Abstract
COVID-19, caused by SARS-CoV-2, is the most consequential pandemic of this century. Since the outbreak in late 2019, animal models have been playing crucial roles in aiding the rapid development of vaccines/drugs for prevention and therapy, as well as understanding the pathogenesis of SARS-CoV-2 infection and immune responses of hosts. However, the current animal models have some deficits and there is an urgent need for novel models to evaluate the virulence of variants of concerns (VOC), antibody-dependent enhancement (ADE), and various comorbidities of COVID-19. This review summarizes the clinical features of COVID-19 in different populations, and the characteristics of the major animal models of SARS-CoV-2, including those naturally susceptible animals, such as non-human primates, Syrian hamster, ferret, minks, poultry, livestock, and mouse models sensitized by genetically modified, AAV/adenoviral transduced, mouse-adapted strain of SARS-CoV-2, and by engraftment of human tissues or cells. Since understanding the host receptors and proteases is essential for designing advanced genetically modified animal models, successful studies on receptors and proteases are also reviewed. Several improved alternatives for future mouse models are proposed, including the reselection of alternative receptor genes or multiple gene combinations, the use of transgenic or knock-in method, and different strains for establishing the next generation of genetically modified mice.
Similar content being viewed by others
Introduction
At the time of writing, the fourth world-wide pandemic declared by WHO on 11 March 2020,1,2 which was caused by a novel coronavirus identified firstly in December 2019,2,3,4,5 is still going on in the spring of the third year. The contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was termed officially as coronavirus disease 2019 (COVID-19) on 11 February 2020 by the WHO.6,7,8 Until now, cumulative confirmed cases exceeded 400 million, and over 5.8 million deaths have been reported globally.
In particular, the highly contagious Omicron variant (B.1.1.529) infected 84 million people in the first month of 2022 alone, equivalent to the number of infections for the whole of 2020 (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports).9,10 Two previous outbreaks of highly contagious coronaviruses in humans before COVID-19 were caused by severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002–2003,11,12 and the Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012.13,14 The transmission of SARS-CoV-2 among humans, which is similar to the previous two coronaviruses but more difficult to control, occurs through direct contact, respiratory droplets, contaminated objects, and aerosols.4,15,16,17,18,19,20 The long-term and large-scale epidemic of SARS-CoV-2 characterized by widespread community transmission,21 while causing large numbers of asymptomatic and mild cases,22,23 and was also transmitted from humans to animals24,25 has brought great pressure on the public health of all countries in the world.
Six coronaviruses that infect humans generally belong to one of two categories. One includes common human coronaviruses known to infect immunocompromised individuals,5 including HCoV-229E (alpha coronavirus), HCoV-NL63 (alpha coronavirus), HCoV-OC43 (beta coronavirus), and HKU1 (beta coronavirus). The other category includes zoonotic species from the Beta coronavirus genus, including MERS-CoV and SARS-CoV.26,27 SARS-CoV-2 is considered the seventh member of the coronavirus family to successfully infect humans (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses 2020).
SARS-CoV-2 has a virion morphology similar to other coronaviruses. Electron micrographs revealed that SARS-CoV-2 virions were roughly spherical with some pleomorphism, ranging in size from 60 to 140 nm in diameter.28 It is an enveloped virus with a positive‐sense single‐stranded RNA genome. The genome sequence of SARS‐CoV‐2 was found to be 79.4% similar with SARS-CoV and 50% identical with MERS-CoV. SARS-CoV-RaTG13 (a bat CoV) was found to share 96% identity with SARS‐CoV‐2.28,29 Genome sequences confirmed the virus as CoV and indicated a common ancestor based on the high similarity between bat CoV and SARS-CoV-2.
Due to the high sequence similarity between SARS-CoV-2 and SARS-CoV-1, their ORFs and nonstructural proteins (nsps) are not significantly different.30 The whole genome of SARS-CoV-2 is ~29.8 kb in length with several open-reading frames (ORFs), the number of which varies across the CoVs.31 The first ORF (ORF1a/b), encoding polyproteins required for viral replication and transcription (nsp1-nsp 16), occupies two-thirds of the viral genome, while the remaining 13 ORFs encode accessory and structural proteins,32 including spike (S), membrane (M), envelope (E), and nucleocapsid (N).33,34,35 Particularly, the receptor-binding domain (RBD) of the spike protein (S) is the primary target of neutralizing antibodies,36 and mutations in S and its RBD largely determine the effectiveness of SARS-CoV-237 vaccines and the transmissibility of SARS-CoV-2.38 By 2022, many variants of concern (VOC) have emerged, accumulating multiple mutations mainly in the spike gene.39 These mutations can enhance the infectivity and virulence of SARS-CoV-2, lead to changes in clinical disease manifestations, or affect the effectiveness of diagnostics, vaccines, and treatment strategies.40
Since the outbreak of the pandemic, considerable efforts have been made to develop effective and safe vaccines, and therapeutic drugs, as well as to understand etiopathogenesis and immunology of SARS-CoV-2 infection. Animal models play crucial roles in all these studies. In the beginning of 2020, the WHO COVID Modeling group (WHO-COM), whose goal was to develop animal models of SARS-CoV-2 infection as soon as possible, was convened quickly by the World Health Organization (WHO) R&D Blueprint.41,42 Various animals were employed to develop COVID-19 disease models, including non-human primates (NHPs), genetically modified mice, wild-type mice sensitized by Ad5 or AAV vectors expressing the hACE2 gene, as well as Syrian hamster, ferret, poultry, and domestic animal models. Here, we summarized the success and deficits of current animal models, as well as the classical and alternative SARS-CoV-2 receptors and proteases. Then, the gaps in current animal models are analyzed and strategies for the development of further COVID-19 animal models are proposed, we strongly believe that it is necessary to develop and reserve animal resources in advance, as well as establish a rapid breeding supply system to be ready for the next pandemic.
Clinical features of COVID-19
General clinical symptoms
Based on the clinical observation of 1198 patients with laboratory-confirmed COIVD-19 from more than 600 hospitals, the characteristic presentation of SARS-CoV-2 infected patients is pneumonia43,44 accompanied by other related symptoms.35,45,46,47,48 The most common symptoms were fever (more than 90%) and cough (more than 80%),49,50 while rhinorrhea and nausea were uncommon.51,52 In another report, clinical symptoms included fever, cough, shortness of breath, fatigue, muscle ache, diarrhea, headache, increased sputum production, sore throat, rhinorrhea, hemoptysis, nausea, and vomiting.26,53,54,55 The clinical symptoms and incidence were summarized in Fig. 1a.
The percentage of clinical symptoms of COVID-19 patients with and without comorbid disease in all cases. a The symptoms of patients with confirmed SARS-CoV-2. b The most common signs of COVID-19 patients with comorbid disease. c Comorbid disease may aggravate COVID-19. The denominator of percentage in this figure is total cases. This represents the percentage of all cases
Variants of concern cause diverse clinical symptoms and have varying incidence.56,57 The alpha VOC was found to result in 3.8-fold higher risk of death or transfer to the ICU compared to the original strain,58,59 while also being characterized by more common loss of smell or taste.59 By the end of 2021, the case fatality rate was ~3% worldwide. Based on a large sample from the general population, without declaring their past medical history. However, there are differences in morbidity and mortality in specific subpopulations, such as pregnant women, babies, or patients with underlying medical conditions, such as diabetes, cancer, or cardiovascular disease.60 According to a report by the Chinese Centre for Disease Control, based on 44,672 patients, the case fatality rate was 7.3% in patients with diabetes as opposed to 2.3% in ono-diabetics.61 In addition, some studies have reported cases with atypical symptoms. For example, Li et al. reported conjunctivitis as the first symptom in two patients,61 while Yesilkaya et al. reported psychosis in two cases.62
Infection in different populations
Everyone is generally susceptible to SARS-CoV-2,63,64 but there are differences in the symptoms of infection among patients of different ages.65 Approximately 80% of older adults aged over 65 years with severe COVID-19 infection are admitted to the ICU, while there are few ICU admissions in patients younger than 19.66,67 However, once children are severely infected, treatment is not easy, medication is limited, and the prognosis is unknown, which should also be paid more attention.68
Older adults, especially those with underlying chronic diseases are more likely to develop severe or critical illness,69,70 resulting in hospitalization, acute respiratory distress syndrome, and death.46,71 Deaths are more common in the elderly over the age of 65 (more than 70%).72
Elderly patients also often have fever (almost 80%) and cough (almost 50%) as the main symptoms,73 followed by dyspnea, asthenia, anorexia, chest tightness, diarrhea, and to a lesser extent myalgia, pharyngitis, nausea, dizziness, headache, abdominal pain, vomiting and other symptoms.35,43,72,74,75,76 In older adults, which are often susceptible to underlying diseases, are also more susceptible to SRAS-CoV-2, which needs special attention.5,77
Pregnant patients with SARS-CoV-2 infection showed fever (7/9), cough (4/9), myalgia (3/9), sore throat (2/9), diarrhea (1/9), and dyspnea (1/9). Fortunately, there were no signs of coronavirus infection in their newborn babies, indicating that there is no evidence of vertical transmission.78,79 In another study, 23 pregnant women were investigated, and all 25 neonates were born alive. Three of the neonates were transiently suspected positive for SARS-CoV-2 after birth, but no newborns developed COVID-19.80
Children represent ~15% of the total number of COVID-19 cases.61 Although the clinical course of COVID-19 is relatively mild in children,81 we cannot ignore it, as the increasing number of cases in children may result in a higher number of patients.68 Children were mainly infected within the family, usually showing typical symptoms of acute respiratory infection,44 including fever (generally below 39 °C) and cough (more than 50%),43,61 Different from adults, the clinical picture of COVID-19 in children includes skin lesions as well as respiratory, gastrointestinal, and also neurological symptoms.82 Numerous reports describe significant abnormalities in a relatively small proportion of children infected with SARS-CoV-2.83,84 In newborns, the screening begins with primary maternal infection (84%). Similarly, most older children were asymptomatic (20%) or had mild (48%) and moderate (20%) signs of clinical infection.85,86 However, a slightly higher proportion of older children became severely ill (12%).87 Dyspnea was the most commonly reported sign in neonates (40%).62,80
The sex disparities in COVID-19 severity and outcome have been described.88 It seems that males are more susceptible to COVID-19 than females presumably because of smoking,89,90 a less effective immune response, and a greater predisposition to thromboembolism, ultimately resulting in severe clinical manifestations in males. In addition, the viral load of the Delta variant was found to be higher in female than in male patients, but that of other variants was almost equal.91
Infection in patients with preexisting diseases
A study of 41 patients, included 32% of patients with underlying chronic conditions such as cardiovascular, diabetes, or hypertension. Similar to the general population without preexisting disease, the chronic patients showed clinical symptoms such as fever, cough, fatigue, increased sputum production, headache, hemoptysis, and diarrhea (Fig. 1b),43 but they had higher rates of fever and fatigue,92 which may be the result of an impaired immune response.46
Patients with comorbidities account for ~30% of all patients with COVID-19, with conditions such as chronic obstructive pulmonary disease, diabetes, hypertension, cardiovascular disease, cerebrovascular disease, hepatitis B infection, cancer, chronic renal disease, and immunodeficiency.46,51,93,94,95,96 The incidence of these comorbidities is shown in Fig. 1c. Diabetes, hypertension, and cardiovascular and cerebrovascular diseases are the most common preexisting disease among COVID-19 patients.15,35,36,43
Diabetes is an important risk factor for adverse outcomes in COVID-19. In addition, cancer patients infected with SARS-CoV-2 have a poor prognosis, especially in case of hematological malignancy, with the state of illness being more evident and resulting in a higher risk of death.97,98 Male sex and aging are high‑risk factors for COVID‑19 patients with cancer.84,99 Patients with two or more comorbidities had significantly escalated risks compared with those who had single comorbidity, while patients with chronic obstructive pulmonary disease, diabetes, hypertension, and malignancy were more likely to reach the composite endpoints than those without.94,100
Infections of the central nervous system
Coronaviruses not only cause respiratory illness but can also invade the central nervous system through a synapse-connected route, which was observed in both patients and the brains of experimental animals. Some early COVID‐19 patients presented neurological signs such as nausea, headache, and vomiting, with fewer neurological symptoms, but with the increase of cases and the emergence of VOC, more cases showed neurological disease symptoms.62,101,102 More than 70% of patients presented related physical symptoms, lasting between 1 and 35 days. The most common reported symptom of psychosis was delusions, hallucinations disorganized thought, behavior, and speech, or even catatonia.103
Anosmia is a common and often the sole symptom of COVID-19 patients,55 and numerous clinicians around the world have reported smell/taste impairment as a symptom of the disease.76 Studies have found that more than 80% of patients have a decreased sense of smell and taste, whereby more than 60% of them have completely lost the sense of smell and taste after general symptoms.104 Notably, studies indicate that the olfactory sensory neurons are affected without getting infected.
