolysis, neuroprotection, angiogenesis, and other aspects also need to be investigated, and these data will provide new diagnostic and treatment strategies for many chronic diseases, such as atherosclerosis and AD. Specifically, studies have revealed the existence and function of nonhistone Kla in plants329,332, and Kla has considerable potential as a target for development in agriculture.
Change history
31 October 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41392-022-01206-5
References
Ferguson, B. S. et al. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl Physiol. 118, 691–728 (2018).
Brooks, G. A. Lactate shuttles in nature. Biochem Soc. Trans. 30, 258–264 (2002).
Brooks, G. A. Cell-cell and intracellular lactate shuttles. J. Physiol. 587, 5591–5600 (2009).
Daw, C. C. et al. Lactate elicits ER-mitochondrial Mg(2+) dynamics to integrate cellular metabolism. Cell 183, 474–489.e417 (2020).
Zhao, Y. et al. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. 33, 108487 (2020).
Brown, T. P. & Ganapathy, V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharm. Ther. 206, 107451 (2020).
Felmlee, M. A. et al. Monocarboxylate transporters (SLC16): Function, regulation, and role in health and disease. Pharm. Rev. 72, 466–485 (2020).
Liberti, M. V. & Locasale, J. W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 41, 211–218 (2016).
Vaupel, P. & Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 599, 1745–1757 (2021).
Li, X. et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol. Cell 61, 705–719 (2016).
Li, M. et al. DDIT3 directs a dual mechanism to balance glycolysis and oxidative phosphorylation during glutamine deprivation. Adv. Sci. 8, e2003732 (2021).
Teng, R. et al. HSP60 silencing promotes Warburg-like phenotypes and switches the mitochondrial function from ATP production to biosynthesis in ccRCC cells. Redox Biol. 24, 101218 (2019).
Certo, M. et al. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 21, 151–161 (2021).
Chen, Z., Liu, M., Li, L. & Chen, L. Involvement of the Warburg effect in non-tumor diseases processes. J. Cell Physiol. 233, 2839–2849 (2018).
Dabral, S. et al. A RASSF1A-HIF1alpha loop drives Warburg effect in cancer and pulmonary hypertension. Nat. Commun. 10, 2130 (2019).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019).
Tong, Y. et al. SUCLA2-coupled regulation of GLS succinylation and activity counteracts oxidative stress in tumor cells. Mol. Cell 81, 2303–2316 e2308 (2021).
Guo, Y. et al. NF- kappa B/HDAC1/SREBP1c pathway mediates the inflammation signal in progression of hepatic steatosis. Acta Pharm. Sin. B 10, 825–836 (2020).
Lv, L. et al. Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol. Cell 42, 719–730 (2011).
Galvan-Pena, S. et al. Malonylation of GAPDH is an inflammatory signal in macrophages. Nat. Commun. 10, 338 (2019).
Harmer, A. R. et al. Sprint training increases muscle oxidative metabolism during high-intensity exercise in patients with type 1 diabetes. Diabetes Care 31, 2097–2102 (2008).
Levy, B. et al. Relation between muscle Na+K+ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 365, 871–875 (2005).
Fantin, V., St-Pierre, J. & Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9, 425–434 (2006).
Bennis, Y. et al. A study of associations between plasma metformin concentration, lactic acidosis, and mortality in an emergency hospitalization context. Crit. Care Med. 48, e1194–e1202 (2020).
Jha, M., Lee, I. & Suk, K. Metabolic reprogramming by the pyruvate dehydrogenase kinase-lactic acid axis: Linking metabolism and diverse neuropathophysiologies. Neurosci. Biobehav. Rev. 68, 1–19 (2016).
Soreze, Y. et al. Mutations in human lipoyltransferase gene LIPT1 cause a Leigh disease with secondary deficiency for pyruvate and alpha-ketoglutarate dehydrogenase. Orphanet J. Rare Dis. 8, 192 (2013).
Luengo, A. et al. Increased demand for NAD relative to ATP drives aerobic glycolysis. Mol. Cell 81, 691–707.e696 (2021).
Emhoff, C. et al. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J. Appl. Physiol. 114, 297–306 (2013).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007).
Halestrap, A. P. The SLC16 gene family-structure, role, and regulation in health and disease. Mol. Asp. Med. 34, 337–349 (2013).
Madaan, A. et al. Lactate produced during labor modulates uterine inflammation via GPR81 (HCA). Am. J. Obstet. Gynecol. 216, 60.e61–60.e17 (2017).
Sun, Z. et al. Activation of GPR81 by lactate inhibits oscillatory shear stress-induced endothelial inflammation by activating the expression of KLF2. IUBMB Life 71, 2010–2019 (2019).
Wu, G. et al. The lactate receptor GPR81 mediates hepatic lipid metabolism and the therapeutic effect of metformin on experimental NAFLDs. Eur. J. Pharmacol. 924, 174959 (2022).
Laroche, S. et al. Participation of L-lactate and its receptor HCAR1/GPR81 in neurovisual development. Cells 10, 1640 (2021).
Lu, J., Tan, M. & Cai, Q. The Warburg effect in tumor progression: Mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 356, 156–164 (2015).
Rabinowitz, J. & Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2, 566–571 (2020).
Dienel, G. A. Brain glucose metabolism: Integration of energetics with function. Physiol. Rev. 99, 949–1045 (2019).
Schurr, A., West, C. A. & Rigor, B. M. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 240, 1326–1328 (1988).
Lhomme, T. et al. Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons. J. Clin. Invest. 131, e140521 (2021).
Gomez-Valades, A. G. et al. Mitochondrial cristae-remodeling protein OPA1 in POMC neurons couples Ca(2+) homeostasis with adipose tissue lipolysis. Cell Metab. 33, 1820–1835.e1829 (2021).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371 e359 (2017).
Titov, D. V. et al. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352, 231–235 (2016).
Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal 10, 179–206 (2008).
Tilton, W. M., Seaman, C., Carriero, D. & Piomelli, S. Regulation of glycolysis in the erythrocyte: Role of the lactate/pyruvate and NAD/NADH ratios. J. Lab Clin. Med. 118, 146–152 (1991).
Quinn, W. J. 3rd et al. Lactate limits T cell proliferation via the NAD(H) redox state. Cell Rep. 33, 108500 (2020).
Wang, C. et al. Malate-aspartate shuttle inhibitor aminooxyacetic acid leads to decreased intracellular ATP levels and altered cell cycle of C6 glioma cells by inhibiting glycolysis. Cancer Lett. 378, 1–7 (2016).
