Abstract
T cells in the immune system protect the human body from infection by pathogens and clear mutant cells through specific recognition by T cell receptors (TCRs). Cancer immunotherapy, by relying on this basic recognition method, boosts the antitumor efficacy of T cells by unleashing the inhibition of immune checkpoints and expands adaptive immunity by facilitating the adoptive transfer of genetically engineered T cells. T cells genetically equipped with chimeric antigen receptors (CARs) or TCRs have shown remarkable effectiveness in treating some hematological malignancies, although the efficacy of engineered T cells in treating solid tumors is far from satisfactory. In this review, we summarize the development of genetically engineered T cells, outline the most recent studies investigating genetically engineered T cells for cancer immunotherapy, and discuss strategies for improving the performance of these T cells in fighting cancers.
Similar content being viewed by others
Introduction
T cells play central roles in cell-mediated adaptive immunity. Since researchers identified the molecular evidence of T cell receptors (TCRs) in the 1980s1,2, the recognition of antigens by TCRs has been heavily investigated, and the molecular mechanisms governing this process have been elucidated3,4, laying the foundation for cancer immunotherapy.
Cancer immunotherapy exploits the body’s own immune system to fight against cancer. This therapy was designated as an annual scientific breakthrough in 2013 by Science magazine and has exhibited promising antitumor efficacy in recent years5,6,7. Cancer immunotherapies are categorized as immune checkpoint inhibitors (ICIs), adoptive cell therapies (ACTs), and tumor vaccines8,9. Numerous patients with advanced tumors have benefited from cancer immunotherapy, and some have achieved complete remission.
ACT has long been used to treat cancers and other diseases. The adoptive transfer of T lymphocytes expanded ex vivo has shown limited antitumor efficacy, as these T lymphocytes lack specificity against tumor cells. To enhance the efficacy of ACT, the infusion of tumor-infiltrating lymphocytes (TILs) with specificity against the tumor cells in patients with preconditioning regimens substantially improved the efficacy of the treatment10,11,12,13. After cloning the TCR gene of the TIL, it is possible to endow T cells with defined specificity by transferring the cloned TCR gene14,15. T cells engineered by viral vectors to express the TCR gene with defined specificity have shown considerable benefit for the treatment of cancers16,17, although there are many limitations of TCR-engineered T (TCR-T) cells, including HLA restriction, side effects, and the lack of a sufficiently broad TCR gene repertoire with defined specificity18,19. Chimeric antigen receptor-modified T (CAR-T) cells, which are genetically engineered to express CAR molecules targeting surface antigens on tumor cells and other cells, can overcome some of the limitations of TCR-T cells20,21. Since the first demonstration of cytotoxicity to target-bearing cells18,20,21,22,23, CAR-T cells have been extensively investigated in preclinical and clinical studies and have exhibited dramatic efficacy in treating hematological malignancies24,25,26,27,28, although moderate effects have been obtained for the treatment of solid tumors29,30,31.
In this review, we summarize the recent investigations of genetically engineered T cells, mostly focusing on CAR construct optimization, clinical efficacy, and strategies to overcome resistance and other limitations, as well as the outlook for future applications of genetically engineered T cells to cancer therapy.
Rationale for the emergence of genetically engineered T cells
T cells gain autoimmune tolerance after the positive selection of thymocytes32 and play pivotal roles in adaptive immunity33. T cells can provide protective immunity through TCR recognition of foreign antigenic peptides presented by antigen-presenting cells (APCs)34,35, by which T cells might combat tumor cells35,36. The adoptive transfer of T cells was first investigated in the treatment of localized and disseminated lymphoma, and tumors regressed after the infusion of T cells in a syngeneic mouse model37; subsequently, studies have investigated the clinical applications of T cells and other immune cells to fight cancers and other diseases. In fact, T cells infused for the treatment of cancers have been manipulated ex vivo by using different strategies: e.g., LAKs (lymphokine-activated killers) are T cells that proliferate after induction with interleukin (IL)-2. To enhance the specificity of transferred T cells, investigators have attempted to activate and induce proliferation in tumor-specific T cells by using dendritic cells exposed to tumor cell lysates38,39,40, although only moderate clinical benefits have been obtained in these clinical trials41,42,43. For the treatment of hematological malignancies, the transfer of allogeneic T cells is an important strategy to induce tumor elimination44, but it damaged normal tissue and visceral organs in recipients, resulting in graft-versus-host disease (GVHD)45,46. The prevention of GVHD by T cell depletion or host-specific allogenic T cell elimination has been proven to be effective and to improve long-time survival47,48,49.
Well-tested ex vivo expansion strategies50,51 warrant sufficient production of the isolated T cells for clinical applications, while the antitumor efficacy of adoptive transfer LAKs and cytokine-induced killer cells is moderate, mainly due to a lack of sufficient effector T cells specifically targeting tumor cells52,53,54. TILs are effector T cells that leave the blood and infiltrate into tumor tissue to attack tumor cells. TILs theoretically load TCRs specific to tumor antigens, and it has been found that TILs expanded ex vivo have an antitumor efficacy that is enhanced 50–100-fold compared with that of IL-2 alone55. Pioneering clinical trials initiated by Rosenberg and colleagues using expanded TILs for the treatment of melanoma and other tumors demonstrated that the adoptive transfer of autologous TILs is efficacious in regressing primary tumor cells and reducing metastasis56. After decades of research43,44,45,46,47, the adoptive transfer of TILs has been demonstrated to be one of the most important cancer immunotherapies for the treatment of melanoma and several other tumors10.