Infection and injury of the gastrointestinal tract
In addition to invading the respiratory system, SARS-COV-2 also invades other organs expressing the cell surface receptor ACE2, and the digestive system is a susceptible target of SARS-COV-2.105,106 More than 80% of COVID-19 patients have one or more digestive symptoms along with fever over the course of the disease.107 In some patients, this was the first, or only, a symptom of the disease.108,109 It was found that COVID-19 patients with gastrointestinal symptoms mostly developed the severe disease, resulting in a high risk of death.110,111 Accordingly, gastrointestinal symptoms may be an important prognostic factor in COVID-19,112,113 and it is valuable to establish an intragastric challenge mouse model.114
Hematological and biochemical parameters
Hematological and biochemical parameters are widely used to diagnose infections, especially those caused by viruses.115,116 Patients infected with SARS-CoV-2 have changed hematological and biochemical parameters, which may be evidence for the infectious type and severity of the disease.117,118 During diagnosis and treatment, hematological indices such as the counts of erythrocytes, leukocytes, lymphocytes, neutrophils, and platelets are monitored. Usually, biomarkers such as IL-6, hemoglobin, serum ferritin, C-reactive protein (CRP), rate of erythrocyte sedimentation, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase and D-dimer are tested as well.75,119,120
In a sample of more than 800 patients, thrombocytopenia was reported in more than 30% of patients with COVID-19,117,121,122 and patients with severe disease had higher levels of thrombocytopenia than those with non-severe disease. Thus, a high platelet count can be used as an indicator of good prognosis.123 A study by Guan et al., based on blood samples of 1099 patients with COVID-19 in China showed that more than 80% of patients had lymphopenia, and the ratio of leucocytes was lower than the normal range in 33.7% of infected patients.121 Reduced hemoglobin levels have been noted in some severe COVID-19 patients, but 20–40% of patients have leucopenia, 3–24% have leukocytosis, and lymphopenia was seen in 30–75% of COVID-19 patients.124
Blood samples collected from 24 asymptomatic carriers with confirmed novel coronavirus infection showed that most of the patients had increased levels of ALT, AST, CPR, D-dimer, and creatine kinase.101,125 Moreover, 16.7% of patients had decreased lymphocyte ratios, which was lower than in the report by Guan and colleagues.121 A study reports that at least 5% of patients have heart failure.126 Among them, the most common cardiac pathology is right ventricular (RV) dilatation (39%), followed by left ventricular (LV) diastolic dysfunction (16%) and left ventricular systolic dysfunction (10%). Although about 60% of patients can recover eventually, patients with clinical heart failure have a high risk of poor prognosis of COVID-19 infection.127
Hemophagocytosis has been noted in the bone marrow aspirates of three severe COVID-19 patients, whereby increased numbers of megakaryocytes in the bone marrow were also found.128,129 Thrombosis also commonly occurs in patients with COVID-19, including pulmonary embolism, venous thromboembolic events such as proximal deep vein and upper extremity thrombosis, as well as arterial thromboembolic events such as ischemic stroke.130,131
Effects on the reproductive system
In an early epidemiological survey, one patient was reported to develop testicular pain,132 and about 40% of patients had low libido, suggesting that SARS-CoV-2 infection may affect the reproductive function of patients. Moreover, it has been reported that erectile failure results from COVID-19-caused cardiovascular dysfunction, and the subsequent treatment may further deteriorate libido.133,134,135
Although the SARS-CoV-2 virus was not found in the testes of COVID-19 patients, testicular microscopy showed spermatocyte abscission and sperm cell elongation, suggesting acute testicular injury.136 Consistently, spermatogenic tubule damage and spermatocyte shedding were observed in the testicular cells of patients infected with COVID-19, but no viral particles were detected in testes137 and semen.136,137
Receptors and cellular proteases that mediate SARS-CoV-2 infection
Cellular receptors mediate viral attachment and the fusion of the viral envelope with the host cell membrane,138,139,140 leading to infection of the host cell. Endogenous proteases,141,142,143 are also required for viral fusion with the cellular membrane and entry into target cells (Table 1). Several classical cellulars and alternative receptors for SARS-CoV-2 have been reported.139,144 The classical receptor human angiotensin-converting enzyme 2 (hACE2)145 is used by both for the first SARS-CoV146,147,148,149 and SARS-CoV-2.150,151,152 Alternative receptors include extracellular matrix metalloproteinase inducer (EMMPRIN or CD147),153 asialoglycoprotein receptor 1 (ASGR1),154 kringle containing transmembrane protein 1 (KREMEN1),154 neuropilin‐1 (Nrp1),155 dipeptidyl peptidase 4 (DPP4/CD26),156 alanyl aminopeptidase (ANPEP/CD13),156 angiotensin II receptor type 2 (AGTR2),157 and glutamyl aminopeptidase (ENPEP).156
Classical receptor and novel receptor candidates
Angiotensin‐converting enzyme 2
Human ACE2 is the most widely recognized receptor for SARS-CoV-2, which has been supported by numerous in vitro158,159,160,161,162 and in vivo experiments.114,144,163,164,165,166 Moreover, it has been identified as a receptor for SARS-COV-1 since 2003.146,147 ACE2 was discovered in early studies as vital regulator of molecular in cardiac function and various other organs via the renin–angiotensin system (RAS).167,168,169 The receptor‐binding domain of the viral S protein binds to ACE2,124,170,171 causing SARS‐CoV‐2 to undergo endocytosis and exposes it to endosomal proteases leading to viral infection of the host.172,173,174 HeLa cells expressing hACE2 are susceptible to infection with SARS‐CoV‐2.175
The main impediment to the infection of wild-type mice with SARS-CoV-2 is lack of appropriate receptors to initiate viral infection.41 The mouse Ace2 gene (mAce2) gene was mapped to chromosome X 70.5 cM. It produces two cDNAs with respective lengths of are 2746 and 1995 bp due to alternative splicing. Notably, mACE2 showed only 83% identity with hACE2,176 resulting in a lack of binding by SARS-CoV-2, preluding infection. The successful development of mouse models by transgenic,165,177 humanization,178 or by Ad5179 transduction have proved the function of ACE2 as a necessary and sufficient factor for infection.
Sequencing of normal human tissues32 and normal human lung samples,180 has demonstrated the expression hACE2 in type II pneumocytes, which appear to support viral replication in humans. However, studies do not indicate that SARS‐CoV‐2 can infect all organs expressing the ACE2 receptor, and other factors, such as cellular proteases that cleave the viral S protein, may also be required.181
CD147
CD147 is a transmembrane glycoprotein, commonly known as an extracellular matrix metalloproteinase inducer (EMMPRIN) or basic immunoglobulin. In earlier studies, CD147 overexpression was found in most cancers, including melanoma, where it was associated with a poor prognosis182 and inflammation.183 Accordingly, CD147 was considered a cancer-associated biomarker with potential role in cancer detection.182 It is found in brain tissue rather than the respiratory system.184
CD147 has been demonstrated to be involved in human immunodeficiency virus (HIV)-1 infection by interacting with virus-associated cyclophilin A.185 Chen et al.186 reported the role of CD147 in invasion of host cells by SARS-CoV-1 in 2005. Recently, CD147-spike protein interaction was revealed as a novel route for SARS-CoV-2 infection of host cells.153 Cells in the central nervous system are more likely to be infected with SARS-CoV-2 through the CD147 receptor and TMPRSS2 protease than through hACE2, since studies revealed that TMPRSS2 and CD147 mRNA levels were higher in the pituitary area, cerebellum, and cortex of the mouse brain.184 This result may explain the mechanism of SARS-CoV-2 infection in the central nervous system.187
A hCD147Tg-NSG mouse model was successfully generated in the NOD- scid IL2Rgamma null (NSG) background.188 In this model, the human CD147 sequence was knocked-in after the endogenous promoter of mouse CD147 (mCD147), resulting in physiological expression of human CD147 protein in appropriate mouse tissues. The novel hCD147Tg-NSG mouse model might allow more detailed studies of the pathogenicity of SARS-CoV-2 in immunocompromised patients. In addition, this mouse model can confirm if CD147 serves as an independent functional receptor or accessory receptor for SARS-CoV-2 entry.188
Asialoglycoprotein receptor 1 and Kringle containing transmembrane protein 1
Asialoglycoprotein receptor 1 (ASGR1) mediates the internalization of galactose-terminated glycoproteins into hepatocytes for degradation in lysosomes. The mouse ASGR genes have been mapped to mouse chromosome 11 using recombinant inbred stains, while the human ASGR 1 and 2 genes, which encode the major Hl and minor H2 receptor polypeptides, are located on chromosome 17p11-13.189 ASGR RNA expression is mainly detected in the liver, with protein expression detectable in the liver, stomach and gallbladder. Kremen proteins are Dickkopf receptors regulate Wnt/β-catenin signaling,76 and are wildly expressed in different tissues.
Genomic receptor profiling identified ASGR1 and KREMEN1, together with ACE2, as potential receptors with diverse S-binding affinities and patterns,154 implying that they may be alternative functional receptors for SARS-CoV-2 cell entry. Cells expressing ACE2/ASGR1/KREMEN1 receptor combinations display a markedly stronger virus susceptibility than those expressing any individual receptor at both the cell and tissue levels. Mouse models transduced with lentiviral particles encoding human ASGR1, KREMEN1 or ACE2 supported SARS-CoV-2 infection. These alternative receptors, independent of ACE2, provide insights into SARS-CoV-2 tropism and pathogenesis, which can inform potential therapeutic strategies.154
The physiological expression pattern of ASGR1, which is not expressed in the lungs, trachea, bronchus, brain and other organs that are affected in clinical cases, makes it a less likely alternative receptor supporting SARS-CoV-2 infection. A lentivirus transduced mouse model provided in vivo evidence, but this conjecture needs further confirmation, for example using transgenic mouse model.
Neuropilin‐1
The neuropilin (NRP) family consists of two members, NRP1 and NRP2.190 As a transmembrane glycoprotein, neuropilin 1 acts as a co-receptor for a number of extracellular ligands, including transforming growth factor beta, class III/IV semaphorins, and certain isoforms of vascular endothelial growth factor.191 NRP1 is expressed on a subset of T regulatory cells and in plasmacytoid dendritic cells, where it aids in priming immune responses. In mice, it is selectively expressed on thymic-derived Tregs and greatly enhances their immunosuppressive function. NRP1 is highly expressed in macrophages and DCs but not CD4+ T cells, serving as an anti-HIV factor to inhibit the infectivity of HIV-1 progeny virions.192 Down-regulated expression of NRP-1 significantly enhanced the transmission of HIV-1 in macrophages and dendritic cells, while also increasing the infectivity of HIV-1 virions.
Recently, it has been observed that SARS-CoV-2 infection promotes liver injury through pathways that may be influenced by previous pathological status and liver expression of NRP1. The cytokine storm in infected patients with severe disease may influence liver sinusoidal-cell phenotype, and facilitating viral invasion.193 Cells expressing NRP1 in the olfactory epithelium and endothelial cells of the olfactory bulb were also found to be infected by the virus.155 The host protease furin cleaves spike protein to produce a polybasic Arg-Arg-Ala-Arg C-terminal sequence on S1, which conforms to a C-end rule motif that binds to cell surface NRP1 and NRP2 receptors, but not the ACE2 receptor.194 Thus, NRP1 may serve as a host factor for SARS-CoV-2 infection and a candidate therapeutic target for the treatment of COVID-19. The binding occurs through the b1/b2 domain on the NRP1 receptor with the polybasic amino acid sequence 682RRAR685.195 A monoclonal antibody targeting NRP1 has been developed,155 but no challenge results have been reported in mouse models to date (Table 1).
hDPP4, AGTR2, ANPEP and ENPEP
MERS-CoV is another coronavirus that is highly pathogenic in humans, causing severe disease and sometimes lethal lower respiratory tract infection.196,197 Dipeptidyl peptidase 4 (DPP4), also known as CD26, has been identified as a functional receptor for MERS-CoV strain EMC.198 As an extracellular peptidase, hDPP4 was detected on the surface of different types of cells such as the liver, capillaries, lungs, kidneys, smooth muscle, and in the immune system.199,200,201 Nevertheless, the homolog of mouse DPP4 (mDPP4) does not play the same role.202 Mice transduced with adenoviral vector expressing hDPP4 could be infected,203 while hDPP4-transgenic or knock-in mice204,205 were susceptible to MERS-CoV infection and developed fatal disease. There is currently insufficient evidence that hDPP4 might support the SARS-CoV-2 infection in vivo. An experiment using HeLa cells showed that SARS-CoV-2 virions do not bind to the DPP4 receptor,175 but another report206 did observe such binding. Based on the similar expression pattern with human ACE2, Qi et al.156 as well as Venkatakrishnan et al.206 inferred that hDDP4 may be a cellular receptor of SARS-CoV-2.
Studies have shown that angiotensin II receptor type 2 (AGTR2), alanyl aminopeptidase (ANPEP) and glutamyl aminopeptidase (ENPEP) may also be receptors of SARS-CoV-2. AGTR2 is a G-protein coupled receptor with high and specific expression in the lungs, and showing a greater binding affinity towards SARS-CoV-2 S protein compared to ACE2. AGTR2 is capable of interacting with ACE2, and gene expression analysis revealed that when ACE2 is significantly downregulated, there is concomitant upregulation of AGTR2.207
Since ANPEP is a known cellular entry receptor for several coronaviruses, such as human CoV-229E, as well as canine and feline CoV,156,208 scientists considered it a candidate receptor for SARS-CoV-2. However, there is only evidence that ANPEP has a similar expression patter in the conjunctiva with ACE2.209
ENPEP plays an important role in regulating blood pressure and remodeling blood vessels. Qi et al.156 found that the expression pattern of ENPEP was similar to that of the ACE2. However, further studies are necessary to confirm the function of ENPEP as an entry receptor, or co-receptor for SARS-CoV-2 infection, since there is no in vivo evidence (Table 1).
Proteases that mediate viral entry of SARS-CoV-2
Transmembrane serine protease 2 (TMPRSS2), the lysosomal proteases cathepsin B and L, the endogenous serine protease furin, and the serine endopeptidase trypsin plays critical roles in the viral entry of SARS-CoV-2.144,181 TMPRSS2 in particular has been widely studied.210,211 SARS-CoV-2 infection requires the maturation of the spike protein, before entry into target cells. Two separate mechanisms of cleavage may be involved, including ACE2 cleavage, which might promote viral uptake, and SARS-S cleavage, which activates the S protein for membrane fusion.212 The arginine and lysine residues among ACE2 amino acids 697 to 716 are essential for cleavage by TMPRSS2.212
TMPRSS2
Several studies have shown that TMPRSS2 can activate and cleave the S protein of SARS-CoV-1 for membrane fusion.210,213 Mechanistic insights into this process of ACE2 cleavage and activation were reported later.212 TMPRSS2 was found to be expressed in the human respiratory system, including the subsegmental bronchial branches and lungs.214 Furthermore, single‐nucleus RNA sequencing revealed that there is higher TMPRSS2 expression in type II pneumocytes, similar to ACE2 expression.114 In agreement with this, TMPRSS2 was found to be co-expressed with ACE2 in several sites, such as in the transient secretory cells of subsegmental bronchial branches and enterocytes in the small intestine,214 which may explain why these organs are vulnerable to SARS‐CoV‐2 infection. Camostat mesylate, which acts as a TMPRSS2 blocker, can partially inhibit SARS-CoV-2 entry in CaCo-2 cells, indicating the essential role of TMPRSS2 as an entry molecule.171 Smoking and exposure to air pollution may cause several comorbidities damaging the lungs, and are associated with more severe COVID-19 disease. Interestingly, smoking is associated with increased expression of TMPRSS2 and ACE2 in the human lungs.215 Notably, a TMPRSS2 deficient mouse model exhibited decreased susceptibility to SARS-CoV-2.212 Overall, the available literature indicates that TMPRSS2 plays crucial roles in the SARS-CoV-2 infection and cell entry.
Cathepsin B, cathepsin L, furin. and trypsin
As main lysosomal proteases, cathepsins B and L are commonly found in lysosomes and endosomes. They are responsible for monitoring the function of lysosomes216,217 and are commonly associated with aging neurons.218 Recently, it was reported that cathepsins B and L take part in SARS‐CoV‐2S protein priming, and are critical proteases for SARS‐CoV‐2 entry into HEK 293/hACE2 cells.219
A furin-like cleavage site was detected at the SARS‐CoV‐2 S1/S2 subunit,220 which is not present in other types of SARS coronaviruses. The ability of furin to cleave the S protein is thought to be the reason for the increased binding affinity of SARS‐CoV‐2 for the ACE2 receptor.221
As a serine endopeptidase, trypsin is highly expressed in respiratory and gastrointestinal cells. Recent reports indicate that trypsin may also be one of the proteases that aid SARS‐CoV‐2 entry.181 Bertram S et al.211 identified that human airway trypsin‐like protease could cleave and activate SARS‐CoV-1 S protein, which might cause viral spread in humans, but this finding requires further confirmation. Other common host factors, such as CSNK2B, GDI2, SLC35B2, DDX51, VPS26A, ARPP-19, C1QTNF7, ALG6, LIMA1, COG3, COG8, BCOR, LRRN2 and TLR9 may also regulate SARS-CoV-2 infection, and were discussed systematically in a recent review.222
Animal models of COVID-19
Non-human primate
Non-human primates share great similarity with humans in terms of physiological characteristics and immune regulation. Intensive efforts have been made to develop COVID-19 disease models in NHPs. Rhesus macaques (Macaca mulatta), Cynomolgus macaques (Macaca fascicularis), African green monkeys (Chlorocebus sabaeus), Baboon (Papio hamadryas) and common marmoset (Callithrix jacchus) have been employed as models of SARS-CoV-2 infection, whereby the first two are the most common selection (Table 2). It is worth mentioning that NHPs are facing enormous demand and soaring costs, so it may be necessary to find alternative models.
Rhesus macaques exhibit mild clinical disease and supporting high level of viral replication in the respiratory tract similar to humans. Mild fever, body weight loss, decreased appetite and hypoxia are common reported symptoms. Occasionally, asthenia, decrease in platelet counts, transient neutropenia and lymphopenia were also reported.223 Rhesus macaques display several histopathological lesions similar to those observed in patients, such as pulmonary discoloration, consolidation, hyperemia, glass opacity, infiltrates, hemorrhage, scar, necrosis and interstitial pneumonia.224 Lesions in the liver and spleen were reported as well. Although Rhesus macaques COVID-19 disease model recapitulates human symptoms most closely,223 some typical clinical symptoms, including acute respiratory distress syndrome, were not observed, which limits its application for mode detailed studies of COVID-19.