Luengo, A. et al. Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol. Cell 81, 691–707 e696 (2021).
Patgiri, A. et al. An engineered enzyme that targets circulating lactate to alleviate intracellular NADH:NAD(+) imbalance. Nat. Biotechnol. 38, 309–313 (2020).
Perry, J. J., Shin, D. S., Getzoff, E. D. & Tainer, J. A. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta 1804, 245–262 (2010).
Shadel, G. S. & Horvath, T. L. Mitochondrial ROS signaling in organismal homeostasis. Cell 163, 560–569 (2015).
Corkey, B. E. & Deeney, J. T. The redox communication network as a regulator of metabolism. Front. Physiol. 11, 567796 (2020).
Yang, S. & Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell Biochem. 467, 1–12 (2020).
Jia, L. et al. Rheb-regulated mitochondrial pyruvate metabolism of Schwann cells linked to axon stability. Dev. Cell 56, 2980–2994 e2986 (2021).
Benjamin, D. et al. Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+depletion in cancer cells. Cell Rep. 25, 3047–3058 e3044 (2018).
Hoy, A. J., Nagarajan, S. R. & Butler, L. M. Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat. Rev. Cancer 21, 753–766 (2021).
Pucino, V., Bombardieri, M., Pitzalis, C. & Mauro, C. Lactate at the crossroads of metabolism, inflammation, and autoimmunity. Eur. J. Immunol. 47, 14–21 (2017).
Pucino, V. et al. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4(+) T cell metabolic rewiring. Cell Metab. 30, 1055–1074 e1058 (2019).
Liu, L. et al. The Glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab. 26, 719–737.e716 (2017).
Jin, E. S., Sherry, A. D. & Malloy, C. R. Lactate contributes to glyceroneogenesis and glyconeogenesis in skeletal muscle by reversal of pyruvate kinase. J. Biol. Chem. 290, 30486–30497 (2015).
Lund, J. et al. Utilization of lactic acid in human myotubes and interplay with glucose and fatty acid metabolism. Sci. Rep. 8, 9814 (2018).
San-Millan, I. & Brooks, G. A. Assessment of metabolic flexibility by means of measuring blood lactate, fat, and carbohydrate oxidation responses to exercise in professional endurance athletes and less-fit individuals. Sports Med. 48, 467–479 (2018).
de Boer, E. et al. Decreased fatty acid oxidation and altered lactate production during exercise in patients with post-acute COVID-19 syndrome. Am. J. Respir. Crit. Care Med. 205, 126–129 (2022).
Fritzen, A. M., Lundsgaard, A. M. & Kiens, B. Tuning fatty acid oxidation in skeletal muscle with dietary fat and exercise. Nat. Rev. Endocrinol. 16, 683–696 (2020).
Wang, T. et al. Acetyl-CoA from inflammation-induced fatty acids oxidation promotes hepatic malate-aspartate shuttle activity and glycolysis. Am. J. Physiol. Endocrinol. Metab. 315, E496–E510, (2018).
Brooks, G. A. The science and translation of lactate shuttle theory. Cell Metab. 27, 757–785 (2018).
Wasserman, K. et al. Lactate, pyruvate, and lactate-to-pyruvate ratio during exercise and recovery. J. Appl Physiol. 59, 935–940 (1985).
Brooks, G. J. C. M. The science and translation of lactate shuttle theory. Cell Metab. 27, 757–785, (2018).
Sahlin, K., Fernstrom, M., Svensson, M. & Tonkonogi, M. No evidence of an intracellular lactate shuttle in rat skeletal muscle. J. Physiol. 541, 569–574 (2002).
Hashimoto, T. et al. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS One 3, e2915 (2008).
Hashimoto, T. & Brooks, G. A. Mitochondrial lactate oxidation complex and an adaptive role for lactate production. Med. Sci. Sports Exerc. 40, 486–494 (2008).
Hashimoto, T., Hussien, R. & Brooks, G. A. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am. J. Physiol. Endocrinol. Metab. 290, E1237–E1244 (2006).
Brooks, G. A. et al. Decreased reliance on lactate during exercise after acclimatization to 4300 m. J. Appl Physiol. 71, 333–341 (1991).
Brooks, G. A. & Gaesser, G. A. End points of lactate and glucose metabolism after exhausting exercise. J. Appl. Physiol. Respir. Environ. Exerc Physiol. 49, 1057–1069 (1980).
Brooks, G. A. Mammalian fuel utilization during sustained exercise. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 120, 89–107 (1998).
Brooks, G. A. Role of the heart in lactate shuttling. Front. Nutr. 8, 663560 (2021).
Gizak, A., McCubrey, J. A. & Rakus, D. Cell-to-cell lactate shuttle operates in heart and is important in age-related heart failure. Aging 12, 3388–3406 (2020).
Sun, Y. et al. Modulation of the astrocyte-neuron lactate shuttle system contributes to neuroprotective action of fibroblast growth factor 21. Theranostics 10, 8430–8445 (2020).
Bisetto, S. et al. New insights into the lactate shuttle: Role of MCT4 in the modulation of the exercise capacity. iScience 22, 507–518 (2019).
Sheikh-Hamad, D. Hints for a kidney lactate shuttle and lactomone. Am. J. Physiol. Ren. Physiol. 320, F1028–F1029 (2021).
Brooks, G. A. The tortuous path of lactate shuttle discovery: From cinders and boards to the lab and ICU. J. Sport Health Sci. 9, 446–460 (2020).
Irizarry-Caro, R. A. et al. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc. Natl Acad. Sci. USA 117, 30628–30638 (2020).
Troutman, T. D. et al. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc. Natl Acad. Sci. USA 109, 273–278 (2012).
Matsumura, T. et al. Identification of BCAP-(L) as a negative regulator of the TLR signaling-induced production of IL-6 and IL-10 in macrophages by tyrosine phosphoproteomics. Biochem. Biophys. Res. Commun. 400, 265–270 (2010).
Sun, S. et al. Lactic acid-producing probiotic saccharomyces cerevisiae attenuates ulcerative colitis via suppressing macrophage pyroptosis and modulating gut microbiota. Front. Immunol. 12, 777665 (2021).
Cui, H. et al. Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone lactylation. Am. J. Respir. Cell Mol. Biol. 64, 115–125 (2021).
Pan, R. Y. et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 34, 634–648 e636 (2022).
Yu, J. et al. Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 22, 85 (2021).