However, the many hurdles facing the use of TILs limit the antitumor capacity of TIL-based immunotherapy. TILs directly recognize antigens presented on the surface of tumor cells in the form of major histocompatibility complex (MHC)–peptide complexes57,58. Because tumor-associated antigen (TAA) is also expressed on self-tissue, immune tolerance occurs when using TILs exposed to p-MHCs derived from TAAs, resulting in unresponsive T cells59. In addition, tumor cells can escape immune surveillance for several reasons, including the downregulation of MHC molecules and autoantigens, leading to immunosuppression and weak immunogenicity60,61,62. In addition, the TILs from a considerable proportion of patients cannot be obtained or expanded enough for infusion, which promotes the engineering of T cells by transducing TCR genes with known specificity to tumor antigens.
TCR-T cells showed strong tumor-killing ability in murine models and mediated the regression of tumors in clinical trials14,17,63, demonstrating that the specificity of T cells can be redirected against the indicated antigens to mediate immunity to cancer. However, the immunity of TILs or TCR-T cell-based cancer immunotherapy is MHC-restricted, since the recognition of MHC-presented antigens by naive or transduced TCRs is the underlying molecular mechanism. In the 1980s, investigators pioneered a method of redirecting the specificity of T cells by introducing genes encoding artificial TCR-like molecules composed of single-chain variable fragments (scFv) of antibodies, spacers, transmembrane domains, and intracellular domains20,21, known as CARs. Unlike TCR, CAR recognizes surface antigens on target cells via the scFv, and CAR-T cells thus elicit cytotoxicity towards target cells in an MHC-independent manner, which broadens the application of genetically engineered T cells. Many gene transfer tools have been exploited for the engineering of T cells, including retroviral vectors64, liposomes65, electroporation66, and other gene-editing strategies67,68,69. The safety and antitumor capacity of gene-engineered T cells have been demonstrated31,70,71,72, making genetically modified T cell-based cancer immunotherapy a promising treatment regimen for hematological and solid tumors.
Genetically engineered T cells for treating hematological malignancies
Use of CAR-T cells for treating hematological malignancies
After the development of CARs, CAR-T cell-based immunotherapy was utilized in the treatment of lymphoma, leukemia, myeloma, and other hematological malignancies (Table 1)21,73. Currently, it is well accepted that CAR-T therapy is efficacious in the treatment of hematological malignancies and exhibits controllable and tolerable toxicity.
Among the CAR-T cells used for treating hematological cancers, CD19 CAR-T cells are the most prevalent, and hundreds of clinical trials using CD19 CAR-T cells are underway. CD19 CAR-T cells, which target conservatively and extensively expressed CD19 in B-cell lymphomas or leukemias74,75,76, have shown promising outcomes in several trials for treating relapsed, refractory B cell (R/R) cancers28,77,78,79, leading the U.S. Food and Drug Administration (FDA) to approve the first CAR-T cell product, tisagenlecleucel (Kymriah or CTL019), for the treatment of acute lymphoblastic leukemia (ALL) in children and adults80. The mostly recently updated data showed that, in a trial of CD19 CAR-T therapy in which 75 evaluable patients participated in the study, 81% of them achieved 3-month overall remission and 76% achieved 12-month overall survival (OS; the median OS was 19 months)81. Furthermore, CTL019 has been tested in R/R chronic lymphocytic leukemia (CLL; 17 evaluable patients; overall response rate (ORR) was 53%, complete response (CR) rate was 35%)82, follicular lymphoma (14 evaluable patients, ORR was 79%, CR was 71% after 6 months)83, multiple myeloma (MM) (10 patients, 1 with cytokine release syndrome (CRS) and 2 with longer progression-free survival)84. In addition to CTL019, another product, known as axicabtagene ciloleucel, exhibited robust efficacy in treating R/R diffuse large B cell lymphoma (DLBCL). Phase II trials demonstrated an ORR of 82%, with a CR rate of 40% at a median follow-up of 15.2 months26. JCAR017, a well-regarded product produced by Juno Therapeutics, resulted in a dramatic ORR of 75% and a 6-month CR of 37% in 68 R/R DLBCL patients85. Overall, CD19 CAR-T therapy showed encouraging therapeutic effects and safety, illustrating that CD19 CAR-T has advantages for the treatment of hematological malignancies, especially B cell malignancies84. Despite the curative efficacies of CD19 CAR-T cells, their side effects, resulting in normal B cell dysplasia, lethal CRS, and neurotoxicity, warrant further study79,84,86,87. In addition, relapse after CR post-CD19 CAR-T infusion is another concern for users of this therapy. Several mechanisms have been uncovered that were involved in relapse, including CD19 loss, CD19 antigen masking, and trogocytosis88,89. CD22, an alternative surface marker expressed by B cell leukemia and lymphoma cells, can be targeted by CAR-T therapy. The sequential infusion of CD22 CAR-T cells post-CD19 CAR-T therapy can lead to the remission of cancer that relapsed after CD19 CAR-T therapy90. In fact, previous studies demonstrated the antitumor capacity of CD22 CAR-T therapy91,92, and earlier clinical studies of CD22 CAR-T cells in ALL were published in 201890. Compared with CD19 CAR-T cells, CD22 CAR-T cells showed comparable antileukemia cytotoxicity90. Meanwhile, there is no evidence for neurological toxicity and seizures resulting from CD22 CAR-T therapy, which has been observed in CD19 CAR-T therapy90,93. To maximize the benefits of CD19 and CD22 CAR-T therapy for hematological malignancies, the sequential or simultaneous combination of the two different CAR-T products should be further studied.