Cynomolgus macaques exhibit limited clinical symptoms, including mild fever and weight loss, nasal discharge and elevated levels of liver-related enzymes when challenged with SARS-CoV-2 via the intranasal or intratracheal route.129,131,132,133,223 Pulmonary consolidation is a common histopathological lesion in Cynomolgus macaques,225 as is observed in clinical patients. Another pathological change exhibited in COVID-19 patients, diffuse alveolar damage (DAD), was also observed in this animal model.225 In addition to the respiratory system, lesions in the liver and spleen also were observed in Cynomolgus macaques.223 Similar to Rhesus macaque, high levels of viral RNA were detected in the respiratory tract of challenged Cynomolgus macaques, even though they had lower viral loads and shorter duration of viral shedding.226
Several clinical signs were also seen in challenged African green monkeys, including transient fever, decreased appetite, hypercapnia, lymphocytopenia and thrombocytopenia, elevated liver-related enzymes, increased monocytes,134,226,227,228 and crucially, acute respiratory distress syndrome (ARDS).228 ARDS, the common and often fatal characteristic sign of severe COVID-19, is sustainably observed in aged African green monkey, while being difficult to replicate in other NHPs. African green monkeys, especially aged animals, are a quite useful model for mirroring severe disease manifestations, notably ARDS.228 Furthermore, viral pneumonia, severe pulmonary consolidation with hemorrhage and infiltration, extensive pulmonary lesions and gastrointestinal abnormalities were also observed in infected animals. Common histopathological lesions include pulmonary discoloration, opacity, bronchiolization, hyperemia and pleural adhesions.227 Compared to Rhesus macaques, African green monkeys exhibit more severe consolidation and edema of lung lobes.226
Compared with macaques, baboons exhibited more severe histopathological lesions, prolonged viral RNA shedding, substantially more lung inflammation,229 as well as showing age-related effects. By contrast, no or only very mild clinical symptoms (mild fever) were reported in common marmosets inoculated with SARS-Cov-2.229 Milder interstitial and alveolar pneumonitis were reported in marmosets, compared to in macaques or baboons.229 It is thought to be less susceptible to COVID-19 infection223,229 (Table 2). In sum, rhesus macaques are the most popular NHPs for COVID-19 disease because they are commercially available and manifest the clinical symptoms quite well. Cynomolgus macaques usually present pulmonary consolidation but show weak clinical symptoms. In contrast, African green monkeys generally exhibit severe symptoms, but their scarcity greatly limit the usage. Nevertheless, natural protective immunity, such as innate as well as humoral and cellular immune responses, can be induced in these NHPs.
Mouse models
Stably inherited genetically modified mouse models
Mice and other rodents are the most widely used experimental animals. However, wild-type rodents are not spontaneously permissive for SARS-CoV-2 infection, because the virus can efficiently bind human ACE2 (hACE2), but not mouse Ace2 (mAce2). The expression of hACE2 was found to be related to cardiovascular and pulmonary diseases230 and it was also found to be a receptor of various coronaviruses including SARS-CoV-1146 and SARS-CoV-2.181 Stably inherited genetically engineered mouse models expressing hACE2, which results in great susceptibility, are critical for the preclinical evaluation of vaccines and drugs against SARS-CoV-2.
Several genetically modified hACE2 mouse models, driven by specific promoters and established via precision knock-in or random transgenic technologies (Table 3), have been adapted for research on cardiovascular disease and coronavirus infection.114,138,231,232,233 These mouse models have various backgrounds and can achieve stable expression of hACE2 in multiple organs. However, differences of hACE2 expression patterns in mouse models, different SARS-CoV-2 strains, as well as diverse infection routes and viral doses, lead to different clinical manifestations and pathological changes (Table 4). SARS-CoV-2 is transmitted through the respiratory tract, so most studies in Table 4 rely on the intranasal route to infect the model animals with SARS-CoV-2, except for hACE2-KI mice which were infected through intragastric inoculation.114 Unlike the upper respiratory tract signs (such as cough and dyspnea) that humans are most likely to develop after infection with SARS-CoV-2,234 weight loss is the most obvious and important clinical sign in mice. However, impaired lung function was observed in certain HFH4-hACE2 and K18-hACE2 mouse infection models (the background for these transgenic mice, see Table 3), including respiratory distress,235 markedly abnormal lung biomechanics236 and labored breathing.163 SARS-CoV-2 infection is usually confined to the respiratory tract in most hACE2 mouse models, but the brain can also be a target organ, suggesting nervous system invasion secondary to respiratory infections.114,163,235,236 Surprisingly, the SARS-COV-2 virus with broad organ tropism replicated in K18-hACE2 mice infected with intermediate viral titers (nCoV-WA1-2020).163 The animals exhibited systemic infection, with virus detectable in the nasal epithelium, trachea, lungs, heart, spleen, liver, kidneys, stomach, large intestine, small intestine and brain. In another K18-hACE2 mouse model, the infection of nasal epithelial cells, especially supporting sustentacular cells may be associated with anosmia as a major manifestation in mild disease.50,164,237 By intranasal inoculation of K18-hACE2 transgenic mice with higher viral doses (2 × 103 and 2 × 104 PFU) or lower doses (2 × 101 and 2 × 102 PFU), a model recapitulating both non-severe and severe COVID-19 infection were established, respectively.238
Lung histopathology check indicated that the hACE2 mouse model is capable of mimicking pneumonia associated with severe SARS-COV-2 infection, with diffuse alveolar damage,237 interstitial pneumonitis114,163,235,239 and inflammatory or lymphocytic infiltrates.114,163,235,236,239,240,241 Notably, the hACE2-KI mouse infection model exhibited distinct vascular system injury and pathological progression related to the age of the mice.114 Moreover, the thrombosis characteristic of critical illness was observed in the K18-hACE2 mouse infection model.164 At present, most studies on immunological processes that influence COVID-19 in mouse models are based on K18-hACE2 mice, which reflect changes in the immune response of the lungs. In particular, several chemokines (CCL2, CCL3, CCL4, CXCL1, and CXCL10) and inflammatory cytokines (TNFα, IL-6, and G-CSF) associated with the severity of COVID-19 disease in humans are highly expressed in these models.221,236,242,243 In aged mice, the interferon and adaptive antibody responses to the SARS-CoV-2 challenge are significantly impaired, resulting in more effective virus replications and severe disease manifestations.244 Importantly, these clinically relevant pathological findings are beneficial for studying the pathogenesis of SARS-CoV-2 in vivo.
Mouse models sensitized by Ad5-hACE2 or AAV-hACE2 transduction
Because the mouse ACE2 receptor does not support viral binding, wild-type mice are not permissive to SARS-CoV-2 infection.170,245,246 To sensitize the wild-type mice, adeno associated vector or adenovirus 5 expressing hACE2 (AAV-hACE2, Ad5-hACE2) were used to transduce mice intratracheally, causing hACE2 expression in lung tissues, which enabled viral entry and infection (Table 5). Different from stably inherited transgenic mice, these sensitized mice express hACE2 in respiratory tract, especially in the lungs, and the expression pattern is different from both transgenic mice and human.41,247,248,249,250 Upon infection with SARS-CoV-2, the virus replicates in the lungs of hACE2 mice for several days to two weeks, depending on the mouse strain used (Table 5).251 Immunodeficient mice such as IFNAR−/− and STAT1−/− presented delayed virus clearance, while in immunocompetent mice quickly cleared the virus.252 The challenged C57BL/6 mice transduced with AAV-hACE2 developed the characteristics of moderate interstitial pneumonia, including inflammatory infiltration in peri-bronchia, diffuse infection within alveolar epithelia, expansion of pulmonary infiltrating myeloid-derived inflammatory cells as well as the recruitment of monocyte-derived macrophages and inflammatory cells.252 Viral infection in the BALB/c or C57BL/6 mice transduced with Ad5-hACE2 induces severe inflammation in the upper and lower respiratory tract, including the infiltration of inflammatory cell from perivascular to interstitial zones necrosis, the aggravation of alveolar edema, vascular congestion and hemorrhage.179 Accompanied by the pathological changes, there were significant clinical signs in the mice transduced with Ad5-hACE2, while there were no significant clinical signs in the mice transduced with AAV-hACE2, which had a lower viral load.252
The immune response is crucial for antiviral defense and lymphopenia is widespread in severe cases of SARS-CoV-2.253 Thus, the models based on immunocompromised mice can mimic the severe outcomes and features of SARS-CoV-2 infection in humans. In the IFNAR knockout mice transduced with AAV-hACE2, the recruitment of Ly6Chi monocytes and monocyte-derived macrophages was abolished, the activation of CD4+, CD8+ or NK cells was inhibited, and the accumulation of neutrophils was increased instead.254 The inflammatory characteristics of IRF3/7−/− mice are similar to IFNAR−/− mice transduced with AAV-hACE2, but the viral load is significantly higher in the latter (Table 5). Ad5-hACE2 transduced STAT1−/− mice are the most permissive to SARS-CoV-2.179 Overall, the outstanding advantage of AAV- or Ad5- transduced mice model is the rapid acquisition of susceptible animal models for emergency needs, but such disease models can only mimic limited physiological features of SARS-CoV-2 infection.
Models based on mouse-adapted strains of SARS-CoV-2
It is of great significance to study the pathogenicity of viruses by analyzing the emerging mutations of mouse-adapted strain.255,256 Huang et al. passaged the SARS-CoV-2 WuHan-Hu-1 strain in 1-year-old BALB/c mice. Lung homogenates were used to intranasally inoculate young mice, and after 11 rounds, the mouse-adapted virus strain WBP-1 was generated. Compared to its ancestral strain, WBP-1 showed increased infectivity in BALB/c mice and caused more severe interstitial pneumonia. Sequence analysis revealed that the Q498H mutation emerged after only one passage, and the Q493K mutation occurred in passage 5, which likely contributed to the high pathogenicity of WBP-1 in mice.257 Further, the two mutations are both located in the RBD, and significantly increased its binding affinity for mouse ACE2. The study tentatively found that the TLR7/8 agonist resiquimod was able to protect mice against WBP-1 challenge, indicating that this mouse-adapted model may be used as a tool for investigating COVID-19 therapies.
The proposed model should s recapitulate the main features of SARS-CoV-2 infection in humans, supporting efficient viral replication in both the upper and lower respiratory tracts.258 Wang et al. intranasally inoculated used 4- to 6-week-old female BALB/c mice with SARS-CoV-2 HRB26, and after for 14 passages the mouse-adapted strain HRB26M was generated. Mild pathological changes were observed in wild-type BALB/c mice when challenged with this strain, demonstrating that SARS-CoV-2 successfully adapted to infect the upper and lower respiratory tract of young BALB/c mice.259 Gu et al. reported a more rapid method, which they used to generate the mouse-adapted strain named MACSP6, after only six passages in BALB/c mice.260 The key RBD mutation (N501Y) was observed in this strain. After an additional 30 passages, a more virulent mouse-adapted strain named MACSP36 was generated, which elicited typical respiratory symptoms in 9-month-old mice, especially tachypnea was exhibited in all moribund animals.256
In addition to accumulating mutations through serial passages, precise reverse genetic technology was employed to rapidly establish mouse-adapted strains (Table 6). Dinnon et al.261 used reverse genetics to remodel the S and mAce2 binding interface, resulting in a recombinant virus, named SARS-CoV-2 MA. This virus utilized mAce2 for entry, and showed more clinically relevant phenotypes than those seen in hACE2 transgenic mice, demonstrating its utility. With further passaging, the strain resulted in a linear decrease of body weight in challenged BALB/c mice. Furthermore, 10-week-old BALB/c mice challenged with the mouse-adapted strain MA10 exhibited a loss of pulmonary function, accompanied by increased morbidity and mortality. A study also reported that 10-week-old C57BL/6 J mice exhibited less severe disease when infected262 (Table 6).
Humanized mouse models with engrafted human tissues or cells
The human immunological mechanisms defining the clinical outcome of SARS-CoV-2 infection remain elusive. Although the animal models susceptible to SARS-CoV-2 have been available,131,133,210,220,230,240 an in-depth understanding of the immunopathogenesis has been hindered by the vast difference in both immune responses and lung environments between humans and animal models.263,264 That said, the SCID-hu lung mouse model has been proved to be an alternative approach to resolve this problem as it shows rapid virus replication, severe lung damage, and robust pro-inflammatory responses.265 To establish this model, human fetal lung tissues were surgically grafted into the dorsal skin of SCID mice and were allowed to grow for about 8 weeks followed by SARS-CoV-2 virus challenge by direct injection into the engrafted lung tissues.265
However, optimal humanized mouse models should carry not only human functional lung tissues but also the human immune system, thereby more precisely recapitulating human immunopathology. A mouse model co-engrafted with human fetal lung tissues and a myeloid-enhanced human immune system was created recently,263 which could serve as a better model than the one solely engrafted with human fetal lung tissues to identify cellular and molecular correlates of lung protection against SARS-CoV-2 infection. These mice exhibit severe inflammatory and histopathological phenotypes, which are associated with macrophage infiltration and differentiation and the upregulation of genes involved in the type I interferon signaling pathway.263 Taken together, engrafted mice models are an alternative and complementary approach to the currently used human lung organoid model265 for the studies on the immunopathology of SARS-CoV-2 infection. However, the limited supply of human tissues and immune cells makes the scale-up difficult. Moreover, the challenge route through direct injection is quite different from the clinical infection.
Syrian hamster models
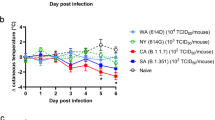
Syrian hamsters have been shown susceptibility to SARS-CoV-1 more than one decade ago, and were proven to be susceptible to variable SARS-CoV-2 isolates recently. In accordance with these findings there is great sequence similarity between human ACE2 and that of hamsters.29,165,233,266,267,268,269,270 Respiratory and pulmonary disease, accompanied by weight loss, occurs in hamsters upon infection.271,272 Weight loss is the main clinical sign which serves as the indicator of infection severity, but is reversed later. Viral load could be detected in the respiratory tract, including bronchial epithelia cells, type I and type II alveolar epithelial cells and macrophages, which are also targets in human lung tissues by SARS-CoV-2.273,274,275,276,277 Virus replication in these tissues reaches the peak at the early stage after infection, followed by a rapid decline. However, almost no viral RNA can be found in the spleen, kidneys, blood, duodenum and brain of infected hamsters, except in some extreme cases.273 Virus propagation is accompanied by inflammation, tissue damage,278,279 and lung abnormalities were found in SARS-CoV-2 challenged hamsters, including interstitial pneumonia, inflammatory cell infiltration, alveolar septal thickening and distinctive vascular system injury.280 Afterwards, a macrophage-dominated pulmonary immune response was induced.278 Sia et al. also observed the infiltration of CD3+ T lymphocytes and monocytes in the peribronchial region after infection.279 After lung damage and histopathological changes caused by virus replication, the viral load decreased rapidly and lung damage resolved in the next days of infection. The histopathological changes in arterial and venous endothelium of hamsters were consistent with endotheliosis in human SARS-CoV-2 infection.273,281
Some studies showed long term damage to other physiological systems including the olfactory sensory system, reproductive system and cardiovascular system.282,283,284,285,286,287 Cardiovascular pathologies were found in female hamsters with late-stage SARS-CoV-2 infection, including myocardial interstitial fibrosis, as well as thickening of ventricular walls and the interventricular septum.284 In addition, SARS-CoV-2 infection changes the serum lipid and metabolite profiles of the hamsters, which was also observed clinically in human patients.284 Another study revealed that SARS-CoV-2 infection caused reproductive problems in male hamsters, including acute decrease of the sperm count and serum testosterone, as well as reduced testicular size and serum sex hormone levels for months after infection.288 The circulating strain Omicron was found to cause similar changes, but vaccination protected male hamsters from the testicular damage.288
According to the clinical manifestations, SARS-CoV-2 causes more severe outcomes in males than in females, and sex differences of acute and chronic damage after infection were also studied in the hamster model.289 In addition, Osterrieder et al. revealed that the progression of SARS-CoV-2 in Syrian hamsters is age-dependent, since viral replication in the upper and lower respiratory tract was different according to the age of hamsters. Consequently, older hamsters exhibited more severe clinical signs, such as weight loss.273
The close contact transmission model was also tested in hamsters, demonstrating that cohousing contact with SARS-CoV-2-carrying hamsters is an efficient way to spread the disease. At the same time, it was found that mRNA-HB27-LNP has a prophylactic effect against SARS-CoV-2 transmission through close contact.280 It was also demonstrated that immunosuppressed hamsters are susceptible to low-dose virus inoculation, while developing more severe and prolonged disease.290,291,292 Brocato et al.203,204 exposed cyclophosphamide immunosuppressed and Rag2 knockout hamsters to SARS-CoV-2 by the respiratory route to test this hypothesis.293,294 Although the ACE2 of hamsters can be used as a cell entry receptor by human SARS-CoV-2, some of the contact residues in hACE2 are not conserved, resulting in reduced susceptibility to infection.295 Halfmann et al.296 used the hACE2 transgenic hamsters to test the infectivity of the Omicron variant. The relevant studies using Syrian hamsters as animal models are summarized in Table 7.