Smith, E. A. & Hodges, H. C. The spatial and genomic hierarchy of tumor ecosystems revealed by single-cell technologies. Trends Cancer 5, 411–425 (2019).
Jiang, J. et al. Lactate modulates cellular metabolism through histone lactylation-mediated gene expression in non-small cell lung cancer. Front. Oncol. 11, 647559 (2021).
Yang, K. et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 29, 133–146 (2022).
Xiong, J. et al. Lactylation-driven METTL3-mediated RNA m(6)A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol. Cell 82, 1660–1677 e1610 (2022).
Caielli, S. et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell 184, 4464–4479 e4419 (2021).
Hagihara, H. et al. Protein lactylation induced by neural excitation. Cell Rep. 37, 109820 (2021).
Xu, X. et al. Autophagic feedback-mediated degradation of IKKalpha requires CHK1- and p300/CBP-dependent acetylation of p53. J. Cell Sci. 133, jcs246868 (2020).
Manickavinayaham, S. et al. E2F1 acetylation directs p300/CBP-mediated histone acetylation at DNA double-strand breaks to facilitate repair. Nat. Commun. 10, 4951 (2019).
Waddell, A. et al. Pharmacological inhibition of CBP/p300 blocks estrogen receptor alpha (ERalpha) function through suppressing enhancer H3K27 acetylation in luminal breast cancer. Cancers 13, 2799 (2021).
Li, L. et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat. Metab. 2, 882–892 (2020).
Moreno-Yruela, C. et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci. Adv. 8, eabi6696 (2022).
Mews, P. et al. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature 546, 381–386 (2017).
Zhou, W. et al. TIGAR promotes neural stem cell differentiation through acetyl-CoA-mediated histone acetylation. Cell Death Dis. 10, 198 (2019).
Gaffney, D. O. et al. Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem. Biol. 27, 206–213.e206 (2020).
Rose, I. A. & Nowick, J. S. Methylglyoxal synthetase, enol-pyruvaldehyde, glutathione, and the glyoxalase system. J. Am. Chem. Soc. 124, 13047–13052 (2002).
Rabbani, N., Xue, M. & Thornalley, P. J. Activity, regulation, copy number and function in the glyoxalase system. Biochem. Soc. Trans. 42, 419–424 (2014).
Pasti, A. P. et al. Human lactate dehydrogenase A undergoes allosteric transitions under pH conditions inducing the dissociation of the tetrameric enzyme. Biosci. Rep. 42, BSR20212654 (2022).
Stolberg, L. et al. d-Lactic acidosis due to abnormal gut flora: Diagnosis and treatment of two cases. N. Engl. J. Med. 306, 1344–1348 (1982).
Ewaschuk, J. B., Naylor, J. M. & Zello, G. A. D-lactate in human and ruminant metabolism. J. Nutr. 135, 1619–1625 (2005).
de Bari, L. et al. D-Lactate transport and metabolism in rat liver mitochondria. Biochem. J. 365, 391–403 (2002).
Flick, M. J. & Konieczny, S. F. Identification of putative mammalian D-lactate dehydrogenase enzymes. Biochem. Biophys. Res. Commun. 295, 910–916 (2002).
de Vrese, M., Koppenhoefer, B. & Barth, C. A. D-lactic acid metabolism after an oral load of DL-lactate. Clin. Nutr. 9, 23–28 (1990).
Oh, M. S. et al. Metabolic utilization and renal handling of D-lactate in men. Metabolism 34, 621–625 (1985).
Schmitt, M. & Greten, F. R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 21, 653–667 (2021).
Rowley, A. H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat. Rev. Immunol. 20, 453–454 (2020).
Frangogiannis, N. G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 11, 255–265 (2014).
Querol, L., Devaux, J., Rojas-Garcia, R. & Illa, I. Autoantibodies in chronic inflammatory neuropathies: Diagnostic and therapeutic implications. Nat. Rev. Neurol. 13, 533–547 (2017).
Gao, B., Ahmad, M. F., Nagy, L. E. & Tsukamoto, H. Inflammatory pathways in alcoholic steatohepatitis. J. Hepatol. 70, 249–259 (2019).
Afonina, I. S., Zhong, Z., Karin, M. & Beyaert, R. Limiting inflammation-the negative regulation of NF-kappaB and the NLRP3 inflammasome. Nat. Immunol. 18, 861–869 (2017).
Wullaert, A., Bonnet, M. C. & Pasparakis, M. NF-kappaB in the regulation of epithelial homeostasis and inflammation. Cell Res. 21, 146–158 (2011).
Hinz, M., Arslan, S. C. & Scheidereit, C. It takes two to tango: IkappaBs, the multifunctional partners of NF-kappaB. Immunol. Rev. 246, 59–76 (2012).
Miao, F., Shan, C. & Ning, D. Walnut oil alleviates LPS-induced intestinal epithelial cells injury by inhibiting TLR4/MyD88/NF-kappaB pathway activation. J. Food Biochem. 45, e13955 (2021).
Qi, S. et al. Silencing of PTX3 alleviates LPS-induced inflammatory pain by regulating TLR4/NF-kappaB signaling pathway in mice. Biosci. Rep. 40, BSR20194208 (2020).
Abebayehu, D. et al. Lactic acid suppresses IgE-mediated mast cell function in vitro and in vivo. Cell Immunol. 341, 103918 (2019).
Peter, K. et al. Lactic acid delays the inflammatory response of human monocytes. Biochem. Biophys. Res. Commun. 457, 412–418 (2015).
Yang, K. et al. Lactate suppresses macrophage pro-inflammatory response to LPS stimulation by inhibition of YAP and NF-kappaB activation via GPR81-mediated signaling. Front. Immunol. 11, 587913 (2020).
Arango Duque, G. & Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 5, 491 (2014).
Ubil, E. et al. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J. Clin. Invest. 128, 2356–2369 (2018).
Sharif, O., Brunner, J. S., Vogel, A. & Schabbauer, G. Macrophage rewiring by nutrient associated PI3K dependent pathways. Front. Immunol. 10, 2002 (2019).
Galvan-Pena, S. & O’Neill, L. A. Metabolic reprograming in macrophage polarization. Front. Immunol. 5, 420 (2014).
Gharib, S. A. et al. Transcriptional and functional diversity of human macrophage repolarization. J. Allergy Clin. Immunol. 143, 1536–1548 (2019).
Kumar, V. Targeting macrophage immunometabolism: Dawn in the darkness of sepsis. Int. Immunopharmacol. 58, 173–185 (2018).