CD20 is a classic target for lymphoma treatment. The targeting of CD20 by rituximab is efficacious for the treatment of non-Hodgkin’s lymphoma (NHL). Efforts have been made to use CAR-T cells targeting CD20 to treat lymphoma. Although the level of CD20 is not comparable to that of CD19, CD20 is also frequently expressed in lymphoid malignancies and B cell acute lymphocytic leukemia (B-ALL)91,92,94. CD20 CAR-T cells have been evaluated for efficacy and safety, and preclinical investigations have demonstrated similar anti-lymphoma activity compared to CD22 CAR-T cell therapy91. In a clinical trial of seven patients with follicular or mantle cell lymphomas treated with first-generation CD20 CAR-T therapy, three patients showed a positive response (one partial response (PR), two CR)16. In a clinical trial for the treatment of R/R NHL, primarily DLBCL, >80% of patients showed an objective response95. These studies have proven that CD20 CAR-T therapy is efficacious with minimal toxicity, but its poor persistence might be an obstacle to sustained antitumor efficacy for first-generation CAR-T cells. Second-generation CD20 CAR-T cells may lead to improved persistence by adding a costimulatory domain (such as 4-1BB, CD28 or a dual costimulatory molecule)96. Clinical data for second-generation CAR-T cells targeting CD20 have shown their robust antitumor capacity and minimal toxicity for the treatment of CD20-expressing lymphoma96,97,98. In leukemia and lymphoma, CD19, CD22, and CD20 have different expressional hierarchies in tumor cells. However, a head-to-head comparison of the antitumor efficacy of CAR-T cells targeting different antigens is needed to determine whether the selection of the antigen affects the clinical efficacy of CAR-T cells.
MM is another hematological malignancy that is derived from plasma cells. MM is one of the most studied hematological cancers that CAR-T therapy targets. MM cells were found to express ultralow levels of CD1924,99, and CD19 CAR-T therapy for treating MM resulted in a durable CR >1 year, despite the absence of CD19 expression post-CD19 CAR-T cell infusion24. B cell mature antigen (BCMA) belongs to the tumor necrosis factor receptor family100,101,102 and is specifically expressed on MM cells, plasma cells, and partial memory B cells100,103,104,105, making BCMA an ideal marker for targeting by CAR-T cells. Two BCMA CAR-T cell products (bb2121 and LCAR-B38M) were subject to clinical trials for the treatment of MM. The data from the bb2121 phase I dose-escalation trial showed that the CR rate reached 71% among the 21 patients treated106. LCAR-B38M treatment in 57 recruited patients produced an ORR of 88% and a CR of 74%107. These initial efforts using BCMA CAR-T cells for MM treatment indicated that more evidence of safety and efficacy is needed. Other targets, such as CD38, CS-1, and CD138, are targeted by CAR-T cells for the treatment of MM. Monoclonal antibodies against CD38 and CS-1 have been approved by the FDA for the treatment of MM. Indatuximab ravtansine, a monoclonal antibody (mAb) against CD138 conjugated with maytansinoid, has been developed for MM treatment. However, CAR-T cells recognizing MM targets need to be meticulously manipulated, especially because of the side effects caused by “on-target, off-tumor” toxicity108,109,110,111.
Receptor tyrosine kinase-like orphan receptor 1 (ROR1) is abnormally expressed on malignant B cells and at lower levels on normal cells and tissue110,112,113,114,115. Although CAR-T cells recognizing ROR1 elicited strong cytotoxicity in tumor cells, toxicity in normal cells expressing low levels of ROR1 was detected92,112,116, and further evidence of safety and efficacy are needed prior to the application of ROR1 CAR-T cells. In human B-cells, the immunoglobulin-κ (Ig κ) and Ig λ light chains are expressed at a certain ratio, which ranges from 4:1 to 0.5:1. Light-chain CAR-T cells can recognize cells histologically and do not target 20–80% of normal B cells and plasma cells110,117. Ten NHL/MM/CLL patients were treated with κ-redirected CAR-T cells in a clinical trial. Among the five patients with relapsed NHL, two achieved complete remission (after two and three infusions), one had a PR, and two progressed. Three MM patients showed stable disease, and the two CLL patients progressed before or shortly after the 6-week evaluation118. CD30, a biomarker targeted by the brentuximab vedotin mAb, is expressed on all Hodgkin’s lymphomas (HLs) and a portion of NHLs. CAR-T cells recognizing CD30 showed antitumor capability in preclinical and clinical studies86,119,120. CAR-T cells specifically recognizing other targets, including CD123 (the IL-3 receptor α chain)121,122,123, CD33124,125, CD44v6, and Lewis Y126 antigen, were subjected to preclinical and clinical investigation.
Use of TCR-engineered T (TCR-T) cells for treating hematological malignancies
The application of TCR-T cells as a novel adoptive immunotherapy127,128 has shown encouraging results in the treatment of several advanced cancers127,129. TCR-T cells were able to detect intracellular antigens more sensitively via the MHC system130. Clinical investigations using TCR-T cells for the treatment of metastatic melanoma, synovial sarcoma, and colorectal cancer have achieved significant success17,70,131,132,133,134. TCR-T cells recognizing New York esophageal squamous cell carcinoma (NY-ESO-1 or CTAG1A) have been used to treat patients with advanced MM and have resulted in durable CR135. In a clinical trial, 80% of patients with MM (20 in total) who received NY-ESO-1 TCR-T cell therapy achieved objective responses without severe toxicity. Although TCR-T cell therapy presents new therapeutic opportunities for MM patients and other tumor patients70,136,137,138, safety should be of primary concern for other targets139,140, especially when the TCRs used for antigen recognition are modified141.