Ferret and mink models
Both ferrets and mink are members of the Mustelidae and are naturally highly susceptible to several human respiratory viruses, including SARS-CoV-1.297,298 Thus, it is reasonable to assume that SARS-CoV-2 infection could also be studied using these models.299 The susceptibility of minks was confirmed by a report on the numerous infections with SARS-CoV-2 across 40 farms in 2020.300 Upon infection, ferrets showed common clinical symptoms such as fever and mild respiratory symptoms, but no death was record.299 Virus replication was detectable in nasal washes, saliva, urine, and feces until the 8th day post infection (dpi), usually peaking on the 3rd dpi and in some studies, declined gradually and completely disappeared by the 14th dpi.243 Ryan et al.301 infected ferrets with three SARS-CoV-2 dosages. In the 5 × 102 PFU dosage group, one of six ferrets displayed viral RNA in the upper respiratory tract, while in the 5 × 104 PFU dosage group, viral RNA could be detected in the upper respiratory tract of all ferrets until the 14th dpi. Intermittent positivity occurred from 14 to 21 dpi in the 5 × 106 PFU dosage group.
Pathological examinations revealed mild bronchial interstitial pneumonia at 7 dpi with 5 × 104 PFU of SARS-CoV-2,301 and no other clinical symptoms or deaths were observed. The lungs of infected ferrets may demonstrate mildly expanded alveolar septa and diffuse interstitial histiocytic pneumonia after infection with 5.4 × 105 TCID50/ml SARS-CoV-2.302 In contrast, infected minks displayed moderate respiratory signs such as labored breathing, bronchiolitis, and diffuse alveolar damage (DAD),303 whereas some minks died of infection.
An outstanding usage of ferrets and mink is for transmission studies.299 Infection was reported following direct inoculation, direct contact and indirect contact, as well as transmission between infected and naïve ferrets and minks.299 Different from other animal models, SARS-CoV-2 can be detected in the nasal cavity of ferrets and they can be infected through indirect contact, indicating that ferrets and minks are capable of spreading virus, which simulates the transmission route of SARS-CoV-2 in humans.24,304,305
Poultry and domestic animals
There are truly vast numbers of poultry and domestic animals in the world. Moreover, they are essential to human life, and have daily close contact with humans. It is therefore necessary to address their potential susceptibility to SARS-CoV-2.24 Cats, dogs, chickens, ducks, pigs24,306 and sheep305 have been tested as potential animal models of COVID-19.41 Sub-adult outbred domestic cats aged 6–9 months were intranasally inoculated with 105 PFU of SARS-CoV-2 strain CTan-H and viral RNA was detected in the upper and lower respiratory tract, but not in the lung samples.24 However, no clinical signs were reported. In addition, cats are susceptible to airborne transmission. It should be noted that cats are not a standard animal model, with limited immunological resources and possible injury to laboratory technicians.24 Due to their aggressiveness, cats are especially difficult to handle in biosafety level-3 containment.41
Available studies show that dogs (Canis lupus familiaris) exhibit only very mild susceptibility to SARS-CoV-2 infection, and no clinical signs were recorded, indicating that dogs are not suitable for the development of in vivo model.24 In addition, chickens, pigs and ducks were challenged with 105 PFU of the CTan-H SARS-CoV-2 strain, but the lack of clear results or clinical signs indicates that they are not permissive to infection.24 The susceptibility of embryonated chicken eggs was also tested, but they could not support virus replication.304
As commonly farmed domestic ruminant animals, sheep were also recently investigated as hosts for SARS-CoV-2 infection,305 further expanding the range of animal models of COVID-19. However, experimentally challenged sheep only supporting limited virus replication according to nasal and oral swabs on 1 and 3 dpi, with virus detectable in the respiratory tract and lymphoid tissues on 4 and 8 dpi. Infection of naïve sentinel sheep may occur via respiratory droplets or aerosol. Interestingly, two SARS-CoV-2 isolates, B.1.1.7-like alpha VOC and its ancestral lineage A, were used to co-infect sheep, whereby the former outcompeted its ancestral lineage A,305 presenting experimental evidence in animal models that VOC alpha has stronger infectivity.
Gaps of available models and future perspective
Animal models for SARS-CoV-2 infection have played crucial roles in the studies of infection mechanism, transmission, and immunity actions, which have fostered the development of COVID-19 vaccines and therapeutics during the pandemic.41,307 Each animal model has its own advantages and limitations. The available genetically modified mouse models were mostly established based on a single receptor gene, which has resulted in a failure to meet all the practical requirements. On the other hand, the global response to COVID-19 is now facing a new phase characterized by the emergence of several SARS-CoV-2 VOC and demands for studies on comorbidities42 as well as vaccine evaluation. It is there for necessary to discuss the strategy of developing the next generation of COVID-19 animal models for the future.
Gaps of available animal models
-
(1)
The development of models that develop more severe disease may be needed. A common limitation of all the available animal models their limited ability to mimic the clinical features of COVID-19.42 The hamster model has emerged as one that more closely mimics moderate disease of humans,279,284,287,308 developing respiratory disease and displaying some other important clinical hallmarks found in patients after SARS-CoV-2 infection. However, it seems imperative to develop more tools to study the immunology if hamsters, since they expected to be an important animal model for severe disease. Such an animal model should present significant clinical symptoms, with the weight loss reaching at least 25%.42
-
(2)
Susceptible animal models supporting long duration of virus replication are still not widely available. The current SARS-CoV-2 models, including NHPs (Table 2), rodents (Tables 3, 4), and other models (Tables 5–7), only support viral infection for a short time, usually less than one week. This duration is not suitable for suboptimal doses of either a monoclonal antibody or a small molecule antiviral, and drug-resistant variants may emerge.309
-
(3)
It is necessary to develop models that recapitulate the effects of comorbidities on SARS-CoV-2 infection. patients with preexisting comorbidities show more severe symptoms when infected,310,311 but there is very limited knowledge on the effects of comorbidities on SARS-CoV-2 infection in animal models. Rodents are a better choice for such models than other species, since it is easier to establish models of SARS-CoV-2 in rats and mice with cardiovascular disease,312 cancer,313 and diabetes.314
-
(4)
It is well known that aged people that have a higher case fatality rate,315 incidence and more severe clinical characteristics. It is therefore necessary to develop animal models that mimic the effect of aging on SARS-CoV-2 infection and immunity.
-
(5)
Animal models that recapitulate the COVID-19 disease progression and the transition from mild to severe illness are urgently needed. With such models, it may be possible to identify novel biomarkers that better predict the course of human disease.316,317
-
(6)
Animal models for SARS-CoV-2 VOC will be essential,318,319,320 to evaluate pathogenicity and transmissibility, to assess whether the available vaccines and therapeutics can protect from VOC infection,321,322 and to further assess the potential risk of vaccine-associated enhanced respiratory disease (VAERD),323 especially in the context of mass vaccination and heterologous vaccination regimens.324,325,326
-
(7)
It may be necessary to develop animal models with Th1 and Th2 responses may be needed. Accordingly, models based on C57BL and BALB/c are both needed.327,328 A humanized ACE2 mouse model with BALB/c background has become available in China (https://www.gempharmatech.com).
-
(8)
Animal models with double and triple humanized gene combinations, rather than a single transgene327 are urgently needed for the next phase of studies. The obvious deficiencies of current mouse models include only using a single transgene, random integration with undetermined transgene copy numbers233 and controversial promoter selections, causing unnatural gene expression profiles and features not found in clinical patients.235 In addition to hACE2, more alternative receptors, co-receptors144 and primary antibody clearance receptors and host factors,222 such as TMRPSS2 and FcγRT were discovered. These prerequisites provide the possibility to establish advanced multigene humanized mouse models.
Developmental strategies for the next generation of COVID-19 animal models
Genetically modified mice with single genes or multi-gene combinations
Fontela et al.42 pointed out some gaps of current animal models, finding that single hACE2 transgenic mice poorly mimic the clinical features of COVID-19.327 It is therefore necessary to develop mouse models with novel single genes or multi-gene combinations. Several new candidate receptors, as well as host proteases (Table 1), and host factors,222 have been found to play crucial roles in SARS-CoVp2 infection. Because mice are easier to genetically modify and are more widely used than other animal species, the modification of novel single or multiple gene(s) in mice should be the first choice. Novel potential receptors including CD147, ASGR1, KREMEN1 and Nrp1 may be worth trying in the construction of mouse models. An NSG immunodeficient mouse expressing the CD147 gene has been reported.329
Jarnagin et al.327 proposed a variety of multi-gene combination modes and implemented them step by step, including hACE2 and hTMPRSS2 double gene combination, hACE2 and hFCγRT double gene combination, or hACE2, hTMPRSS2 and hFCγRT triple gene combination. The authors also proposed the combination of hTMPRSS2 and hFCγRT.327 We assume that further humanizing CD147, ASGR1 and host protease hTMPRSS2, based on the available humanized hACE2-KI mice,179 may provide mouse models that can duplicate severe COVID-19 disease, which are urgently needed for future studies. On the other hand, antibody-dependent enhancement (ADE) poses a great threat to vaccine and antibody therapy. Yet, the methods to evaluate ADE in animal models are still needed to be explored. Recently, Okuya et al.330 reported that ADE could be mediated by two host factors, the Fcγ receptor and the complement component, C1q. Therefore, an FCγR and hACE2 double humanized mouse model would be a useful tool for ADE evaluation in vivo. However, it remains challenging to co-express multiple human genes in one mouse model.
Mice with T, B or NK cells deficiency are susceptible to a variety of viruses and support long duration of viral replication.331,332,333 Since one of the obvious defects of the current COVID-19 animal models is typically a limited duration of viral replication, they are not suitable for exploring whether drug-resistant variants may emerge. Therefore, either animal strains with natural immunodeficiencies or the experimental induction of immunodeficiencies by knockout of Rag2 gene would be worth exploring, which may prolong the in vivo replication of SARS-CoV-2.293,329,334 Animal models supporting the long duration of SARS-CoV-2 infection may be used in the studies on the sequela of COVID-19, namely long COVID-19.335
An important finding is that older COVID-19 patients present more severe disease,308,336 as well as those with preexisting comorbidities309 such as cardiac disease,337,338 diabetes,339,340,341 and cancer. However, there is very limited knowledge on the effect of comorbidities in animal models. Available humanized or transgenic hACE2 mice can be used to construct mouse models suitable for the study of different complications by additional disease model induction or cross-breeding with available genetically models such as diabetic mice,314 aging mice,342 cardiovascular disease mice343,344,345 and tumor mice.
Selection of transgenic or knock-in strategy
Animal models humanized for the ACE2 receptor or other genes were generated by inserting hACE2 receptor or other foreign genes, driven by intrinsic the mouse promoter, into the locus of mAce2 or analogous genes. The first advantage of such models over random insertion is that the expression pattern of the transgene closely mimics natural expression.179,346,347,348 Secondly, the interference of mouse orthologs is excluded, and thirdly, transgenes are integrated as single copy. However, intrinsic mouse promoters usually lead to a lower expression of transgenes, resulting in poor susceptibility and weak clinical symptoms (Table 4). The K18 promoter164,235,242 and HFH-4 promoter349 may result in high expression of transgenes, but the expression pattern is non-physiological.235 New models are increasingly being established using precision knock-in, rather than random insertion.350,351 Safe harbor sites, such as Rosa26352,353 and Hipp11178,354 are increasingly being preferred for the insertion of heterologous genes.355
Powerful promoters, such as the CAG promoter,356,357,358 may be employed instead of intrinsic mouse promoters, whereby the transgene expression cassette is still inserted into the locus of the respective mouse ortholog to remove interference while still guaranteeing strong expression. This strategy may be used to construct animal models of infection with other pathogens.
Genetic background of animal models
The genetic background has a strong effect on numerous characteristics of animal models,359 including immunity even if the same transgene is modified.360 C57BL/6 and BALB/c mice have different immune responses, the former being biased toward the Th1 type and the latter toward the Th2 type. BALB/c mice expressing hACE2 may allow modeling the lung pathology and late sequelae of COVID-19.327 Wild-type hamsters are permissive for SARS-CoV-2 infection, but may benefit from enhanced susceptibility if the hACE2 gene is introduced.361 Similarly, wild-type ferrets can be infected with SARS-CoV-2, and if there native Ace2 gene is humanized, more severe symptoms may be generated. However, it is much more challenging and expensive to genetically modify hamster or ferrets than mouse, since mice benefit from a vast array of mature technology. Nonetheless, some research teams have taken the first step forward in genetically modifying these alternative animal modes.295,361
Reserving animal models in advance, a lesson from the COVID-19 pandemic
Animal production and breeding takes a long time, and the construction of novel genetically modified animal models is even more time consuming. By contrast, the outbreak of an emerging infectious disease is urgent and unpredictable. It is therefore very necessary to maintain a population of naturally susceptible animal models, such as NHPs, ferrets and Syrian hamsters in advance, and to establish genetically modified mouse models for those pathogens lacking susceptible animal models. According to the characteristics of the pathogens and its receptor, genetically modified animal models may be developed by introducing host receptors or co-receptor into mice, resulting in susceptible mouse models. In addition, mice deficient in T, B or NK cell function,331 or lacking the interferon (IFN) receptor362 are permissive to infection by multiple pathogens. Accordingly, knocking out the host rag 2 gene,363,364,365 or IFN receptor gene may result in broad-spectrum susceptible animal models.362,366,367
In view of the rapid spread and great harm of respiratory pathogens, more attention should be paid to the construction and reserve of animal models for respiratory pathogens, such as coronaviruses, respiratory syncytial viruses, or enteroviruses that can be transmitted by respiratory routes, such as EV-D68.368
To maintain a stable population of genetically modified mouse models requires large amounts of resources and labor, while cryo-preservation of embryos and sperm costs much less. It is a big challenge to produce thousands of mice for experiments in one or two months when starting with a handful of mice. In vitro fertilization (IVF) technology369,370,371 may achieve this goal.372 In one example, sperm from 1 or 2 male hACE2-KI mice114 was collected, and hundreds of wild-type C57BL/6 mice were super-ovulated to collect oocytes. Fertilized oocytes were transplanted into recipient mice, and hACE2 positive mice were screened among the offspring (Fig. 2). Over 1000 hACE2 mice were successfully produced in two months in our laboratory (Table 8) in the first half year of 2020, when hACE2 transgenic mice were in great and urgent demand. Such a rapid breeding system was a crucial tool in the initial phase of the COVID-19 pandemic.