Costa Leite, T. et al. Lactate favours the dissociation of skeletal muscle 6-phosphofructo-1-kinase tetramers down-regulating the enzyme and muscle glycolysis. Biochem. J. 408, 123–130 (2007).
Zhang, J. et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab. 31, 1136–1153 e1137 (2020).
Selleri, S. et al. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget 7, 30193–30210 (2016).
Kolkhir, P. et al. Understanding human mast cells: Lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 22, 294–308 (2022).
Syed, M. et al. Lactic acid suppresses MRGPRX2 mediated mast cell responses. Cell Immunol. 368, 104422 (2021).
Ranganathan, P. et al. GPR81, a cell-surface receptor for lactate, regulates intestinal homeostasis and protects mice from experimental colitis. J. Immunol. 200, 1781–1789 (2018).
Xu, J. et al. Lactate up-regulates the expression of PD-L1 in kidney and causes immunosuppression in septic Acute Renal Injury. J. Microbiol. Immunol. Infect. 54, 404–410 (2021).
Shan, T. et al. M2TAM subsets altered by lactic acid promote Tcell apoptosis through the PDL1/PD1 pathway. Oncol. Rep. 44, 1885–1894 (2020).
Hoque, R. et al. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146, 1763–1774 (2014).
Caslin, H. L. et al. Lactic acid inhibits lipopolysaccharide-induced mast cell function by limiting glycolysis and ATP availability. J. Immunol. 203, 453–464 (2019).
Rajendran, P. et al. The multifaceted link between inflammation and human diseases. J. Cell Physiol. 233, 6458–6471 (2018).
Droge, W., Roth, S., Altmann, A. & Mihm, S. Regulation of T-cell functions by L-lactate. Cell Immunol. 108, 405–416 (1987).
Haas, R. et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 13, e1002202 (2015).
Souto-Carneiro, M. M. et al. Effect of increased lactate dehydrogenase A activity and aerobic glycolysis on the proinflammatory profile of autoimmune CD8+T cells in rheumatoid arthritis. Arthritis Rheumatol. 72, 2050–2064 (2020).
Xie, N. et al. Metabolic characterization and RNA profiling reveal glycolytic dependence of profibrotic phenotype of alveolar macrophages in lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 313, L834–L844 (2017).
Reyfman, P. A. et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 199, 1517–1536 (2019).
Imtiyaz, H. Z. et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Invest. 120, 2699–2714 (2010).
Carmona-Fontaine, C. et al. Metabolic origins of spatial organization in the tumor microenvironment. Proc. Natl Acad. Sci. USA 114, 2934–2939 (2017).
De Vlaeminck, Y. et al. Single-domain antibody fusion proteins can target and shuttle functional proteins into macrophage mannose receptor expressing macrophages. J. Control Release 299, 107–120 (2019).
Guo, L. et al. Induction of breast cancer stem cells by M1 macrophages through Lin-28B-let-7-HMGA2 axis. Cancer Lett. 452, 213–225 (2019).
Jackute, J. et al. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 19, 3 (2018).
Chen, Y., Zhang, S., Wang, Q. & Zhang, X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J. Hematol. Oncol. 10, 36 (2017).
Li, W. et al. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis. 10, 918 (2019).
Zhao, S. et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 13, 156 (2020).
Mu, X. et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle 17, 428–438 (2018).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Peters, A. et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 35, 986–996 (2011).
Angelin, A. et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293 e1287 (2017).
Ivashkiv, L. B. The hypoxia-lactate axis tempers inflammation. Nat. Rev. Immunol. 20, 85–86 (2020).
Errea, A. et al. Lactate inhibits the pro-inflammatory response and metabolic reprogramming in murine macrophages in a GPR81-independent manner. PLoS One 11, e0163694 (2016).
Samuvel, D. J. et al. Lactate boosts TLR4 signaling and NF-kappaB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J. Immunol. 182, 2476–2484 (2009).
Fischbeck, A. J. et al. Tumor lactic acidosis: Protecting tumor by inhibiting cytotoxic activity through motility arrest and bioenergetic silencing. Front. Oncol. 10, 589434 (2020).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Paolini, L. et al. Lactic acidosis together with GM-CSF and M-CSF induces human macrophages toward an inflammatory protumor phenotype. Cancer Immunol. Res. 8, 383–395 (2020).
Fernandez, S. F. et al. Low pH environmental stress inhibits LPS and LTA-stimulated proinflammatory cytokine production in rat alveolar macrophages. Biomed. Res. Int. 2013, 742184 (2013).
Gottfried, E. et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 107, 2013–2021 (2006).
Noormandi, A., Khalili, H., Mohammadi, M. & Abdollahi, A. Effect of magnesium supplementation on lactate clearance in critically ill patients with severe sepsis: A randomized clinical trial. Eur. J. Clin. Pharm. 76, 175–184 (2020).
Wani, S. J. et al. Combination of vitamin C, thiamine and hydrocortisone added to standard treatment in the management of sepsis: Results from an open label randomised controlled clinical trial and a review of the literature. Infect. Dis. 52, 271–278 (2020).
Zhang, Y. et al. Efficacy of continuous renal replacement on acute renal injury developed in severe sepsis. J. Biol. Regul. Homeost. Agents 33, 525–530 (2019).
Jones, A. E. et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: A randomized clinical trial. JAMA 303, 739–746 (2010).
Lee, S. M. et al. Lactate clearance and vasopressor seem to be predictors for mortality in severe sepsis patients with lactic acidosis supplementing sodium bicarbonate: A retrospective analysis. PLoS One 10, e0145181 (2015).
Zhou, X. et al. Use of stepwise lactate kinetics-oriented hemodynamic therapy could improve the clinical outcomes of patients with sepsis-associated hyperlactatemia. Crit. Care 21, 33 (2017).
Chen, H. et al. Early lactate-guided resuscitation of elderly septic patients. J. Intensive Care Med. 37, 686–692 (2022).
During, J. et al. Lactate, lactate clearance and outcome after cardiac arrest: A post-hoc analysis of the TTM-Trial. Acta Anaesthesiol. Scand. 62, 1436–1442 (2018).
Besnier, E. et al. Hypertonic sodium lactate improves microcirculation, cardiac function, and inflammation in a rat model of sepsis. Crit. Care 24, 354 (2020).
van Hall, G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. 199, 499–508 (2010).
Dienel, G. A. Brain lactate metabolism: The discoveries and the controversies. J. Cereb. Blood Flow. Metab. 32, 1107–1138 (2012).