Bispecific antibodies for treating hematological malignancies
Bispecific T cell engagers (BiTE) are engineered mAbs consisting of two scFvs with affinity for CD3 and tumor antigens142. BiTE shares many similarities with CAR-T cells and exhibits remarkable efficacy as a cancer therapy. The promising efficacy of BiTEs in the treatment of hematological malignancies has been demonstrated in clinical trials143,144. Blinatumomab (or MT103), which is specific for CD3 and CD19 and was the first BiTE approved by the FDA, has been used in several trials to treat lymphoma, ALL, and MM145,146,147. Its promising performance, as shown by the clinical data (especially in R/R B-ALL), has made it known as an “off-the-shelf” product that is easy to utilize compared with CD19 CAR-T cells148. However, adverse events usually accompany the administration of blinatumomab. The most common side effects are neurotoxicity and CRS149,150,151,152,153,154. Moreover, the half-life of BiTE is shorter, resulting in a more complicated treatment process. CD20/CD3 BiTEs (TBTA05 and CD20Bi) have been evaluated in clinical trials145. CD3/BCMA and CD3/CD38 BiTEs have completed preclinical evaluation103,155,156. CD3 linked with CD123 and CD33 BiTEs has been evaluated in clinical trials for AML patients145,157. In the near future, BiTEs targeting different antigens expressed by hematological malignancies may provide alternatives for patients, and the comparison of BiTE with CAR-T cells in the treatment of the same targets will improve our understanding of the treatment of cancer by immunotherapy.
Use of genetically engineered natural killer (NK) cells for treating hematological malignancies
NK cells are mainly derived from bone marrow CD34 lymphocytes158,159. NK cells recognize and elicit rapid responses against virus-infected cells and tumor cells and serve as important innate effector cells160,161,162. NK cell-based cancer immunotherapy, including the adoptive transfer of NK cells combined with NK cells and mAbs (such as ICIs), can be used to induce antibody-specific cytotoxicity, and genetically engineered NK cells can be used to induce antitumor effects. Compared with CAR-T cells, CAR-NK cells mainly produce interferon (IFN)-γ and granulocyte macrophages colony-stimulating factor (GM-CSF), thereby reducing the potential CRS163,164. NK cells have a shorter survival time than CAR-T cells, and the incidence of side effects is further reduced, although it is likely that the antitumor efficacy is decreased165.
Natural killer T cells (NKTs) are a group of special T cell subsets that express both T cell surface markers and NK cell surface markers. Although NKTs in the body account for only 1/1000 of the total number of immune cells, the antitumor activity of NKTs is robust, but the reason for this is not fully understood. Preclinical data suggest that NKTs express the surface markers of both NK cells and CD8 T cells and produce more cell killing enzymes that kill tumor cells166. Evidence of the role of NKT cells in tumor immune surveillance and the antitumor potential of ligand-activated NKT cells have been demonstrated in a mouse model, increasing the enthusiasm for conducting further antitumor potential investigations161,167,168.
Studies have investigated the engineering of NKT cells to improve their antitumor potential. Hecezy et al. demonstrated that NKT cells modified by CARs with specificity for GD2 showed specific toxicity against GD2-positive tumor cells, increases in in vivo survival, and potent antitumor activity in a mouse model of a solid tumor163. GD2 CAR-NKT cell therapy is now being tested in clinical trials to determine its safety and efficacy for treating neuroblastoma (NCT03294954). NKT cells were also engineered for treating lymphoma. Tian et al. documented that CD62L is crucial for the antitumor activity of CAR-NKT cells169. CD62L is the receptor of sulfated sialyl Lewis X antigen and functions in peripheral lymph node homing. It has been demonstrated that CD62L-positive but not CD62L-negative CD19 CAR-NKT cells exhibit prolonged in vivo persistence and superior anti-lymphoma activity169. It is hoped that CD62L-positive CD19 CAR-NKT cells can be tested for safety and antitumor potential to provide alternative engineered immune cells for treating hematological malignancies. All of these therapies are summarized in Table 1.
Use of genetically engineered T cells for treating solid tumors
Genetically engineered T cells have long been employed to treat solid tumors17,30,170. The clinical efficacy of this type of treatment is far from satisfactory compared with that achieved in treating hematological malignancies24,171,172,173. Considerable research has been conducted in an attempt to enhance the antitumor activities of CAR-T and TCR-T cells, and different strategies aiming to determine the efficacy and safety of CAR-T therapy are being tested in clinical trials for the treatment of cancers, such as breast cancer, sarcoma, and neuroblastoma (Table 2). The first clinical application of CAR-T therapy for cancer treatment was the use of CAR-T cells recognizing carbonic anhydrase IX (CAIX) for the treatment of metastatic renal cell carcinoma, which showed moderate antitumor activity30. Other results of clinical trials that used genetically engineered T cells to treat solid tumors have been barely comparable to those achieved with CAR-T therapy for leukemia and lymphoma174.
The most commonly used targets for CAR-T therapy are surface antigens, such as carcinoembryonic antigen (CEA) for colorectal adenocarcinoma175,176, fibroblast activation protein for malignant pleural mesothelioma177, diganglioside GD2 for neuroblastoma178, glioblastoma179, melanoma180, and osteosarcoma181, human epidermal growth factor receptor 2 (HER2) for HER2-positive sarcoma182, mesothelin for pancreatic cancer183, IL-13 receptor α (IL-13Rα) for glioma184, and mutant αvβ6 integrin for pancreatic tumors185. TCR-engineered T cells always target the p-HLA complex. In addition, the p-HLA complex can also be recognized by CAR-T cells whose scFv for binding was derived from a TCR-like antibody186. For these clinical trials, some outcomes have been published. Pule et al. generated EBV-specific T cells to recognize GD2 and infused these GD2 CAR-T cells into patients to treat neuroblastoma187. It was found that virus-specific GD2 CAR-T cells persisted and exhibited moderate anti-neuroblastoma activity. HER2 is thought to be an ideal target for cancer therapy, and many strategies have targeted HER2 to successfully treat breast cancer, gastric cancer, and other tumors. In 2010, Morgan et al. reported that the infusion of HER2 CAR-T cells to treat metastatic colon cancer caused severe adverse effects, likely due to the large number of third-generation CAR-T cells used and “on-target, off-tumor” toxicity188. The meticulous redesign of the clinical strategies used, including the splitting of the HER2 CAR-T cells infusion, the use of a second-generation CAR construct with different scFvs, and a reduction in the total number of CAR-T cells, effectively improved safety while maintaining antitumor efficacy182,189. Epidermal growth factor receptor (EGFR) is widely expressed in normal epithelial tissue, but a mutant EGFR variant, which is a biomarker of lung cancer and breast cancer, can be targeted by CAR-T cells. It was reported that EGFR CAR-T cells used for treating 11 non-small cell lung cancer patients showed efficacy in 2 patients with PRs and 5 with stable disease190. CEA CAR-T cells used for liver cancer treatment also showed moderate antitumor efficacy with minimal toxicity191.