Rapid breeding of hACE2-KI model mice using in vitro fertilization (IVF) technology. a The main steps of IVF, including a collection of mouse sperm and oocytes, insemination and implantation of fertilized oocytes into recipient foster mice. b A single humanized hACE2-KI mouse and 100 C57BL/6 wild-type mice could produce 638 offspring by IVF technology in a single round, among which 296 hACE2 positive mice were screened by PCR. This rapid breeding technique greatly accelerated the production of humanized hACE2-KI mice
Conclusions
Multiple kinds of COVID-19 disease models have been developed to date, using a wide range of animal models such as NHPs, genetically modified mouse, AAV- or Ad5 transduced mice, as well as wild-type mice infected with adapted strains. In addition, Syrian hamsters, ferrets, as well as livestock or poultry,24 including pigs, cats,306 dogs, sheep,305 chickens and ducks24 have also been used as models for SARS-CoV-2 infection. These existing models play crucial roles in the development of vaccines and therapeutics. Nevertheless, outstanding deficiencies have emerged, especially when facing novel requirements. Facing the new phase of COVID-19 pandemic, the scientific community should focus on establishing multi-gene genetically modified animal models and models of VOC infection.373 Compared with other species, mice are more easily genetically modified, making it easier to establish complex multi-gene models, and there is an abundant immunological resource. It therefore stands to reason that mouse models will become state-of-the-art tools for replicating complex clinical features and disease progressions of COVID-19.327 Finally, there is an urgent need to reserve animal resources and to prepare well for novel emerging infectious diseases in the future, particularly those caused by respiratory pathogens.
References
Koyama, T., Platt, D. E. & Parida, L. Variant analysis of SARS-CoV-2 genomes. Bull. World Health Organ. 98, 495–504 (2020).
Wang, W., Tang, J. & Wei, F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 92, 441–447 (2020).
Li, Q. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382, 1199–1207 (2020).
Novel Coronavirus (2019-nCoV) situation report, 1. https://apps.who.int/iris/handle/10665/330760 (World Health Organization, 2020).
Zhu, N. et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733 (2020).
Shibo, J. & Zheng-Li The first disease X is caused by a highly transmissible acute respiratory syndrome coronavirus. Virol. Sin. 35, 3 (2020).
Pollard, C. A., Morran, M. P. & Nestor-Kalinoski, A. L. The COVID-19 pandemic: a global health crisis. Physiol. Genomics 52, 549–557 (2020).
Feng, D. et al. Management of urology during COVID-19 pandemic: a perspective from Sichuan Province, China. Int J. Surg. 81, 115–121 (2020).
Abbas Khan, A. W. R. et al. Blocking key mutated hotspot residues in the RBD of the omicron variant (B.1.1.529) with medicinal compounds to disrupt the RBD-hACE2 complex using molecular screening and simulation approaches. RSC Adv. 12, 7318–7327 (2022).
Sabrina, L. et al. SARS-CoV-2 BA.1 variant is neutralized by vaccine booster-elicited serum, but evades most convalescent serum and therapeutic antibodies. Sci. Transl. Med. 5, eabn8543 (2022).
Stadler, K. et al. SARS-beginning to understand a new virus. Nat. Rev. Microbiol. 1, 209–218 (2003).
Hsueh, P. R. & Yang, P. C. Severe acute respiratory syndrome (SARS) - an emerging infection of the 21st century. J. Formos. Med. Assoc. 102, 825–839 (2003).
Wit, E. D., Doremalen, N. V., Falzarano, D. & Munster, V. J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 14, 523–534 (2016).
Zhou, J., Chu, H., Chan, J. F. & Yuen, K. Y. Middle East respiratory syndrome coronavirus infection: virus-host cell interactions and implications on pathogenesis. Virol. J. 12, 218 (2015).
Liu, K. et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 133, 1025–1031 (2020).
Bardellini, E., Amadori, F., Veneri, F., Conti, G. & Majorana, A. Coronavirus Disease-2019 and dental practice: a project on the use of ozonized water in the water circuit of the dental armchair. Stomatologija 21, 35–38 (2020).
Liu, Y. et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 582, 557–560 (2020).
Blocken, B. et al. Ventilation and air cleaning to limit aerosol particle concentrations in a gym during the COVID-19 pandemic. Build Environ. 193, 107659 (2021).
Han, Y. & Yang, H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): a Chinese perspective. J. Med. Virol. 92, 639–644 (2020).
Rothan, H. A. & Byrareddy, S. N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 109, 102433 (2020).
Clayton, E., Rohaim, M. A., Bayoumi, M. & Munir, M. The molecular virology of coronaviruses with special reference to SARS-CoV-2. Adv. Exp. Med. Biol. 1352, 15–31 (2021).
Zhou, J. et al. Observation and analysis of 26 cases of asymptomatic SARS-COV2 infection. J. Infect. 81, e69–e70 (2020).
Chen, J., Vitetta, L., Henson, J. D. & Hall, S. The intestinal microbiota and improving the efficacy of COVID-19 vaccinations. J. Funct. Foods 87, 104850 (2021).
Shi, J. et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368, 1016–1020 (2020).
Leroy, E. M., Ar Gouilh, M. & Brugere-Picoux, J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health 10, 100133 (2020).
Cui, J., Li, F. & Shi, Z. L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 17, 181–192 (2019).
Mallick, R. & Duttaroy, A. K. Origin and structural biology of novel coronavirus (SARS-CoV-2). Adv. Exp. Med. Biol. 1352, 1–13 (2021).
Shereen, M. A., Khan, S., Kazmi, A., Bashir, N. & Siddique, R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 24, 91–98 (2020).
Lau, S. K. P. et al. Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 26, 1542–1547 (2020).
Kumar, S., Maurya, V. K., Prasad, A. K., Bhatt, M. & Saxena, S. K. Structural, glycosylation and antigenic variation between 2019 novel coronavirus (2019-nCoV) and SARS coronavirus (SARS-CoV). VirusDisease 31, 13–21 (2020).
Kadam, S. B., Sukhramani, G. S., Bishnoi, P., Pable, A. A. & Barvkar, V. T. SARS-CoV-2, the pandemic coronavirus: molecular and structural insights. J. Basic Microbiol. 61, 180–202 (2021).
Li, M. Y., Li, L., Zhang, Y. & Wang, X. S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 9, 45 (2020).
Xu, X. et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life sci. 63, 4 (2020).
Wu, C. et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 10, 766–788 (2020).
Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513 (2020).
Ju, B. et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 584, 115–119 (2020).
Mohammadi, M., Shayestehpour, M. & Mirzaei, H. The impact of spike mutated variants of SARS-CoV2 [Alpha, Beta, Gamma, Delta, and Lambda] on the efficacy of subunit recombinant vaccines. Braz. J. Infect. Dis. 25, 101606 (2021).
Pru, B. M. Variants of SARS CoV-2: mutations, transmissibility, virulence, drug resistance, and antibody/vaccine sensitivity. Front. Biosci. 27, 65 (2022).
Magazine, N. et al. Mutations and evolution of the SARS-CoV-2 spike protein. Viruses 14, 640 (2022).
Boehm, E., Kronig, I., Neher, R. A., Eckerle, I. & Kaiser, L. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin. Microbiol. Infect. 27, 1109–1117 (2021).
Munoz-Fontela, C. et al. Animal models for COVID-19. Nature 586, 509–515 (2020).
Munoz-Fontela, C. et al. Advances and gaps in SARS-CoV-2 infection models. PLoS Pathog. 18, e1010161 (2022).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).
Fwca, B. et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395, 514–523 (2020).
Zheng, J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J. Bio Sci. 16, 1678–1685 (2020).
Wang, D. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323, 1061–1069 (2020).
Vetter, P., Vu, D. L., L’Huillier, A. G., Schibler, M. & Jacquerioz, F. Clinical features of Covid-19. BMJ 369, m1470 (2020).
Qu, J. M., Cao, B. & Chen, R. C. Clinical features of COVID-19. COVID-19. pp.13–39 (2021).
Kaur, A., Bhalla, V., Salahuddin, M., Rahman, S. O. & Pottoo, F. H. COVID-19 infection: epidemiology, virology, clinical features, diagnosis and pharmacological treatment. Curr. Pharm. Des. 27, 3551–3565 (2021).
Elmokadem, A. H. et al. Mimickers of novel coronavirus disease 2019 (COVID-19) on chest CT: spectrum of CT and clinical features. Insights Imaging 12, 12 (2021).
Eastin, C. & Eastin, T. Clinical characteristics of coronavirus disease 2019 in China. J. Emerg. Med. 58, 711–712 (2020).
Sisó-Almirall, A. et al. Clinical features of Covid-19 in Barcelona City. Preprint at https://hdl.handle.net/2027.42/155329 (2020).
Zhang, H. et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in patients out of Wuhan from China: a case control study. BMC Infect. Dis. 21, 207 (2021).
Rogier, T., Eberl, I., Moretto, F., Sixt, T. & Piroth, L. COVID-19 or not COVID-19? Compared characteristics of patients hospitalized for suspected COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 40, 2023–2028 (2021).
Gao, Y., Chen, Y., Liu, M., Niu, M. & Tian, J. Nervous system diseases are associated with the severity and mortality of patients with COVID-19: a systematic review and meta-Analysis. Epidemiol. Infect. 149, 1–38 (2021).
Khanmohammadi, S., Rezaei, N., Khazaei, M. & Shirkani, A. A case of autosomal recessive interferon alpha/beta receptor alpha chain (IFNAR1) deficiency with severe COVID-19. J. Clin. Immunol. 42, 19–24 (2021).
Yadav, P. D. et al. Neutralization of Beta and Delta variant with sera of COVID-19 recovered cases and vaccinees of inactivated COVID-19 vaccine BBV152/Covaxin. J. Travel Med. 28, 7 (2021).
Pradier, C. Patients admitted for variant Alpha COVID-19 have poorer outcomes than those infected with the old strain. J. Clin. Med. 10, 3550 (2021).
Yesilkaya, U. H., Sen, M. & Karamustafalioglu, N. New variants and new symptoms in COVID-19: first episode psychosis and Cotard’s Syndrome two months after infection with the B.1.1.7 variant of coronavirus. Schizophr. Res. 243, 315–316 (2022).
Mastrangelo, A., Bonato, M. & Cinque, P. Smell and taste disorders in COVID-19: from pathogenesis to clinical features and outcomes. Neurosci. Lett. 748, 135694 (2021).
Wu, Z. & Mcgoogan, J. M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323, 1239–1242 (2020).
Yesilkaya, U. H., Sen, M. & Karamustafalioglu, N. New variants and new symptoms in COVID-19: first episode psychosis and Cotard’s syndrome two months after infection with the B.1.1.7 variant of coronavirus. Schizophr. Res. S0920-9964, 00213–00219 (2021).
Su-Qiong, J. et al. Characteristics of immune and inflammatory responses among different age groups of pediatric patients with COVID-19 in China. World J. Pediatr. 17, 375–384 (2021).
Fatima, K., Lakshmi, L. J., Unnisa, S. M. & Lakshmi, G. J. Comparison of COVID-19 RT-PCR results in different age groups during 6 months period at a tertiary care centre. Indian J. Med. Microbiol. 39, S63–S64 (2021).
Fleitas, P. E., Almazan, M. C., Cortez, S. D., Paz, J. A. & Krolewiecki, A. J. Association between comorbidities and death from COVID-19 in different age groups. Ann. Intern. Med. 174, 308–315 (2021).
Weaver, R. H., Jackson, A., Lanigan, J., Power, T. G. & Weybright, E. Health behaviors at the onset of the COVID-19 pandemic. Am. J. Health Behav. 45, 44–61 (2021).
Flaatten, H. et al. The importance of revealing data on limitation of life sustaining therapy in critical ill elderly Covid-19 patients. J. Crit. Care 67, 147–148 (2022).
Badal, S. et al. Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: a systematic review and meta-analysis. J. Clin. Virol. 135, 104715 (2021).
Vrillon, A. et al. COVID-19 in adults with dementia: clinical features and risk factors of mortality-a clinical cohort study on 125 patients. Alzheimers Res. Ther. 13, 77 (2021).
Jiang, N. et al. Clinical features and risk factors associated with severe COVID-19 patients in China. Chin. Med. J. 134, 944–953 (2021).
Deng, L. et al. Course of illness and outcomes in older COVID-19 patients treated with HFNC: a retrospective analysis. Aging 13, 15801–15814 (2021).
Wu, Z. & Mcgoogan, J. M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72, 314 cases from the chinese center for disease control and prevention. JAMA 323, 1239–1242 (2020).
Rozzini, R. et al. Delirium: clinical presentation and outcomes in older COVID-19 patients. Front. Psychiatry 11, 586686 (2020).
Zeyaullah, M. et al. COVID-19 and SARS-CoV-2 variants: current challenges and health concern. Front. Genet. 12, 693916 (2021).
Waleed, R. M., Sehar, I., Iftikhar, W. & Khan, H. S. Hematologic parameters in coronavirus infection (COVID-19) and their clinical implications. Discoveries 8, e117 (2020).
Mao, L. et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 77, 683–690 (2020).
Neumann-Podczaska, A., Chojnicki, M., Karbowski, L. M., Al-Saad, S. R. & Wieczorowska-Tobis, K. Clinical characteristics and survival analysis in a small sample of older COVID-19 patients with defined 60-day outcome. Int. J. Env. Res. Pub. He. 17, 8362 (2020).
Chen, H., Guo, J., Wang, C., Luo, F. & Zhang, Y. Clinical characteristics and intrauterine vertical transmissions potential of COVID-19 infection in 9 pregnant women: a retrospective review of medical records. Obstet. Anesthe. Dig. 41, 51–51 (2021).
Nori, W., Hameed, B. H., Thamir, A. R. & Fadhil, A. COVID-19 in pregnancy: implication on platelets and blood indices. Rev. Bras. Ginecol. Obstet. 43, 595–599 (2021).
Yu, X., Chen, H., Luo, F., Guo, J. & Zhang, Y. Further evaluation of the mother-to-child transmission potential of SARS-CoV-2 infection during pregnancy: a retrospective study. Preprint at https://doi.org/10.21203/rs.3.rs-77490/v1 (2020).
Das, A., Sonkawade, N., Valvi, C., Kulkarni, R. & Kinikar, A. Hematological profile of Covid-19 in children. IOSR-JDMS, 28–34 (2021).
Borrelli, M., Corcione, A., Castellano, F., Nastro, F. F. & Santamaria, F. Coronavirus disease 2019 in Children. Front. Pediatr. 9, 66484 (2021).
Oualha, M., Bendavid, M., Berteloot, L., Corsia, A. & Renolleau, S. Severe and fatal forms of COVID-19 in children. Arch. Pédiatrie. 27, 235–238 (2020).
Kahn, A. R., Schwalm, C. M., Wolfson, J. A., Levine, J. M. & Johnston, E. E. COVID-19 in children with cancer. Curr. Oncol. Rep. 24, 295–302 (2022).
Chb, J. & Salvatore, C. COVID-19 infection in newborns. Clin. Perinatol. 49, 73–92 (2021).
Ghema, K. et al. Outcomes of newborns to mothers with COVID-19. Infect. Dis. Now. 51, 435–439 (2021).
Taheri, L., Gheiasi, S. F., Taher, M., Basirinezhad, M. H. & Nayeri, N. D. Clinical features of COVID-19 in newborns, infants, and children: a systematic review and meta-analysis. Compr. Child Adolesc. Nurs. 1–19 (2021).