Halestrap, A. P. The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life 64, 1–9 (2012).
Descalzi, G. et al. Lactate from astrocytes fuels learning-induced mRNA translation in excitatory and inhibitory neurons. Commun. Biol. 2, 247 (2019).
Veloz Castillo, M. F., Magistretti, P. J. & Cali, C. l-Lactate: Food for thoughts, memory, and behavior. Metabolites 11, 548 (2021).
Yang, J. et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl Acad. Sci. USA 111, 12228–12233 (2014).
Wang, J. et al. Brain endothelial cells maintain lactate homeostasis and control adult hippocampal neurogenesis. Cell Stem Cell 25, 754–767 e759 (2019).
Alberini, C. M. et al. Astrocyte glycogen and lactate: New insights into learning and memory mechanisms. Glia 66, 1244–1262 (2018).
Lu, W. T. et al. Curcumin ameliorates memory deficits by enhancing lactate content and MCT2 expression in APP/PS1 transgenic mouse model of Alzheimer’s disease. Anat. Rec. 302, 332–338 (2019).
El Hayek, L. et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 39, 2369–2382 (2019).
Morland, C. et al. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. J. Neurosci. Res. 93, 1045–1055 (2015).
Wilkinson, H. N. & Hardman, M. J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 10, 200223 (2020).
Haller, H. L. et al. Oxygen, pH, lactate, and metabolism—How old knowledge and new insights might be combined for new wound treatment. Medicina 57, 1190 (2021).
DeBerardinis, R. J. & Chandel, N. S. We need to talk about the Warburg effect. Nat. Metab. 2, 127–129 (2020).
Trabold, O. et al. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen. 11, 504–509 (2003).
Hunt, T. K., Aslam, R., Hussain, Z. & Beckert, S. Lactate, with oxygen, incites angiogenesis. Adv. Exp. Med. Biol. 614, 73–80 (2008).
Hunt, T. K. et al. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid. Redox Signal 9, 1115–1124 (2007).
Hunt, T. K., Conolly, W. B., Aronson, S. B. & Goldstein, P. Anaerobic metabolism and wound healing: an hypothesis for the initiation and cessation of collagen synthesis in wounds. Am. J. Surg. 135, 328–332 (1978).
Milovanova, T. N. et al. Lactate stimulates vasculogenic stem cells via the thioredoxin system and engages an autocrine activation loop involving hypoxia-inducible factor 1. Mol. Cell Biol. 28, 6248–6261 (2008).
Liu, Q. et al. A Fenton reaction at the endoplasmic reticulum is involved in the redox control of hypoxia-inducible gene expression. Proc. Natl Acad. Sci. USA 101, 4302–4307 (2004).
Vural, E. et al. Skin graft take rates, granulation, and epithelialization: Dependence on myeloid cell hypoxia-inducible factor 1alpha. Arch. Otolaryngol. Head. Neck Surg. 136, 720–723 (2010).
Savitri, C. et al. M2 macrophage-derived concentrated conditioned media significantly improves skin wound healing. Tissue Eng. Regen. Med. 19, 617–628 (2022).
Zhang, S. M. et al. M2-polarized macrophages mediate wound healing by regulating connective tissue growth factor via AKT, ERK1/2, and STAT3 signaling pathways. Mol. Biol. Rep. 48, 6443–6456 (2021).
Gu, S. et al. AKT3 deficiency in M2 macrophages impairs cutaneous wound healing by disrupting tissue remodeling. Aging 12, 6928–6946 (2020).
Fu, J. et al. Quercetin promotes diabetic wound healing via switching macrophages from M1 to M2 polarization. J. Surg. Res. 246, 213–223 (2020).
Vagesjo, E. et al. Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc. Natl Acad. Sci. USA 115, 1895–1900 (2018).
Ohnstedt, E. et al. Accelerated wound healing in minipigs by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Pharmaceutics 14, 229 (2022).
Davis, F. M. & Gallagher, K. Time heals all wounds… but wounds heal faster with lactobacillus. Cell Host Microbe 23, 432–434 (2018).
Kinney, H. C. et al. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol. 110, 563–578 (2005).
Baartscheer, A. et al. Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc. Res. 57, 1015–1024 (2003).
Slepkov, E. R., Rainey, J. K., Sykes, B. D. & Fliegel, L. Structural and functional analysis of the Na+/H+exchanger. Biochem. J. 401, 623–633 (2007).
Andersson, A. K., Ronnback, L. & Hansson, E. Lactate induces tumour necrosis factor-alpha, interleukin-6 and interleukin-1beta release in microglial and astroglial-enriched primary cultures. J. Neurochem. 93, 1327–1333 (2005).
Shen, Z. et al. Inhibition of G protein-coupled receptor 81 (GPR81) protects against ischemic brain injury. CNS Neurosci. Ther. 21, 271–279 (2015).
Berthet, C., Castillo, X., Magistretti, P. J. & Hirt, L. New evidence of neuroprotection by lactate after transient focal cerebral ischaemia: Extended benefit after intracerebroventricular injection and efficacy of intravenous administration. Cerebrovasc. Dis. 34, 329–335 (2012).
Tassinari, I. D. et al. Lactate administration reduces brain injury and ameliorates behavioral outcomes following neonatal hypoxia-ischemia. Neuroscience 448, 191–205 (2020).
Haege, E. R., Huang, H. C. & Huang, C. C. Identification of lactate as a cardiac protectant by inhibiting inflammation and cardiac hypertrophy using a zebrafish acute heart failure model. Pharmaceuticals 14, 261 (2021).
Molstrom, S. et al. Bedside microdialysis for detection of early brain injury after out-of-hospital cardiac arrest. Sci. Rep. 11, 15871 (2021).
Theodoraki, K. et al. Transhepatic lactate gradient in relation to liver ischemia/reperfusion injury during major hepatectomies. Liver Transpl. 12, 1825–1831 (2006).
Marion, D. W. et al. Effect of hyperventilation on extracellular concentrations of glutamate, lactate, pyruvate, and local cerebral blood flow in patients with severe traumatic brain injury. Crit. Care Med. 30, 2619–2625 (2002).
Frydland, M. et al. Lactate is a prognostic factor in patients admitted with suspected ST-elevation myocardial infarction. Shock 51, 321–327 (2019).
Hajjar, L. A. et al. High lactate levels are predictors of major complications after cardiac surgery. J. Thorac. Cardiovasc. Surg. 146, 455–460 (2013).