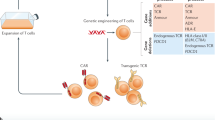
The reason for the moderate clinical efficacy of earlier CAR-T cells used for the treatment of solid tumors is multifactorial. Unlike hematological malignancies, solid tumors present many obstacles to CAR-T cells resulting from short-term persistence or insufficient infiltration. As a fundamental prerequisite for therapeutic efficacy, CAR-T cells need to be trafficked to the tumor lesion. Once they accumulate in the vicinity, they must efficiently infiltrate into the tumor. After migrating into the solid tumor lesion, CAR-T cells must overcome hostile immunosuppressive elements to elicit specific cytotoxicity192, as shown in Fig. 1.
T cell trafficking to tumor sites
Efforts to enhance CAR-T cell trafficking have been made. Investigators have modified CAR T cells with chemokine receptors that specifically bind chemokines produced by tumors, aiming to improve the homing of CAR-T cells to tumor sites. It has been demonstrated that enhanced CCR2b expression in mesothelin CAR-T cells and GD2 CAR-T cells leads to improved antitumor effects in malignant pleural mesothelioma and neuroblastoma due to the increased migration of CAR-T cells to tumor lesions193,194. In addition to CCR2 expression, the transgenic coexpression of CCR4 improved the homing of CD30 CAR-T cells to CD30+ HL that secreted CCL17 (the ligand for CCR4) and thereby improved the anti-lymphoma effects195.
Intravenous infusion is currently considered the standard method for adoptive cell therapy. Therefore, CAR-T cells must migrate to the area where solid tumors are present. One strategy to improve T cell trafficking is the local administration of CAR-T cells to tumor sites, including intratumoral (NCT02587689), intracranial (NCT00730613), pleural (NCT02414269), and hepatic artery (NCT01373047) delivery. In a preclinical model, Adusumillii et al.196 evaluated two routes of administration for mesothelin-targeted T cells using M28z CARs and demonstrated that the intrapleurally administration of CAR-T cells required 30-fold fewer M28z T cells to induce long-term complete remission compared to systemically infused CAR-T cells. Intrapleural administration resulted in enhanced antitumor efficacy and functional T cell persistence for 200 days, while intravenously delivered CAR-T cells did not achieve comparable activation, tumor eradication, or persistence196. The central nervous system is an immune-privileged site secondary to the blood–brain barrier (BBB). It is difficult for intravenously infused CAR-T cells to traffic to brain tumors. The local delivery of T cells via methods such as intracranial infusion could circumvent the BBB and improve the immunotherapy of brain tumors197. Preclinical and clinical studies have confirmed the efficacy of locally delivered CAR-T cells in treating primary and metastatic brain cancers31,198,199,200.
Immunosuppressive microenvironment
In solid tumors, CAR-T cells face a hostile tumor microenvironment (TME), even though CAR-T cells are able to migrate to tumor sites. Solid tumors are usually infiltrated by an abundance of immune-suppressor cells, including M2 tumor-associated macrophages, myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Tregs) and B cells, which protect malignant cells from the antitumor activity of the immune system201,202,203. In addition, immunosuppressive cytokines and inhibitory immune checkpoints play a crucial role in tumor pathogenesis and metastasis and limit the therapeutic potential of cancer immunotherapies204,205.
Preclinical investigations have demonstrated that the incorporation of costimulatory molecules, such as CD28, into CARs may help engineered CAR-T cells overcome the immunosuppressive TME mediated by Treg cells206,207,208. It has been recently reported that engineered CAR-T cells with inducible IL-18 tip the balance within the immune cell landscape toward the Th1 acute-phase response, thereby reducing the number of immunosuppressive cells, such as Tregs and CD206+ macrophage cells, and resulting in an augmented immune attack against large established tumors. Notably, this effect was achieved in fully immune-competent mice with advanced pancreatic adenocarcinoma209. Depleting MDSCs can also boost T cell responses. A study showed that the administration of GD2 CAR-T cells in combination with MDSC depletion led to significant antitumor efficacy in a xenograft sarcoma model, while CAR-T cells alone elicited minimal antitumor activity210. In addition, Noman et al.211 demonstrated that programmed death ligand 1 (PD-L1) blockade prevented T cell suppression by MDSCs. It was also reported that the blockade of PD-L1+ MDSCs and Tregs in the TME enhanced CEA CAR-T cell antitumor capability212. Overall, the findings of these studies indicated that immunosuppression was mediated by suppressor cells and supported the rationale of cell preconditioning to enhance the antitumor activity of CAR-T cells.