Pivonello, R. et al. Sex disparities in COVID-19 severity and outcome: are men weaker or women stronger? Neuroendocrinology 111, 1066–1085 (2021).
Guan, W. J., Liang, W. H., Zhao, Y., Liang, H. R. & He, J. X. Comorbidity and its impact on 1,590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 55, 2000547 (2020).
Made, C., Simons, A., Schuurs-Hoeijmakers, J., Heuvel, G. & Hoischen, A. Presence of genetic variants among young men with severe COVID-19. JAMA 324, 663–673 (2020).
Acer, O., Genc Bahce, Y. & Ozudogru, O. Association of viral load with age, gender, disease severity, and death in severe acute respiratory syndrome coronavirus 2 variants. J. Med. Virol. 94, 3063–3069 (2022).
Zhou, M. et al. Inflammatory profiles and clinical features of COVID-19 survivors three months after discharge in Wuhan, China. J. Infect. Dis. 224, 1473–1488 (2021).
Karcioglu, O., Yeniocak, S. & Hosseinzadeh, M. Comorbid Diseases and COVID-19: COPD, Smoking, Obesity, Diabetes, Cancer, and Kidney Failure. (Demystifying COVID-19: Understanding the Disease, Its Diagnosis and Treatment, 2021).
Guan, W., Liang, W. H., Zhao, Y., Liang, H. R. & He, J. X. Comorbidity and its impact on 1,590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 55, 1–23 (2020).
Verma, N., Duseja, A. & Singh, V. Impact of pre-existing chronic liver disease on the outcome of patients with COVID-19 disease. Gastroenterology 40, 2515–2521 (2020).
Ahmad, A., Ishtiaq, S. M., Khan, J. A., Aslam, R. & Arshad, M. I. COVID-19 and comorbidities of hepatic diseases in a global perspective. World J. Gastroenterol. 27, 1296–1310 (2021).
Yang, K. et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 21, 904–913 (2020).
Russell, B. et al. COVID-19 risk factors for cancer patients: a first report with comparator data from COVID-19 negative cancer patients. Cancers 13, 2479 (2021).
Strang, P., Hedman, C., Adlitzer, H. & Schultz, T. Dying from cancer with COVID-19: age, sex, socio-economic status, and comorbidities. Acta Oncol. 60, 1019–1024 (2021).
Mirandola, M. et al. Cancer and Covid-19: a preliminary study on the trauma aspects of coronavirus in cancer patients. Psychooncology. Preprint at https://doi.org/10.1002/pon.5936 (2022).
Chachques, J. C. et al. Cardiovascular, hematological and neurosensory impact of COVID-19 and variants. Eur. Rev. Med. Pharm. Sci. 25, 3350–3364 (2021).
Alba, L. et al. New-onset psychosis: a case report of brief psychosis related to COVID-19 infection. Psychiatry Res. 301, 113975 (2021).
Cmsa, B. et al. COVID-19-associated psychosis: a systematic review of case reports - ScienceDirect. Genl Hosp. Psychiat. 73, 84–100 (2021).
Lechien, J. R., Chiesa-Estomba, C. M. & De Siati, D. R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Oto-Rhino-L. 277, 2251–2261 (2020).
Ramaraj, A. B. & Rice-Townsend, S. E. Inaccurate nasopharyngeal testing in a patient with gastrointestinal COVID19: a case report. Am. J. Surg. 221, 1293–1294 (2021).
Kariyawasam, J. C., Jayarajah, U., Riza, R., Abeysuriya, V. & Seneviratne, S. L. Gastrointestinal manifestations in COVID-19. Trans. R. Soc. Trop. Med. Hyg. 115, 1362–1388 (2021).
Wong, S. H., Lui, R. N. & Sung, J. J. Covid-19 and the digestive system. J. Gastroenterol. Hepatol. 35, 744–748 (2020).
Zhan, T., Tang, Y., Han, Z., Zhu, Q. & Tian, X. Clinical characteristics of 195 cases of COVID-19 with gastrointestinal symptoms COVID-19 with gastrointestinal symptoms. Turk. J. Gastroenterol. 32, 148–154 (2021).
Tarik, A. et al. Gastrointestinal manifestations with COVID-19 virus infection: a Moroccan prospective study. Arab. J. Gastroenterol. 22, 305–309 (2021).
Jin, X. et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 69, 1002–1009 (2020).
Faruqui, S. et al. Cholangiopathy after severe COVID-19: clinical features and prognostic implications. Am. J. Gastroenterol. 116, 1414–1425 (2021).
Middleton, C. E., Varshney, N. & Roland, D. Histopathological findings in the gastrointestinal tract of patients with COVID-19. Am. J. Clin. Pathol. 156, S57–S58 (2021).
Leal, T., Costa, E., Arroja, B., Goncalves, R. & Alves, J. Gastrointestinal manifestations of COVID-19: results from a European centre. Eur. J. Gastroenterol. Hepatol. 33, 691–694 (2021).
Sun, S. H. et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe 28, 124–133.e124 (2020).
Najim, R. H. & Kadhim, S. R. Biochemical and hematological parameters as a predictor for COVID -19 infection in 65 patients diagnosed by real time -PCR in Kirkuk city. Syst. Rev. Pharm. 11, 797–799 (2020).
Erdinc, B., Sahni, S. & Gotlieb, V. Hematological manifestations and complications of COVID-19. Adv. Clin. Exp. Med. 30, 101–107 (2021).
Yang, X. et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 18, 1469–1472 (2020).
Sdb, A. et al. Clinical features and prognostic factors in Covid-19: a prospective cohort study. EBioMedicine 67, 103378 (2021).
Al-Saadi, E. & Abdulnabi, M. A. Hematological changes associated with COVID-19 infection. J. Clin. Lab Anal. 36, e24064 (2022).
Bairwa, M., Kumar, R., Beniwal, K., Kalita, D. & Bahurupi, Y. Hematological profile and biochemical markers of COVID-19 non-survivors: a retrospective analysis. Clin. Epidemiol. Glob. Health 11, 100770 (2021).
Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H. & Zhong, N. S. Clinical characteristics of coronavirus disease 2019 in China. NEJM 382, 1861–1862 (2020).
Lippi, G., lebani, M. P. & Henry, B. M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta 506, 145–148 (2020).
Mina, A., Besien, K. V. & Platanias, L. C. Hematological manifestations of COVID-19. Leukemia Lymphoma 61, 2790–2798 (2020).
Huang, I. & Pranata, R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J. Intensive Care 8, 36 (2020).
Hu, Z. et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 63, 706–711 (2020).
Rey, J. R., Caro-Codón, J. & Rosillo, S. O. Heart failure in Covid‐19 patients: prevalence, incidence and prognostic implications. Eur. J. Heart Fail. 22, 2205–2215 (2020).
Standl, E. & Schnell, O. Heart failure outcomes and Covid-19. Diabetes Res. Clin. Pr. 175, 108794 (2021).
Debliquis, A. et al. Haemophagocytosis in bone marrow aspirates in patients with COVID-19. Br. J. Haematol. 190, e70–e73 (2020).
Li, Q. et al. Hematological features of persons with COVID-19. Leukemia 34, 2163–2172 (2020).
Cui, S., Chen, S., Li, X., Liu, S. & Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 18, 1421–1424 (2020).
Yihua, Z. et al. Lymphocyte cell population as a potential hematological index for early diagnosis of COVID-19. Cell Mol. Biol. 66, 202–206 (2020).
Jk, A., Tta, D., Nsa, C. & Ajgab, D. Abdominal and testicular pain: an atypical presentation of COVID-19. Am. J. Emerg. Med. 38, 1542.e1–1542.e3 (2020).
Andrea, V., Marco, V., Alessandro, A. & Giuseppe, B. How did COVID-19 affect medical and cardiology journals? A pandemic in literature. J. Cardiovasc. Med. 22, 840–847 (2021).
Venturelli, A., Vitolo, M., Albini, A. & Boriani, G. How did COVID-19 affect medical and cardiology journals? A pandemic in literature. J. Cardiovasc. Med. 22, 840–847 (2021).
Patel, D. P., Punjani, N., Guo, J., Alukal, J. P. & Hotaling, J. M. The impact of SARS-CoV-2 and COVID-19 on male reproduction and men’s health. Fertility Sterility 115, 813–823 (2021).
Iakymenko, O. A., Ramasamy, R. & Kryvenko, O. N. Testicular changes associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Arch. Pathol. Lab. Med. 145, 781a–781 (2021).
Yang, M., Chen, S., Huang, B., Zhong, J. M. & Nie, X. Pathological Findings in the Testes of COVID-19 Patients: Clinical Implications. EU Focus 6, 1124–1129 (2020).
Leveringhaus, E., Cagatay, G. N., Hardt, J., Becher, P. & Postel, A. Different impact of bovine complement regulatory protein 46 (CD46bov) as a cellular receptor for members of the species Pestivirus H and Pestivirus G. Emerg. Microbes Infect. 11, 60–72 (2022).
Eslami, N. et al. SARS-CoV-2: REceptor and Co-receptor Tropism Probability. Curr. Microbiol. 79, 133 (2022).
Anwar, M. N. et al. The interactions of flaviviruses with cellular receptors: implications for virus entry. Virology 568, 77–85 (2022).
Conwell, S. E., White, A. E., Harper, J. W., Knipe, D. M. & Sandri-Goldin, R. M. Identification of TRIM27 as a novel degradation target of herpes simplex virus 1 ICP0. J. Virol. 89, 220–229 (2015).
Kourjian et al. Sequence-specific alterations of epitope production and presentation by HIV protease inhibitors. Mol. Immunol. 193, 3496–3506 (2015).
Essalmani, R., Jain, J., Susan-Resiga, D., Andreo, U. & Seidah, N. G. Implications of Spike-glycoprotein processing at S1/S2 by Furin, at S2′ by Furin and/or TMPRSS2 and shedding of ACE2: cell-to-cell fusion, cell entry and infectivity of SARS-CoV-2. Biorxiv 11, 6655–6663 (2021).
Masre, S. F., Jufri, N. F., Ibrahim, F. W., & Abdul Raub, S. H. Classical and alternative receptors for SARS-CoV-2 therapeutic strategy. Rev. Med. Virol. 31, 1–9 (2021).
Shang, J. et al. Structural basis of receptor recognition by SARS-CoV-2. Nature 581, 221–224 (2020).
Li, W. et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454 (2003).
Kuhn, J. H., Li, W., Choe, H. & Farzan, M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol. Life Sci. 61, 2738–2743 (2004).
Wong, S. K., Li, W., Moore, M. J., Choe, H. & Farzan, M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 279, 3197–3201 (2004).
Towler, P. et al. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 279, 17996–18007 (2004).
Chatterjee, S. Important steps to control COVID-19/SARS-CoV-2 infection. SN Compre. Clin. Med. 2, 381–382 (2020).
Sternberg, A., McKee, D. L. & Naujokat, C. Novel drugs targeting the SARS-CoV-2/COVID-19 machinery. Curr. Top. Med. Chem. 20, 1423–1433 (2020).
Sun, Z. et al. Potent neutralization of SARS-CoV-2 by human antibody heavy-chain variable domains isolated from a large library with a new stable scaffold. MAbs 12, 1778435 (2020).
Wang, K. et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target Ther. 5, 10 (2020).
Gu, Y. et al. Receptome profiling identifies KREMEN1 and ASGR1 as alternative functional receptors of SARS-CoV-2. Cell Res. 32, 14 (2022).
Cantuti-Castelvetri, L. et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370, 856–860 (2020).
Qi, F., Qian, S., Zhang, S. & Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 526, 135–140 (2020).
Cui, C., Huang, C., Zhou, W., Ji, X. & Cui, Q. AGTR2, one possible novel key gene for the entry of SARS-CoV-2 into human cells. IEEE/ACM T Comput. BI. PP, 1-1 (2020).
Caly, L., Druce, J. D., Catton, M. G., Jans, D. A. & Wagstaff, K. M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 178, 104787 (2020).
Wyler, E., Msbauer, K., Franke, V., Diag, A. & Landthaler, M. Bulk and single-cell gene expression profiling of SARS-CoV-2 infected human cell lines identifies molecular targets for therapeutic intervention. iScience 24, 102151 (2021).
Huang, J., Song, W., Huang, H. & Sun, Q. Pharmacological therapeutics targeting RNA-dependent RNA polymerase, proteinase and spike protein: from mechanistic studies to clinical trials for COVID-19. J. Clin. Med. 9, 1131 (2020).
Shajahan, A. et al. Comprehensive characterization of N- and O- glycosylation of SARS-CoV-2 human receptor angiotensin converting enzyme 2. Glycobiology 31, 410–424 (2021).
Stanifer, M. L., Kee, C., Cortese, M., Triana, S. & Boulant, S. Critical role of type III interferon in controlling SARS-CoV-2 infection, replication and spread in primary human intestinal epithelial cells. Cell Rep. 3, 107863 (2020).
Yinda, C. K. et al. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog. 17, e1009195 (2021).
Zheng, J. et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 589, 603–607 (2021).
Bao, L. et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583, 830–833 (2020).
Wu, Y., Wang, F., Shen, C., Peng, W. & Liu, L. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 368, eabc2241 (2020).
Patel, V. B., Zhong, J. C., Grant, M. B. & Oudit, G. Y. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ. Res. 118, 1313–1326 (2016).
Burrell, L. M., Johnston, C. I., Tikellis, C. & Cooper, M. E. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol. Metab. 15, 166–169 (2004).
Grzegrzolka, J. et al. ACE and ACE2 expression in normal and malignant skin lesions. Folia Histochem. Cytobiol. 51, 232–238 (2013).
Letko, M., Marzi, A. & Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 5, 562–569 (2020).
Hoffmann, M., Kleine-Weber, H. & Phlmann, S. A Multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell 78, 779–784.e5 (2020).
Simmons, G. et al. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl Acad. Sci. USA 102, 11876–11881 (2005).
Tay, M. Z., Poh, C. M., Renia, L., MacAry, P. A. & Ng, L. F. P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 20, 363–374 (2020).
Fábio, L., Falco, M., Da, L., Pontes, S. & Quaresma, A. S. The complexity of respiratory disease associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: from immunopathogenesis to respiratory therapy. Rev. Med. Virol. e2167, 1–15 (2020).
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).
Komatsu, T. et al. Molecular cloning, mRNA expression and chromosomal localization of mouse angiotensin-converting enzyme-related carboxypeptidase (mACE2). DNA Seq. 13, 217–220 (2002).
Moreau, G. B., Burgess, S. L., Sturek, J. M., Donlan, A. N. & Mann, B. J. Evaluation of K18-hACE2 mice as a model of SARS-CoV-2 infection. Am. J. Trop. Med. Hyg. 103, 1215–1219 (2020).
Sun, H., Chen, M., Zhang, A. B., Zhang, J. & Weinstein, L. S. Liver-specific expression of constitutively active Gsα leads to hyperglycemia with impaired insulin secretion. Endocr. J. 5, A446–A447 (2021).
Sun, J. et al. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell 182, 734–743.e735 (2020).
Zhao, Y. et al. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 202, 756–759 (2020).
Ou, X., Liu, Y., Lei, X., Li, P. & Qian, Z. Characterization of spike glycoprotein of 2019-nCoV on virus entry and its immune cross-reactivity with spike glycoprotein of SARS-CoV. Nat. Commun. 11, 1620 (2020).
Li, Y. et al. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology 54, 677–687 (2009).
Tomoki, K., Kayaho, M., Waichi, S., Shoichi, M. & Kenji, K. CD147 (EMMPRIN/Basigin) in kidney diseases: from an inflammation and immune system viewpoint. Nephrol. Dial. Transpl. 30, 1097–1103 (2015).