Li, B., Chen, R., Huang, R. & Luo, W. Clinical benefit of cardiac ischemic postconditioning in corrections of tetralogy of Fallot. Interact. Cardiovasc. Thorac. Surg. 8, 17–21 (2009).
Li, L. et al. TAp73-induced phosphofructokinase-1 transcription promotes the Warburg effect and enhances cell proliferation. Nat. Commun. 9, 4683 (2018).
Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011).
Roland, C. L. et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 74, 5301–5310 (2014).
Brown, T. P. et al. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene 39, 3292–3304 (2020).
Feng, J. et al. Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene 36, 5829–5839 (2017).
Estrella, V. et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 73, 1524–1535 (2013).
Parks, S. K., Chiche, J. & Pouyssegur, J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 13, 611–623 (2013).
Martinez-Zaguilan, R. et al. pH and drug resistance. I. Functional expression of plasmalemmal V-type H+-ATPase in drug-resistant human breast carcinoma cell lines. Biochem. Pharm. 57, 1037–1046 (1999).
Wang, H. et al. LAMC2 modulates the acidity of microenvironments to promote invasion and migration of pancreatic cancer cells via regulating AKT-dependent NHE1 activity. Exp. Cell Res. 391, 111984 (2020).
Mo, L. et al. Shikonin suppresses the epithelial-to-mesenchymal transition by downregulating NHE1 in bladder cancer cells. J. Cancer 12, 6814–6824 (2021).
Sun, Z. et al. NHE1 mediates 5-Fu resistance in gastric cancer via STAT3 signaling pathway. Onco Targets Ther. 13, 8521–8532 (2020).
Xie, R. et al. NHE1 is upregulated in gastric cancer and regulates gastric cancer cell proliferation, migration, and invasion. Oncol. Rep. 37, 1451–1460 (2017).
Lee, S. et al. Na(+),HCO3(-)-cotransporter NBCn1 (Slc4a7) accelerates ErbB2-induced breast cancer development and tumor growth in mice. Oncogene 37, 5569–5584 (2018).
Lee, S. et al. Disrupting Na(+), HCO(3)(-)-cotransporter NBCn1 (Slc4a7) delays murine breast cancer development. Oncogene 35, 2112–2122 (2016).
Lauritzen, G. et al. The Na+/H+exchanger NHE1, but not the Na+, HCO3(-) cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Lett. 317, 172–183 (2012).
De Milito, A. & Fais, S. Tumor acidity, chemoresistance, and proton pump inhibitors. Future Oncol. 1, 779–786 (2005).
Flinck, M. et al. The acid-base transport proteins NHE1 and NBCn1 regulate cell cycle progression in human breast cancer cells. Cell Cycle 17, 1056–1067 (2018).
Andersen, A. P. et al. The net acid extruders NHE1, NBCn1, and MCT4 promote mammary tumor growth through distinct but overlapping mechanisms. Int. J. Cancer 142, 2529–2542 (2018).
Vegran, F. et al. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 71, 2550–2560 (2011).
Lee, D. C. et al. A lactate-induced response to hypoxia. Cell 161, 595–609 (2015).
Li, L. et al. Hypoxia-induced GBE1 expression promotes tumor progression through metabolic reprogramming in lung adenocarcinoma. Signal Transduct. Target Ther. 5, 54 (2020).
Park, M. J. et al. HIF1-alpha regulates acinar cell function and response to injury in mouse pancreas. Gastroenterology 154, 1630–1634.e1633 (2018).
Lu, H. et al. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J. Biol. Chem. 280, 41928–41939 (2005).
Vollmann-Zwerenz, A. et al. Tumor cell invasion in glioblastoma. Int. J. Mol. Sci. 21, 1932 (2020).
Savagner, P. Epithelial-mesenchymal transitions: From cell plasticity to concept elasticity. Curr. Top. Dev. Biol. 112, 273–300 (2015).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
Schulz, M. C., Wagenbrett, L., Schwerdt, G. & Gekle, M. Influence of extracellular acidosis on matrix protein homeostasis in tumour cells and fibroblasts. Adv. Exp. Med. Biol. 1072, 213–217 (2018).
Ogunwobi, O. O., Wang, T., Zhang, L. & Liu, C. Cyclooxygenase-2 and Akt mediate multiple growth-factor-induced epithelial-mesenchymal transition in human hepatocellular carcinoma. J. Gastroenterol. Hepatol. 27, 566–578 (2012).
Ahn, J. H., Choi, Y. S. & Choi, J. H. Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol. Hum. Reprod. 21, 792–802 (2015).
Ohno, Y. et al. Lactate increases myotube diameter via activation of MEK/ERK pathway in C2C12 cells. Acta Physiol. 223, e13042 (2018).
Lagares-Tena, L. et al. Caveolin-1 promotes Ewing sarcoma metastasis regulating MMP-9 expression through MAPK/ERK pathway. Oncotarget 7, 56889–56903 (2016).
Niu, D. et al. Lactic acid in tumor invasion. Clin. Chim. Acta 522, 61–69 (2021).
Boedtkjer, E. & Pedersen, S. F. The acidic tumor microenvironment as a driver of cancer. Annu. Rev. Physiol. 82, 103–126 (2020).
Nakagawa, Y. et al. Effects of extracellular pH and hypoxia on the function and development of antigen-specific cytotoxic T lymphocytes. Immunol. Lett. 167, 72–86 (2015).
Fischer, K. et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109, 3812–3819 (2007).
Almeida, L. et al. CD4(+) T-cell differentiation and function: Unifying glycolysis, fatty acid oxidation, polyamines NAD mitochondria. J. Allergy Clin. Immunol. 148, 16–32 (2021).
Peng, M. et al. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354, 481–484 (2016).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002).
Teijeira, A. et al. Metabolic consequences of T-cell costimulation in anticancer immunity. Cancer Immunol. Res. 7, 1564–1569 (2019).
Xu, K. et al. Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science 371, 405–410 (2021).
Mendler, A. N. et al. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int. J. Cancer 131, 633–640 (2012).
Calcinotto, A. et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 72, 2746–2756 (2012).
Navarro, F. et al. Overcoming T cell dysfunction in acidic pH to enhance adoptive T cell transfer immunotherapy. Oncoimmunology 11, 2070337 (2022).
Noman, M. Z. et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211, 781–790 (2014).
Bian, Y. et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature 585, 277–282 (2020).
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 24, 657–671 (2016).
Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 12, 325–338 (2012).