Various immunosuppressive cytokines, such as transforming growth factor (TGF)-β and IL-10, are involved in the inhibition of engineered T cell-based cancer immunotherapy203,213,214. In a recent study, investigators engineered prostate-specific membrane antigen CAR-T cells to coexpress a dominant-negative TGF-β receptor and observed increased proliferation, enhanced cytokine secretion, resistance to exhaustion, long-term in vivo persistence, and the induction of tumor eradication by CAR-T cells in aggressive human prostate cancer215. Then a phase I clinical trial was initiated to assess CAR-T cells coexpressing TGF-βRII in patients with relapsed and refractory metastatic prostate cancer (NCT03089203). In addition, engineered CAR-T cells with the enhanced secretion of cytokines, known as TRUCKs (T cells redirected for universal cytokine killing), have been investigated216,217,218. CAR-T cells targeting MUC16 with IL-12 secretion have eradicated ovarian cancers in a preclinical trial. Engineered CAR-T cells with autocrine IL-12 expression exhibited improved antitumor activity by promoting CD8+ T cell function and potentially circumventing the inhibitory TME219. MUC16 TRUCKs are currently being evaluated in a clinical trial for treating solid tumors (NCT02498912)219. The local delivery of the chemokine RANTES (regulated and normal T cell expressed and secreted) and the cytokine IL-15 by an oncolytic virus strikingly increased the persistence of anti-GD2 CAR-T cells and enhanced the survival of established neuroblastoma cells mice220. We generated CAR-T cells specific for human vascular endothelial growth factor receptor 1 (VEGFR-1) and T cells that genetically expressed IL-15, and the VEGFR-1-specific CAR-T cells delayed tumor growth and formation and suppressed pulmonary metastasis in a xenograft tumor model221. Indeed, numerous studies have demonstrated that γ-chain (γc) family cytokines could be used to enhance the immunity of CAR-T cells. IL-2, IL-4, IL-7, IL-15, and IL-21 have been shown to mitigate the effects of immunosuppressive factors in the TME and produced remarkable enhancement of CAR-T efficacy221,222,223,224,225,226,227,228,229.
T cells have also been demonstrated to express coinhibitory receptors, termed markers of T cell exhaustion, to reduce the antitumor activities of CAR-T cells. In recent years, PD-1 has been studied as a potential target to promote CAR-T cell efficacy230. Strategies to manipulate PD-1 expression on CAR-T cells include the coadministration of a PD-1/PD-L1 blocking antibody, the genetic removal of PD-1 from T cells and the secretion of autocrine molecules to induce PD-1/PD-L1 blockade by CAR-T cells. Cherkassky et al. demonstrated that PD-1/PD-L1 blockade restored the antitumor activity of CD28 CAR-T cells in an orthotopic pleural mesothelioma mouse model, mechanistically confirming the role of the PD-1/PD-L1 axis in CAR-T cell exhaustion231. Ren et al. genetically depleted PD-1 in prostate stem cell antigen (PSCA) CAR-T cells by using the CRISPR/Cas9 gene-editing system and demonstrated their enhanced antitumor efficacy, both in vitro and in vivo, in a murine prostate cancer model232. Furthermore, several clinical trials of CAR-T cells expressing PD-1 antibody for the treatment of solid tumors are underway (NCT03030001, NCT02873390, NCT03179007, NCT03182816, NCT03182803, NCT03615313). Additionally, a clinical trial (NCT03399448) sponsored by University of Pennsylvania is evaluating the antitumor efficacy of NY-ESO-1-specific TCR-T cells with the elimination of PD-1 and endogenous TCR by the CRISPR/Cas9 gene-editing system for the treatment of MM, melanoma, and other tumors. It should be noted that systemic checkpoint blockade diseases, such as pneumonitis, colitis, hepatitis, and other adverse events, have occurred in many patients after PD-1 blockade treatment. In a PD-1 monotherapy clinical trial, Suzanne et al. observed grade 3 or 4 drug-related adverse events in 14% of patients, and 3 patients died from pulmonary toxicity out of a total of 296 patients233. Therefore, side effects should be considered when combining CAR-T cells with PD-1/PD-L1 blockade therapy. However, when PD-1 blockade was combined with CAR-T cells, no damage was observed in normal tissues, while the enhancement of the anti-breast cancer activity of CAR-T cells was maintained234. CAR-T cells secreting anti-PD-1 ScFv also produced minimal side effects235. The reduced side effects might be ascribed to the restricted secretion of PD-1 blockade antibodies in areas of CAR-T distribution or the selective elimination of PD-1 specifically on CAR-T cells. It is expected that the combination of CAR-T therapy with anti-PD-1 therapy will increase the efficacy of cancer immunotherapy.
Evolution of genetically engineered T cells
The first clinical application of CAR-T cells was for the treatment of metastatic renal cell carcinoma by the infusion of CAR-T cells recognizing CAIX236. CAIX CAR-T cells successfully targeted CAIX-expressing tumor cells but also recognized CAIX-expressing normal tissues, resulting in so-called “on-target, off-tumor” toxicity and grade 2-4 enzyme disturbances236. The antitumor efficacy of CAIX CAR-T was moderate, mainly due to the construction of a CAR with an intracellular CD3ζ motif, which failed to induce robust in vivo antitumor effects after engaging with tumor cells236,237. To obtain ideal antitumor efficacy and prevent severe adverse effects, the CAR construct was optimized. The CAR molecule consists of four parts: the extracellular scFv, the transmembrane domain, the costimulatory domain and the CD3ζ signal domain, which are intracellular238.
Binding domain
Each part of the CAR construct can be modified to improve the biological function of CAR-T cells. The scFv or other molecule used for the binding domain exerts pivotal effects on the targeting function of CAR-T cells and can be further optimized239,240. This approach may employ human scFvs for CAR construct design to improve the long-term survival of CAR-T cells, whereas a potential human anti-mouse antibody reaction may eliminate CAR-T cells that use a murine scFv for binding and lead to poor persistence. In recent years, efforts have been made to replace scFvs with nanobodies derived from camelids241, and preclinical studies have shown that nanobody-based CAR-T cells showed improved flexibility in antigen recognition and achieved comparable efficacy when treating different tumors242,243. CAR-T cells attack cells expressing the CAR-recognized antigens, which are always TAAs. TAAs are also expressed on normal tissues at lower levels compared with those of tumors. To maximally mitigate “on-target, off-tumor” toxicity, the affinity of the scFv in the CAR construct is meticulously tuned to selectively recognize tumor cells having higher levels of TAAs while sparing normal tissues with low levels of antigen244,245. CAR-T cells always recognize the antigens on the surfaces of the target cells. The TCR-like antibody specifically binds the p-HLA resembling the TCR, since it binds neither the presented peptide nor the HLA molecule alone. CAR-T cells that use TCR-like scFvs for binding can recognize intracellular antigens246. CAR-T cells with TCR-like scFvs recognizing alpha fetoprotein have shown robust tumor-inhibiting effects in the treatment of liver cancer186. Indeed, some other molecules have been used for binding, e.g., ligands or cytokines binding to a specific receptor, which are antigens that the targeted cells can recognize247,248.