Qiao, J. et al. The expression of SARS-CoV-2 receptor ACE2 and CD147, and protease TMPRSS2 in human and mouse brain cells and mouse brain tissues. Biochem. Biophys. Res. Commun. 533, 867–871 (2020).
Pushkarsky, T. et al. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc. Natl Acad. Sci. USA 98, 6360–6365 (2001).
Chen, Z. et al. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 191, 755–760 (2005).
Zalpoor, H. et al. The roles of Eph receptors, neuropilin 1, P2X7, and CD147 in COVID 19 associated neurodegenerative diseases: infammasome and JaK inhibitors as potential promising therapies. Cell Mol. Biol. Lett. 27, 10 (2022).
Badeti, S., Tseng, H. C., Romanienko, P., Yehia, G. & Liu, D. Development of a novel human CD147 transgenic NSG mouse model to test SARS-CoV-2 infection and immune responses. Cell Biosci. 12, 88 (2022).
Sanford, J. P., Eddy, R. L., Doyle, D. & Shows, T. B. Assignment of human asialoglycoprotein receptor gene (ASGR1) to chromosome 17p11-13. Genomics 11, 779–781 (1991).
Geretti, E., Shimizu, A. & Klagsbrun, M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis 11, 31–39 (2008).
Chaudhary, B., Khaled, Y. S., Ammori, B. J. & Elkord, E. Neuropilin 1: function and therapeutic potential in cancer. Cancer Immunol. Immunother. 63, 81–99 (2014).
Dai, X. et al. Ablation of neuropilin 1 in myeloid cells exacerbates high-fat diet-induced insulin resistance through Nlrp3 inflammasome in vivo. Diabetes 66, 2424 (2017).
Benedicto, A., García-Kamiruaga, I. & Arteta, B. Neuropilin-1: a feasible link between liver pathologies and COVID-19. World J. Gastroenterol. 27, 3516–3529 (2021).
Daly, J. L., Simonetti, B., Antón-Plágaro, C., Williamson, M. K. & Yamauchi, Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 370, 861–865 (2020).
Moutal, A. et al. SARS-CoV-2 spike protein co-opts VEGF-A/neuropilin-1 receptor signaling to induce analgesia. Pain 162, 243–252 (2021).
Zaki, A. M., van Boheemen, S., Bestebroer, T. M., Osterhaus, A. D. & Fouchier, R. A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367, 1814–1820 (2012).
Bermingham, A., Ma, C., Brown, C., Aarons, E. & Zambon, M. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Eurosurveillance 17, 20290 (2012).
Raj, V. S. et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495, 251–254 (2013).
Lambeir, A. M., Durinx, C., Scharpe, S. & De Meester, I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab Sci. 40, 209–294 (2003).
Lei, Y. et al. Dipeptidyl peptidase-IV inhibition for the treatment of cardiovascular disease- recent insights focusing on angiogenesis and neovascularization. Circ. J. 81, 770–776 (2017).
Yannick, W., Lesley, B., Kaat, K., Anne-Marie, L. & Ingrid, D. M. The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front. Immunol. 6, 387- (2015).
Fan, C. et al. A human DPP4-knockin mouse’s susceptibility to infection by authentic and pseudotyped MERS-CoV. Viruses 10, 9 (2018).
Zhao, J. et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl Acad. Sci. USA 111, 4970 (2014).
Li, K. et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis. 213, 712–722 (2016).
Li, K. et al. Mouse-adapted MERS coronavirus causes lethal lung disease in human DPP4 knockin mice. Proc. Natl Acad. Sci. USA 114, E3119–E3128 (2017).
Vankadari, N. & Wilce, J. A. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 9, 601–604 (2020).
Karuppan, M., Devadoss, D., Nair, M., Chand, H. S. & Lakshmana, M. K. SARS-CoV-2 infection in the central and peripheral nervous system-associated morbidities and their potential mechanism. Mol. Neurobiol. 58, 2465–2480 (2021).
Yeager, C. L. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 357, 6377 (1992).
Leonardi, A., Rosani, U. & Brun, P. Ocular surface expression of SARS-CoV-2 receptors. Ocul. Immunol. Infla. 28, 735–738 (2020).
Matsuyama, S. et al. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 84, 12658–12664 (2010).
Bertram, S. et al. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J. Virol. 85, 13363–13372 (2011).
Heurich, A. et al. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 88, 1293–1307 (2014).
Glowacka, I. et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 85, 4122–4134 (2011).
Lukassen, S. et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 39, e105114 (2020).
Milne, S., Timens, W., Chen, X. Y., Bossé, Y. & Sin, D. D. SARS-CoV-2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Resp. Med. 8, e50–e51 (2020).
Cermak, S. et al. Loss of cathepsin B and L leads to lysosomal dysfunction, NPC-like cholesterol sequestration and accumulation of the key Alzheimer’s proteins. PLoS ONE 11, e0167428 (2016).
Millet, J. K. & Whittaker, G. R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 202, 120–134 (2015).
Nakanishi, H. Neuronal and microglial cathepsins in aging and age-related diseases. Aging Res. Rev. 2, 367–381 (2003).
Ou, X., Liu, Y., Lei, X., Li, P. & Qian, Z. Characterization of spike glycoprotein of 2019-nCoV on virus entry and its immune cross-reactivity with spike glycoprotein of SARS-CoV. Nat. Commun. 11, 1620 (2019).
Xia, S. et al. The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Signal Transduct. Target Ther. 5, 92 (2020).
Zhou, Z. et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe 27, 883–890.e882 (2020).
Lata, S. et al. Where all the roads meet? A Crossover perspective on host factors regulating SARS-CoV-2. Infect. - ScienceDirect. J. Mol. Biol. 434, 167403 (2022).
Lu, S. et al. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduct. Target Ther. 5, 157 (2020).
Munster, V. J. et al. Subtle differences in the pathogenicity of SARS-CoV-2 variants of concern B.1.1.7 and B.1.351 in rhesus macaques. Sci. Adv. 7, eabj3627 (2021).
Rockx, B. et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368, 1012–1015 (2020).
Johnston, S. C. et al. Development of a coronavirus disease 2019 nonhuman primate model using airborne exposure. PLoS ONE 16, e0246366 (2021).
Woolsey, C. et al. Establishment of an African green monkey model for COVID-19 and protection against re-infection. Nat. Immunol. 22, 86–98 (2021).
Blair, R. V. et al. Acute respiratory distress in aged, SARS-CoV-2-infected African green monkeys but not rhesus macaques. Am. J. Pathol. 191, 274–282 (2021).
Singh, D. K. et al. Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nat. Microbiol. 6, 73–86 (2021).
Jia, H., Yue, X. & Lazartigues, E. ACE2 mouse models: a toolbox for cardiovascular and pulmonary research. Nat. Commun. 11, 5165 (2020).
Menachery, V. D. et al. SARS-like WIV1-CoV poised for human emergence. Proc. Natl Acad. Sci. USA 113, 3048–3053 (2016).
McCray, P. B. Jr. et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 81, 813–821 (2007).
Yang, X. H. et al. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp. Med. 57, 450–459 (2007).
Guan, W. J. & Zhong, N. S. Clinical characteristics of Covid-19 in China. Reply. N. Engl. J. Med. 382, 1861–1862 (2020).
Jiang, R. D. et al. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell 182, 50–58.e58 (2020).
Winkler, E. S. et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 21, 1327–1335 (2020).
Spinato, G. et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 323, 2089–2090 (2020).
Dong, W. et al. The K18-human ACE2 transgenic mouse model recapitulates non-severe and severe COVID-19 in response to an infectious dose of the SARS-CoV-2 virus. J. Virol. 96, e0096421 (2022).
Bao, L., Deng, W., Huang, B., Gao, H. & Qin, C. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583, 830–833 (2020).
Moreau, G. B., Burgess, S. L., Sturek, J. M., Donlan, A. N. & Mann, B. J. Evaluation of K18-hACE2 mice as a model of SARS-CoV-2 infection. Am. J. Tropical Med. Hyg. 103, 1215–1219 (2020).
Wu, Y. et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 368, 1274–1278 (2020).
Oladunni, F. S. et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat. Commun. 11, 6122 (2020).
Blanco-Melo, D. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036–1045.e1039 (2020).
Chen, Y. et al. Age-associated SARS-CoV-2 breakthrough infection and changes in immune response in a mouse model. Emerg. Microbes Infect. 11, 368–383 (2022).
Wang, Q. et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 181, 894–904.e899 (2020).
Wan, Y., Shang, J., Graham, R., Baric, R. S. & Li, F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 94, e00127–20 (2020).
Hassan, A. O. et al. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell 182, 744–753.e744 (2020).
Jawalagatti, V. et al. A simplified SARS-CoV-2 mouse model demonstrates protection by an oral replicon-based mRNA vaccine. Front. Immunol. 13, 811802 (2022).
Sefik, E. et al. A humanized mouse model of chronic COVID-19. Nat. Biotechnol. 40, 906–920 (2021).
Gary, E. N. et al. A novel mouse AAV6 hACE2 transduction model of wild-type SARS-CoV-2 infection studied using synDNA immunogens - ScienceDirect. iscience 24, 102699 (2021).
Israelow, B., Mao, T., Klein, J., Song, E. & Iwasaki, A. Adaptive immune determinants of viral clearance and protection in mouse models of SARS-CoV-2. Sci. Immunol. 6, eabl4509 (2021).
Israelow, B. et al. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J. Exp. Med. 217, e20201241 (2020).
Kang, S. et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl Acad. Sci. USA 117, 22351–22356 (2020).
Yang, M. S. et al. Non-invasive administration of AAV to target lung parenchymal cells and develop SARS-CoV-2-susceptible mice. Mol. Ther. S1525-0016, 00010–00017 (2022).
Hc, A. & Fwcab, C. A lethal mouse model using a mouse-adapted SARS-CoV-2 strain with enhanced binding to mouse ACE2 as an important platform for COVID-19 research. EBioMedicine 68, 103406 (2021).
Sun, S., Gu, H., Cao, L., Chen, Q. & Qin, C. Characterization and structural basis of a lethal mouse-adapted SARS-CoV-2. Nat. Commun. 12, 5654 (2021).
Huang, K. et al. Q493K and Q498H substitutions in Spike promote adaptation of SARS-CoV-2 in mice. EBioMed 67, 103381 (2021).
Muruato, A., Vu, M. N., Johnson, B. A., Davis-Gardner, M. E. & Menachery, V. D. Mouse Adapted SARS-CoV-2 protects animals from lethal SARS-CoV challenge. Preprint at bioRxiv 2021.2005.2003.442357.
Wang, J. et al. Mouse-adapted SARS-CoV-2 replicates efficiently in the upper and lower respiratory tract of BALB/c and C57BL/6J mice. Protein Cell 11, 776–782 (2020).
Gu, H. et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science 369, 1603–1607 (2020).
Dinnon, K. H. et al. A mouse-adapted SARS-CoV-2 model for the evaluation of COVID-19 medical countermeasures. Nature 586, 560–566 (2020).
Leist, S. R. et al. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 183, 1070–1085 (2020).
Kenney, D. J. et al. Humanized mice reveal a macrophage-enriched gene signature defining human lung tissue protection during SARS-CoV-2 infection. Cell Rep. 39, 110714 (2022).
Douam, F., Ploss, A., Opin, C. & Virol. The use of humanized mice for studies of viral pathogenesis and immunitys. Curr. Opin. Virol. 29, 62–71 (2018).
Fu, W. et al. A SCID mouse-human lung xenograft model of SARS-CoV-2 infection. Theranostics 11, 6607–6615 (2021).
Chan, J. F. et al. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 71, 2428–2446 (2020).
Chu, H., Chan, J. F. & Yuen, K. Y. Animal models in SARS-CoV-2 research. Nat. Methods 19, 392–394 (2022).
Braxton, A. M. et al. Hamsters as a model of severe acute respiratory syndrome coronavirus-2. Comp. Med. 71, 398–410 (2021).
Rosenke, K. et al. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg. Microbes Infect. 9, 2673–2684 (2020).
Zhou, P. et al. Addendum: a pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 588, E6 (2020).
Zhang, Y. N. et al. Different pathogenesis of SARS-CoV-2 Omicron variant in wild-type laboratory mice and hamsters. Signal Transduct. Target Ther. 7, 62 (2022).
Kaptein, S., Jacobs, S., Langendries, L., Seldeslachts, L. & Delang, L. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc. Natl Acad. Sci. USA 117, 26955–26965 (2020).
Osterrieder, N. et al. Age-dependent progression of SARS-CoV-2 infection in Syrian hamsters. Viruses 12, 779 (2020).
Hui, K. P. Y. et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir. Med. 8, 687–695 (2020).
Schafer, A. et al. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J. Exp. Med. 218, e20201993 (2021).
Kreye, J. et al. A therapeutic non-self-reactive SARS-CoV-2 antibody protects from lung pathology in a COVID-19 hamster model. Cell 183, 1058–1069.e1019 (2020).
Imai, M., Iwatsuki-Horimoto, K., Hatta, M., Loeber, S. & Kawaoka, Y. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl Acad. Sci. USA 117, 202009799 (2020).
Mulka, K. R. et al. Progression and resolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in golden Syrian hamsters. Am. J. Pathol. 192, 195–207 (2022).
Sia, S. F. et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 583, 834–838 (2020).
Deng, Y. Q. et al. Lipid nanoparticle-encapsulated mRNA antibody provides long-term protection against SARS-CoV-2 in mice and hamsters. Cell Res. 32, 375–382 (2022).
Ackermann, M. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 383, 120–128 (2020).
Gupta, A. et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 26, 1017–1032 (2020).
To, K. K. et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg. Microbes Infect. 10, 507–535 (2021).
Rizvi, Z. A. et al. Golden Syrian hamster as a model to study cardiovascular complications associated with SARS-CoV-2 infection. Elife 11, e73522 (2022).
Campos, R. K. et al. SARS-CoV-2 infects hamster testes. Microorganisms 9, 1318 (2021).
Dejucq-Rainsford, N. Is SARS-CoV-2-induced testicular damage in hamsters relevant? Nat. Rev. Urol. 1–2 (2022).
Zhang, A. J. et al. Severe acute respiratory syndrome coronavirus 2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin. Infect. Dis. 73, e503–e512 (2021).
Li, C. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections by intranasal or testicular inoculation induces testicular damage preventable by vaccination in golden Syrian hamsters. Clin. Infect. Dis. ciac142 (2022).
Dhakal, S. et al. Sex differences in lung imaging and SARS-CoV-2 antibody responses in a COVID-19 golden Syrian hamster model. mBio 12, e0097421 (2021).
Racnik, J. et al. Transmission of SARS-CoV-2 from human to domestic ferret. Emerg. Infect. Dis. 27, 2450–2453 (2021).
Dileepan, M. et al. Seroprevalence of SARS-CoV-2 (COVID-19) exposure in pet cats and dogs in Minnesota, USA. Virulence 12, 1597–1609 (2021).
Haagmans, B. L. & Koopmans, M. P. G. Spreading of SARS-CoV-2 from hamsters to humans. Lancet 399, 1027–1028 (2022).
Brocato, R. L. et al. Disruption of adaptive immunity enhances disease in SARS-CoV-2-infected Syrian hamsters. J. Virol. 94, e01683–20 (2020).
Yen, H. L. et al. Transmission of SARS-CoV-2 delta variant (AY.127) from pet hamsters to humans, leading to onward human-to-human transmission: a case study. Lancet 399, 1070–1078 (2022).
Damas, J. et al. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc. Natl Acad. Sci. USA 117, 22311–22322 (2020).
Halfmann, P. J. et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature 603, 687–692 (2022).
Subbarao, K. & Roberts, A. Is there an ideal animal model for SARS? Trends Microbiol. 14, 299–303 (2006).