Erra Diaz, F., Dantas, E. & Geffner, J. Unravelling the interplay between extracellular acidosis and immune cells. Mediators Inflamm. 2018, 1218297 (2018).
Gu, J. et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-beta signaling in regulatory T cells. Cell Rep. 39, 110986 (2022).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Wang, R. et al. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J. Exp. Clin. Cancer Res. 30, 62 (2011).
Pollari, M. et al. PD-L1(+) tumor-associated macrophages and PD-1(+) tumor-infiltrating lymphocytes predict survival in primary testicular lymphoma. Haematologica 103, 1908–1914 (2018).
Rodriguez, P. C. et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849 (2004).
Yang, L. et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 5, 4436 (2014).
Wang, S. & Zhang, Y. HMGB1 in inflammation and cancer. J. Hematol. Oncol. 13, 116 (2020).
Zhang, Q. Y. et al. Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2. Oncol. Rep. 33, 1630–1638 (2015).
Pistoia, V. & Pezzolo, A. Involvement of HMGB1 in resistance to tumor vessel-targeted, monoclonal antibody-based immunotherapy. J. Immunol. Res. 2016, 3142365 (2016).
Guan, X., Rodriguez-Cruz, V. & Morris, M. E. Cellular uptake of MCT1 inhibitors AR-C155858 and AZD3965 and their effects on MCT-mediated transport of L-lactate in murine 4T1 breast tumor cancer cells. AAPS J. 21, 13 (2019).
Vlachostergios, P. J., Oikonomou, K. G., Gibilaro, E. & Apergis, G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomark. 15, 725–734 (2015).
Wei, Y. et al. Prognostic significance of serum lactic acid, lactate dehydrogenase, and albumin levels in patients with metastatic colorectal cancer. Biomed. Res. Int. 2018, 1804086 (2018).
Walenta, S. et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 60, 916–921 (2000).
Matsumura, A. et al. Non-invasive quantification of lactate by proton MR spectroscopy and its clinical applications. Clin. Neurol. Neurosurg. 107, 379–384 (2005).
Charles-Edwards, G. D. et al. Non-invasive detection and quantification of human foetal brain lactate in utero by magnetic resonance spectroscopy. Prenat. Diagn. 30, 260–266 (2010).
Isobe, T. et al. Lactate quantification by proton magnetic resonance spectroscopy using a clinical MRI machine: a basic study. Australas. Radio. 51, 330–333 (2007).
Wang, Z. J. et al. Hyperpolarized (13)C MRI: State of the art and future directions. Radiology 291, 273–284 (2019).
Cheung, S. M. et al. Lactate concentration in breast cancer using advanced magnetic resonance spectroscopy. Br. J. Cancer 123, 261–267 (2020).
Giskeodegard, G. F. et al. Lactate and glycine-potential MR biomarkers of prognosis in estrogen receptor-positive breast cancers. NMR Biomed. 25, 1271–1279 (2012).
Xu, H. N. et al. Is higher lactate an indicator of tumor metastatic risk? A pilot MRS study using hyperpolarized (13)C-pyruvate. Acad. Radio. 21, 223–231 (2014).
Wolpaw, A. J. & Dang, C. V. Exploiting metabolic vulnerabilities of cancer with precision and accuracy. Trends Cell Biol. 28, 201–212 (2018).
Miao, P. et al. Lactate dehydrogenase A in cancer: A promising target for diagnosis and therapy. IUBMB Life 65, 904–910 (2013).
Ippolito, L., Morandi, A., Giannoni, E. & Chiarugi, P. Lactate: A metabolic driver in the tumour landscape. Trends Biochem. Sci. 44, 153–166 (2019).
Maekawa, M. et al. Promoter hypermethylation in cancer silences LDHB, eliminating lactate dehydrogenase isoenzymes 1–4. Clin. Chem. 49, 1518–1520 (2003).
Leiblich, A. et al. Lactate dehydrogenase-B is silenced by promoter hypermethylation in human prostate cancer. Oncogene 25, 2953–2960 (2006).
Brown, N. J. et al. Lactate dehydrogenase-B is silenced by promoter methylation in a high frequency of human breast cancers. PLoS One 8, e57697 (2013).
Cui, J. et al. Suppressed expression of LDHB promotes pancreatic cancer progression via inducing glycolytic phenotype. Med. Oncol. 32, 143 (2015).
Wu, H. et al. Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct. Target Ther. 6, 242 (2021).
Maeda, M. et al. Genetic and drug inhibition of LDH-A: Effects on murine gliomas. Cancers 14, 2306 (2022).
Zhang, W. et al. Inhibition of LDHA suppresses cell proliferation and increases mitochondrial apoptosis via the JNK signaling pathway in cervical cancer cells. Oncol Rep. 47, 77 (2022).
Jin, L. et al. Phosphorylation-mediated activation of LDHA promotes cancer cell invasion and tumour metastasis. Oncogene 36, 3797–3806 (2017).
Hou, X. et al. LDHA induces EMT gene transcription and regulates autophagy to promote the metastasis and tumorigenesis of papillary thyroid carcinoma. Cell Death Dis. 12, 347 (2021).
Le, A. et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl Acad. Sci. USA 107, 2037–2042 (2010).
Mohammad, G. H. et al. Targeting pyruvate kinase M2 and lactate dehydrogenase A is an effective combination strategy for the treatment of pancreatic cancer. Cancers 11, 1372 (2019).
Manerba, M. et al. Galloflavin (CAS 568-80-9): A novel inhibitor of lactate dehydrogenase. ChemMedChem 7, 311–317 (2012).
Vettraino, M., Manerba, M., Govoni, M. & Di Stefano, G. Galloflavin suppresses lactate dehydrogenase activity and causes MYC downregulation in Burkitt lymphoma cells through NAD/NADH-dependent inhibition of sirtuin-1. Anticancer Drugs 24, 862–870 (2013).
Cui, B. et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Invest. 129, 1030–1046 (2019).
Maekawa, M., Sudo, K., Kanno, T. & Li, S. S. Molecular characterization of genetic mutation in human lactate dehydrogenase-A (M) deficiency. Biochem. Biophys. Res. Commun. 168, 677–682 (1990).
Kanno, T. et al. Lactate dehydrogenase M-subunit deficiency: A new type of hereditary exertional myopathy. Clin. Chim. Acta 173, 89–98 (1988).
Elia, I. et al. Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature 568, 117–121 (2019).