Antigens for TCR-T cells have been derived from melanoma antigens, such as GP100, MART1249, NY-ESO-1250, MAGE family members251, and WT1252, and the clinical application of TCR-T cell therapy has achieved substantial efficacy in the treatment of melanoma17. Similar to CAR-T therapy, the TME often inhibits the efficacy of TCR-T cell therapy. Preconditioning strategies, including lymphodepletion by nonmyeloablative chemotherapy or intensive myeloablative chemoradiotherapy, have been demonstrated to improve the antitumor efficacy of TILs253. Preconditioning chemotherapy with cyclophosphamide plus fludarabine has shown promise in improving the efficacy of TCR-T and CAR-T cell therapy26,83. The affinity of TCRs are significantly associated with TCR-T cell efficacy. Affinity optimization produced improved tumor-killing ability ex vivo. However, the modification of affinity must be meticulously implemented, since improper modification can lead to cross-reactions and result in lethal adverse effects141,254,255.
Intracellular domain
Distinct from the extracellular binding domain, the intracellular domain of CAR results in signaling transduction initiated by the binding of the extracellular domain to the antigen. Recently, the evolution of CAR-T cells has focused on the costimulatory intracellular domain and resulted in the substantial enhancement of antitumor efficacy. First-generation CAR-T cells, which contain a CAR construct with only CD3ζ in the intracellular domain, showed limited antitumor efficacy in clinical trials16,256, mainly due to the lack of costimulatory molecules that are essential for T cell responses to antigens257,258. In fact, the intracellular signal transduction domain always includes the indispensable CD3ζ chain, which contains immunoreceptor tyrosine-based activation motifs (ITAMs) that function in signal transduction259, and one or more intracellular costimulatory molecules, such as CD28, 4-1BB (CD137), or CD27, to transmit activation signals180,260,261,262,263,264,265,266,267,268,269,270. In terms of the intracellular signal domain, CARs have evolved from the first to the fourth generation. The intracellular signal transduction domain of first-generation CARs consisted of ITAMs from the CD3ζ chain of the TCR. It has been demonstrated that first generation of CAR-T cells persist in the recipient without long-term survival and robust proliferation29,256. Then costimulatory signal molecules, including CD28, CD137, CD27, and OX40, were integrated into CAR constructs to enhance the persistence, proliferation, and cytokine secretion of T cells271,272,273,274. Second-generation CAR-T cells, bearing one costimulatory domain and CD3ζ, have achieved remarkable clinical efficacy in treating B-ALL275,276,277, although the optimal costimulatory molecules for signaling transduction in CAR constructs remain to be determined. The third-generation CAR construct includes CD3ζ and two costimulatory molecules from among CD28, 4-1BB, and OX40, further improving the proliferation and survival of CAR-T cells after infusion8,91,278,279. Fourth-generation CAR-T cells are TRUCKs, and TRUCKs contain CAR molecules and a CAR-inducible cytokine (IL-12), which can deposit cytokines in the targeted tumor area and recruit a second wave of immune cells, such as macrophages and NK cells217,280,281. Currently, second- and third-generation CAR-T cells are mainly used for clinical applications.
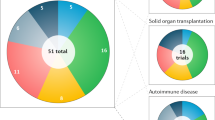
Although many intracellular domains in costimulatory molecules can improve the tumor-killing activities of CAR-T cells, CD28 and 4-1BB intracellular domains are the most commonly used costimulatory domains, and CAR-T cells with either CD28 or the 4-1BB intracellular domain have shown dramatic antitumor capability and tolerable toxicity in clinical trials28,271,272,275. When analyzing the mostly recently updated CAR-T clinical trials (342 in total; 156 CAR constructs are available) worldwide at https://clinicaltrials.gov, it was found that most CAR constructs used in the CAR-T clinical trials are second-generation CAR constructs. Twenty clinical trials are using the third-generation CAR construct, and five are using fourth-generation CAR constructs, into which additional elements, such as an inducible caspase-9 gene (iCas9) that can lead to self-destruction by apoptosis, were integrated (Fig. 2a). Among the clinical trials using a second-generation CAR construct, 4-1BB is more prevalent in the CAR construct. A total of 101 clinical trials (64.7%) utilized the 4-1BB costimulatory molecule, and 30 trials used CAR constructs with the CD28 intracellular signaling domain (19.2%). Among the clinical trials using third-generation CAR-T cells, approximately half of the trials (14) used the CD28-4-1BB CAR construct. Other constructs, including CD28-OX40, CD28-4-1BB-CD27, NKG2D-DAP10, CD28-TLR2, or iMyD88/CD40, were used in the CAR-T cells (Fig. 2b).