Shi, Z. & Hu, Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res 133, 74–87 (2008).
Kim, Y. I. et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 27, 704–709.e702 (2020).
Oreshkova, N. et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveill 25, 2001005 (2020).
Ryan, K. A., Bewley, K. R., Fotheringham, S. A., Brown, P. & Carroll, M. W. Dose-dependent response to infection with SARS-CoV-2 in the ferret model: evidence of protection to re-challenge. 12, 81 (2020).
Kreft, I. C. et al. Absence of COVID-19-associated changes in plasma coagulation proteins and pulmonary thrombosis in the ferret model. Thromb. Res. 210, 6–11 (2022).
Ritter, J. M. et al. Histopathology and localization of SARS-CoV-2 and its host cell entry receptor ACE2 in tissues from naturally infected US-farmed mink (Neovison vison). Vet. Pathol. 59, 681–695 (2022).
Schlottau, K. et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe. 1, e218–e225 (2020).
Gaudreault, N. N. et al. Susceptibility of sheep to experimental co-infection with the ancestral lineage of SARS-CoV-2 and its alpha variant. Emerg. Microbes Infect. 11, 662–675 (2022).
Bosco-Lauth, A., Hartwig, A. E., Porter, S., Gordy, P. & Bowen, R. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc. Natl. Acad. Sci. USA 117, 26382–26388 (2020).
Caldera-Crespo, L. A. et al. Experimental models of COVID-19. Front. Cell Infect. Microbiol. 11, 792584 (2021).
Osterrieder, N., Bertzbach, L. D., Dietert, K., Ab Delgawad, A. & Trimpert, J. Age-dependent progression of SARS-CoV-2 infection in Syrian hamsters. Viruses 12, 779 (2020).
Caldera-Crespo, L. A. et al. Experimental models of COVID-19. Front. Cell Infect. Microbiol. 11, 792584 (2022).
Izquierdo-Domínguez, A., Rojas-Lechuga, M. J., Chiesa-Estomba, C., Calvo-Henríquez, C. & Alobid, I. Smell and taste dysfunctions in COVID-19 are associated with younger age in ambulatory settings - a multicenter cross-sectional study. J. Invest. Allerg. Clin. Immunol. 30, 346–357 (2020).
Medetalibeyoglu, A. et al. Older adults hospitalized with Covid-19: clinical characteristics and early outcomes from a single center in Istanbul, Turkey. J. Nutr. Health Aging 24, 928–937 (2020).
Aravani, D., Kassi, E., Chatzigeorgiou, A. & Vakrou, S. Cardiometabolic syndrome: an update on available mouse models. Thromb. Haemost. 121, 703–715 (2021).
Kaltenbacher, T. et al. CRISPR somatic genome engineering and cancer modeling in the mouse pancreas and liver. Nat. Protoc. 17, 1142–1188 (2022).
Ahmad, A. et al. Adiponectin homolog novel osmotin protects obesity/diabetes-induced NAFLD by upregulating AdipoRs/PPARalpha signaling in ob/ob and db/db transgenic mouse models. Metab 90, 31–43 (2019).
Asensio, A. et al. Epidemiology of Clostridioides difficile infection in hospitalized patients in Spain: an eight-year review (2012-2019). Enferm. Infecc. Microbiol. Clin. 20, S0213–0005X (2021).
Temiz, M. Z. et al. Altered kidney function induced by SARS-CoV-2 infection and acute kidney damage markers predict survival outcomes of COVID-19 patients: a prospective pilot study. Ren. Fail 44, 233–240 (2022).
Asier Fernández-Pato et al. Plasma miRNA profile at COVID-19 onset predicts severity status and mortality. Emerg. Microbes Infect. 11, 676–688 (2022).
Abdelnabi, R., Boudewijns, R., Foo, S., Seldeslachts, L. & Kai, D. Comparative infectivity and pathogenesis of emerging SARS-CoV-2 variants in Syrian hamsters. EBioMed 68, 103403 (2021).
Altmann, D. M. Narrating the natural history of live infection by SARS CoV-2 VOC in animal models. EBioMed 74, 103704 (2021).
Corbett, K. S. et al. Protection against SARS-CoV-2 Beta variant in mRNA-1273 vaccine-boosted nonhuman primates. Science 374, 1343–1353 (2021).
Jingwen, A. et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 11, 337–343 (2022).
Han-Yi, Huang et al. Vaccination with SARS-CoV-2 spike protein lacking glycan shields elicits enhanced protective responses in animal models. Sci. Transl. Med. 14, 639 (2022).
Dipiazza, A. T., Leist, S. R., Abiona, O. M., Moliva, J. I. & Ruckwardt, T. J. COVID-19 vaccine mRNA-1273 elicits a protective immune profile in mice that is not associated with vaccine-enhanced disease upon SARS-CoV-2 challenge. Immunity 54, 1869–1882 (2021).
Liu, X. et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet 398, 856–869 (2021).
Atmar, R. L. et al. Homologous and Heterologous Covid-19 Booster Vaccinations. N. Engl. J. Med. 386, 1046–1057 (2022).
Spencer, A. J. et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat. Commun. 12, 2893 (2021).
Jarnagin, K., Alvarez, O., Shresta, S. & Webb, D. R. Animal models for SARS-Cov2/Covid19 research-A commentary. Biochem. Pharm. 188, 114543 (2021).
Mariana, D. Z., Lucía, J., Magdalena, H., Ricardo, H. V. & Abraham, L. Protein expression profile of Taenia crassiceps cysticerci related to Th1- and Th2-type responses in the mouse cysticercosis model. Acta Tropica 212, 105696 (2020).
Badeti, S., Tseng, H. C., Romanienko, P., Yehia, G. & Liu, D. Development of a novel human CD147 transgenic NSG mouse model to test SARS-CoV-2 infection and immune responses. Res. Sq. https://doi.org/10.21203/rs.3.rs-396257/v1 (2021).
Okuya, K. et al. Multiple routes of antibody-dependent enhancement of SARS-CoV-2 infection. Microbiol. Spectr. 10, e0155321 (2022).
Liu, Q. et al. Biodistribution and residence time of adenovector serotype 5 in normal and immunodeficient mice and rats detected with bioluminescent imaging. Sci. Rep. 7, 3597 (2017).
Michaely, L. M. et al. NSG-mice reveal the importance of a functional innate and adaptive immune response to overcome RVFV infection. Viruses 14, 350 (2022).
Quitt, O. et al. T-cell engager antibodies enable T cells to control HBV infection and to target HBsAg-positive hepatoma in mice. J. Hepatol. 75, 1058–1071 (2021).
Low, B. E., Hosur, V., Lesbirel, S. & Wiles, M. V. Efficient targeted transgenesis of large donor DNA into multiple mouse genetic backgrounds using bacteriophage Bxb1 integrase. Sci. Rep. 12, 5424 (2022).
Sudre, C. H. et al. Attributes and predictors of long COVID. Nat. Med. 27, 626–631 (2021).
Strang, P., Hedman, C., Adlitzer, H. & Schultz, T. Dying from cancer with COVID-19: age, sex, socio-economic status, and comorbidities. Acta Oncol. 60, 1019–1024 (2021).
Seecheran, R. et al. Atrial arrhythmias in a patient presenting with coronavirus disease-2019 (COVID-19) infection. J. Investig. Med. High. Impact Case Rep. 8, 2324709620925571 (2020).
Chen, L. et al. Novel coronavirus-induced right ventricular failure and point of care echocardiography: a case report. Cardiology 145, 467–472 (2020).
Wu, H. et al. Secular trends in all-cause and cause-specific mortality rates in people with diabetes in Hong Kong, 2001-2016: a retrospective cohort study. Diabetology 63, 757–766 (2020).
Morra, M. E. et al. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta-analysis. Rev. Med. Virol. 28, e1977 (2018).
Kulcsar, K. A., Coleman, C. M., Beck, S. E. & Frieman, M. B. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight 4, e131774 (2019).
Kiso, M., Manabe, N., Komatsu, K., Shimabe, M. & Miyamoto, H. Abnormal structural luteolysis in ovaries of the senescence accelerated mouse (SAM): expression of Fas ligand/Fas-mediated apoptosis signaling molecules in luteal cells. J. Reprod. Dev. 49, 457–463 (2003).
Nemir, M. et al. The Notch pathway controls fibrotic and regenerative repair in the adult heart. Eur. Heart J. 35, 2174–2185 (2014).
Lievens, D. et al. Abrogated transforming growth factor beta receptor II (TGFbetaRII) signalling in dendritic cells promotes immune reactivity of T cells resulting in enhanced atherosclerosis. Eur. Heart J. 34, 3717–3727 (2013).
Liao, Y. et al. CB1 cannabinoid receptor deficiency promotes cardiac remodeling induced by pressure overload in mice. Int. J. Cardiol. 167, 1936–1944 (2013).
Rojas, C. et al. Humanized mouse models for the study of periodontitis: an opportunity to elucidate unresolved aspects of its immunopathogenesis and analyze new immunotherapeutic strategies. Front. Immunol. 12, 663328 (2021).
Corchero, J. et al. The CYP2D6 humanized mouse: effect of the humanCYP2D6 transgene and HNF4α on the disposition of debrisoquine in the mouse. Mol. Pharmacol. 60, 1260 (2001).
Southwell, A. L., Warby, S. C., Carroll, J. B., Doty, C. N. & Hayden, M. R. A fully humanized transgenic mouse model of Huntington disease. Hum. Mol. Genet. 22, 18–34 (2012).
Tichelaar, J. W., Lim, L., Costa, R. H. & Whitsett, J. A. HNF-3/forkhead homologue-4 influences lung morphogenesis and respiratory epithelial cell differentiation in vivo. Dev. Biol. 213, 405–417 (1999).
Duan, W. et al. Knockin of SV40 Tag oncogene in a mouse adenocarcinoma of the prostate model demonstrates advantageous features over the transgenic model. Oncogene 24, 1510–1524 (2005).
Gabril, M. Y. et al. Prostate targeting: PSP94 gene promoter/enhancer region directed prostate tissue-specific expression in a transgenic mouse prostate cancer model. Gene Ther. 9, 1589–1599 (2002).
Niu, J. et al. Human cytomegalovirus IE2 may impair the cognitive ability of the hippocampus through the GluNRs/CaMKIIα/CREB signaling pathway in the Rosa26-LSL-IE2/Cre mouse. Behav. Brain Res. 15, 149 (2022).
Kohlhepp, R. L., Hegge, L. F., Nett, J. E. & Moser, A. R. ROSA26 mice carry a modifier of Min-induced mammary and intestinal tumor development. Mamm. Genome 11, 1058–1062 (2000).
Li, Y. S. et al. Generation of H11-albumin-rtTA transgenic mice: a tool for inducible gene expression in the liver. G3 (Bethesda) 9, 591–599 (2019).
Sun, Y. et al. The acidic domain of Hmga2 and the domain’s linker region are critical for driving self-renewal of hematopoietic stem cell. Int. J. Hematol. 115, 553–562 (2022).
Yamano, K. et al. Identification of the functional expression of adenosine A3 receptor in pancreas using transgenic mice expressing jellyfish apoaequorin. Transgenic Res. 16, 429–435 (2007).
Suzuki et al. The nanos3-3’UTR is required for germ cell specific NANOS3 expression in mouse embryos. PLoS ONE 5, e9300 (2010).
Zariwala, H. A. et al. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J. Neurosci. 32, 3131–3141 (2012).
Aricibasi, M., Jung, A., Heller, E. D. & Rautenschlein, S. Differences in genetic background influence the induction of innate and acquired immune responses in chickens depending on the virulence of the infecting infectious bursal disease virus (IBDV) strain. Vet. Immunol. Immunopathol. 135, 79–92 (2010).
Liu, S. et al. Generation of a uniform thymic malignant lymphoma model with C57BL/6J p53 gene deficient mice. J. Toxicol. Pathol. 35, 25–36 (2022).
Halfmann, P. J. et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature 603, 687–692 (2022).
Broek, M., Müller, U., Sui, H., Zinkernagel, R. M. & Aguet, M. Immune defence in mice lacking type I and/or type ii interferon receptors. Immunol. Rev. 148, 5–18 (2010).
Halford, W. P. et al. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: implications for the regulation of viral latency. Virol. J. 3, 44 (2006).
Berges, B. K., Akkina, S. R., Folkvord, J. M., Connick, E. & Akkina, R. Vaginal and rectal mucosal transmission of R5 and X4 tropic HIV-1 in humanized Rag2−/−γc−/− (RAG-hu) mice. J. Virol. 373, 342 (2008).
Diane et al. Immune response-mediated protection of adult but not neonatal mice from neuron-restricted measles virus infection and central nervous system disease. J. Virol. 73, 1795–1801 (1999).
Vicente, A. C., Guedes-Da-Silva, F. H., Carlos, H., Dumard & Silva, J. L. Yellow fever vaccine protects resistant and susceptible mice against Zika virus infection. 13, e0007072 (2019).
Lin, T. H. et al. Immunodomination of serotype-specific CD4+ T-cell epitopes contributed to the biased immune responses induced by a tetravalent measles-vectored dengue vaccine. Front. Immunol. 11, 546 (2020).
Hixon, A. M. et al. A mouse model of paralytic myelitis caused by enterovirus D68. PLoS Pathog. 13, e1006199 (2017).
Kanatsu-Shinohara, M. et al. Restoration of fertility in infertile mice by transplantation of cryopreserved male germline stem cells. Hum. Reprod. 18, 2660–2667 (2003).
Kohaya, N., Fujiwara, K., Ito, J. & Kashiwazaki, N. Generation of live offspring from vitrified mouse oocytes of C57BL/6J strain. PLoS ONE 8, e58063 (2013).
Nakagawa, Y. et al. Application of oocyte cryopreservation technology in TALEN-mediated mouse genome editing. Exp. Anim. 63, 349–355 (2014).
Ogura, A. et al. In vitro fertilization and microinsemination with round spermatids for propagation of nephrotic genes in mice. Theriogenology 45, 1141–1149 (1996).
Lazarevic, I., Pravica, V., Miljanovic, D. & Cupic, M. Immune evasion of SARS-CoV-2 emerging variants: what have we learnt so far? Viruses 13, 1192 (2021).
Acknowledgements
We are grateful for the support by the National Key R&D Program of China (2021YFC2301700) and National Science and Technology Major Projects of Infectious Disease funds (2017ZX103304402). We also would like to acknowledge the supports from Ms Chunming Sun, who drew the Figures.
Author information
Authors and Affiliations
Contributions
C.F.F. and Y.C.W. designed this work, Y.W., R.X., Y.S.Y., and S.N.L. searched the information and wrote the draft, C.L. and S.S.L were responsible for editing and revision. C.F.F. and Y.C.W. oversaw the entire work. All authors have read and approved the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fan, C., Wu, Y., Rui, X. et al. Animal models for COVID-19: advances, gaps and perspectives. Sig Transduct Target Ther 7, 220 (2022). https://doi.org/10.1038/s41392-022-01087-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-022-01087-8
This article is cited by
-
Cost-effectiveness analysis of COVID-19 intervention policies using a mathematical model: an optimal control approach
Scientific Reports (2024)
-
Omicron subvariant BA.5 efficiently infects lung cells
Nature Communications (2023)
-
A tissue specific-infection mouse model of SARS-CoV-2
Cell Discovery (2023)
-
Gas chromatography-mass spectrometry (GC–MS) profiling of aqueous methanol fraction of Plagiochasma appendiculatum Lehm. & Lindenb. and Sphagnum fimbriatum Wilson for probable antiviral potential
Vegetos (2022)
-
Organoid Technologies for SARS-CoV-2 Research
Current Stem Cell Reports (2022)