Doherty, J. et al. Blocking lactate export by inhibiting the Myc target MCT1 Disables glycolysis and glutathione synthesis. Cancer Res. 74, 908–920 (2014).
Gan, L. et al. Metabolic targeting of oncogene MYC by selective activation of the proton-coupled monocarboxylate family of transporters. Oncogene 35, 3037–3048 (2016).
Khan, A. et al. Targeting metabolic activity in high-risk neuroblastoma through Monocarboxylate Transporter 1 (MCT1) inhibition. Oncogene 39, 3555–3570 (2020).
Huang, H. K. et al. Isoorientin decreases cell migration via decreasing functional activity and molecular expression of proton-linked monocarboxylate transporters in human lung cancer cells. Am. J. Chin. Med. 48, 201–222 (2020).
Wang, N. et al. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 184, 370–383 e313 (2021).
Buyse, C. et al. Evaluation of syrosingopine, an MCT inhibitor, as potential modulator of tumor metabolism and extracellular acidification. Metabolites. 12, 557 (2022).
Benjamin, D. et al. Syrosingopine sensitizes cancer cells to killing by metformin. Sci. Adv. 2, e1601756 (2016).
Benyahia, Z. et al. In vitro and in vivo characterization of MCT1 inhibitor AZD3965 confirms preclinical safety compatible with breast cancer treatment. Cancers 13, 569 (2021).
Dana, P. et al. CD147 augmented monocarboxylate transporter-1/4 expression through modulation of the Akt-FoxO3-NF-kappaB pathway promotes cholangiocarcinoma migration and invasion. Cell Oncol. 43, 211–222 (2020).
Zhang, D. et al. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 355, 176–183 (2014).
Raez, L. E. et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother. Pharm. 71, 523–530 (2013).
Bizjak, M. et al. Combined treatment with Metformin and 2-deoxy glucose induces detachment of viable MDA-MB-231 breast cancer cells in vitro. Sci. Rep. 7, 1761 (2017).
Sukumar, M. et al. Inhibiting glycolytic metabolism enhances CD8+T cell memory and antitumor function. J. Clin. Invest. 123, 4479–4488 (2013).
Dunbar, E. M. et al. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest. N. Drugs 32, 452–464 (2014).
Powell, S. F. et al. Phase II study of dichloroacetate, an inhibitor of pyruvate dehydrogenase, in combination with chemoradiotherapy for unresected, locally advanced head and neck squamous cell carcinoma. Invest. N. Drugs 40, 622–633 (2022).
Li, M. et al. miR-564: A potential regulator of vascular smooth muscle cells and therapeutic target for aortic dissection. J. Mol. Cell Cardiol. 170, 100–114 (2022).
Yang, Y. et al. The lncRNA punisher regulates apoptosis and mitochondrial homeostasis of vascular smooth muscle cells via targeting miR-664a-5p and OPA1. Oxid. Med. Cell Longev. 2022, 5477024 (2022).
Li, X. et al. Multistage-responsive nanocomplexes attenuate ulcerative colitis by improving the accumulation and distribution of oral nucleic acid drugs in the colon. ACS Appl. Mater. Interfaces 14, 2058–2070 (2022).
Qi, H. Y. et al. Glucose-responsive nanogels efficiently maintain the stability and activity of therapeutic enzymes. Nanotechnol. Rev. 11, 1511–1524 (2022).
Dancy, B. M. & Cole, P. A. Protein lysine acetylation by p300/CBP. Chem. Rev. 115, 2419–2452 (2015).
Huang, H. et al. The regulatory enzymes and protein substrates for the lysine beta-hydroxybutyrylation pathway. Sci Adv. 7, eabe2771 (2021).
Sabari, B. R., Zhang, D., Allis, C. D. & Zhao, Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 18, 90–101 (2017).
Yang, Y. et al. Global insights into lysine acylomes reveal crosstalk between lysine acetylation and succinylation in streptomyces coelicolor metabolic pathways. Mol. Cell Proteom. 20, 100148 (2021).
Meng, X., Baine, J. M., Yan, T. & Wang, S. Comprehensive analysis of lysine lactylation in rice (Oryza sativa) grains. J. Agric Food Chem. 69, 8287–8297 (2021).
Shvedunova, M. & Akhtar, A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 23, 329–349 (2022).
James, A. M. et al. Non-enzymatic N-acetylation of lysine residues by acetylCoA often occurs via a proximal S-acetylated thiol intermediate sensitive to glyoxalase II. Cell Rep. 18, 2105–2112 (2017).
Gao, M., Zhang, N. & Liang, W. Systematic analysis of lysine lactylation in the plant fungal pathogen Botrytis cinerea. Front. Microbiol. 11, 594743 (2020).
Acknowledgements
We thank Jingjie Biotechnology Co., LTD., for providing technical support for the manuscript. This work was supported by The National Natural Science Foundation of China (grant no. 81870331), The Natural Science Foundation of Shandong Province (grant no. ZR2020MH045), and The Qingdao Municipal Science and Technology Bureau Project (grant no. 21-1-4-rkjk-12-nsh).
Author information
Authors and Affiliations
Contributions
T.Y., Z.W., and Y.Y. designed the study. X.L., X.L., and X.X. collated the data, carried out the data analyses, and wrote the initial draft of the manuscript. X.L. and Y.Y. contributed to drafting the manuscript. T.Y., X.L., Y.Y., T.Z., Y.Z., P.L., B.Z., and Z.W. reviewed and approved the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Yang, Y., Zhang, B. et al. Lactate metabolism in human health and disease. Sig Transduct Target Ther 7, 305 (2022). https://doi.org/10.1038/s41392-022-01151-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-022-01151-3
This article is cited by
-
MCT4-dependent lactate transport: a novel mechanism for cardiac energy metabolism injury and inflammation in type 2 diabetes mellitus
Cardiovascular Diabetology (2024)
-
RBBP7, regulated by SP1, enhances the Warburg effect to facilitate the proliferation of hepatocellular carcinoma cells via PI3K/AKT signaling
Journal of Translational Medicine (2024)
-
WNT-inhibitory factor 1-mediated glycolysis protects photoreceptor cells in diabetic retinopathy
Journal of Translational Medicine (2024)
-
Association between hypomagnesemia and serum lactate levels in patients with sepsis: a retrospective observational study
Journal of Anesthesia, Analgesia and Critical Care (2024)
-
Lactylation stabilizes DCBLD1 activating the pentose phosphate pathway to promote cervical cancer progression
Journal of Experimental & Clinical Cancer Research (2024)