The data were obtained from https://clinicaltrials.gov, which was accessed on January 30, 2019. a Diagram of clinical trials of CAR-T cells from different generations. There are 342 registered trials categorized as CAR-T cell trials (second generation: 133, third generation: 20, fourth generation: 5, NA, not available). b Diagram of clinical trials of CAR-T cells using different costimulatory molecules. A total of 156 available CAR constructs with different costimulatory molecules were specifically indicated, and a pie diagram is presented that shows the percentages of trials using cells with different costimulatory domains (4-1BB: 64.7%, CD28: 19.2%, CD28+4-1BB: 9%, CD28+OX40: 2%, CD28+TLR2: 0.6%, CD28+4-1BB+CD27: 2%, CD27: 0.6%, iMyD88/CD40: 0.6%, NKG2D and DAP10: 1.2%)
Among CAR-T cells using these different intracellular constructs, a comparison of CAR-T cells using CD28 or 4-1BB as the costimulatory molecule has been made, showing that CD28 and 4-1BB CAR-T cells vary in function and effectiveness261,270,272,282,283,284,285,286. CD28ζ and 4-1BBζ CARs show increased secretion of cytokines, including IFN-γ, IL-2, TNF-α, and GM-CSF, compared to first-generation CARs150,276,277,287,288,289,290. CAR T cells incorporating the 4-1BB costimulatory domain are more persistent and are likely to be more resistant to exhaustion than those incorporating CD28261,282. The incorporation of 4-1BB in the CAR construct may endow T cells with long-lasting survival and more central memory compartments than that of CD28286,291,292. 4-1BBζ CARs affect downstream signaling pathways through TNF receptor-associated factor 2 (TRAF2), while CD28ζ CARs affect signaling through nuclear factor (NF)-κB, AKT, extracellular signal–regulated kinase, NF of activated T cells, and Bcl-XL260,293,294,295,296,297,298. In clinical trials, CD28ζ CAR and 4-1BBζ CAR-T cells showed similar antitumor activities and remission rates27,275. Preclinical studies have shown that third-generation CAR-T cells exhibit improved antitumor effects compared with second-generation CAR-T cells299,300. Ramos et al.301 designed a clinical trial in which second- (CD28ζ) and third-generation (28BBζ) CD19 CAR-T cells were simultaneously infused into patients, and the third-generation CAR-T cells showed superior expansion and longer persistence than the second-generation CAR-T cells, suggesting the improved antitumor efficacy of the third-generation CAR-T cells compared to the second-generation CAR-T cells. Haso et al.302 reported that second-generation CAR-T cell immunotherapy was superior to third-generation CAR-T therapy with an anti-CD22 CAR in B-ALL, in contrast to the results of Ramos et al.’s research301. In fact, CAR functionality is not solely determined by the cytoplasmic signal domains, as other structural features may affect its overall function. CAR function varies depending on the expression level of the antigen on the target cells and the CAR in the T cells, the affinity of the CAR for the antigen, and signal pathway transduction by the CAR. To further determine the optimal choice of CAR-T for cancer patients, more clinical evidence is needed. For instance, a clinical trial (NCT01853631) is underway to compare the use of second- and third-generation CD19 CAR-T cells for the treatment of hematological malignancies, which will provide particular value for selection of second- and third-generation CAR-T cells.
CAR-T cells bearing costimulatory molecules other than CD28 or 4-1BB have been heavily investigated to determine their advantages, and some of these CAR-T cells are being tested in clinical trials for the treatment of cancers. CAR-T cells targeting CD33 with OX40 or CD28 costimulatory intracellular domains have shown similar effects to those with the 4-1BBζ construct in terms of T cell proliferation and cytokine secretion303. Third-generation GD2-specific CAR-T cells with OX40 and CD28 intracellular costimulatory domains were assessed (NCT02107963, NCT01822652, NCT01953900). ICOSζ CAR-T cells have increased phosphoinositide-3 kinase activation and IFN-γ production and secrete increased amounts of IL-17A, IL-17F, and IL-22 compared to 4-1BBζ CAR-T cells and express CD161, but their antitumor effects were not greater than those of CD28ζ and 4-1BBζ303,304,305 cells. CAR-T cells using a third-generation CAR construct containing inducible T cell co-stimulator and a 4-1BB intracellular costimulatory domain were demonstrated to have robust antitumor efficacy in solid tumor models compared with second-generation CAR-T cells having a 4-1BB domain275,306,307. Adding CD27 to the second-generation CAR construct increased T cell expansion, BCL-XL upregulation, and IFN-γ production, and the resulting cells showed comparable in vivo efficacy compared with CD28ζ and 4-1BBζ CAR-T cells308. In addition, clinical data demonstrated that CD28-CD27-CD3ζ CAR-T cells were safe when infused to treat leukemia262,308. The intracellular domain of NKG2D, either individually or in combination with CD28/4-1BB domains, enhanced many properties of CAR-T cells, including the secretion of IFN-γ, TNF-α, and GM-CSF, and improved antitumor efficacy in vivo309,310,311,312,313. CAR-T cells containing a MyD88/CD40-based inducible costimulatory molecule promoted T cell proliferation and cytokine production267. A clinical trial (NCT02744287) exploited MyD88/CD40 CAR-T cells to target PSCA for the treatment of prostate adenocarcinoma and other cancers. Toll-like receptors (TLRs) and cytokine receptors can stimulate T cells to proliferate and produce inflammatory cytokines. The TLR2 and IL15Rα intracellular domains were also incorporated into the CAR construct, and the CAR-T cells were able to induce T cell expansion, cytokine production, and antitumor activity in vivo266,268. A CAR-T cell bearing the TLR-2 costimulatory domain was tested in a clinical trial to treat leukemia (NCT02822326). It is well accepted that second- or third-generation CAR constructs containing CD28 or 4-1BB intracellular costimulatory domains improved the persistence and survival of CAR-T cells. To overcome the insufficient persistence of TCR-T cells314, the addition of the CD28 or 4-1BB intracellular region was used to improve the tumor-killing capacity and long-term survival315.
Similar to CARs in CAR-T cells, the structures of TCRs in TCR-T cells have been optimized. TCR-T cells recognize tumor antigens depending on the HLA molecule used316,317. TCRs, which are composed of transgenic α chains and β chains, must bind to CD3 molecules. In genetically modified TCR-T cells, transgenic α or β chains can be mismatched with endogenous TCR α or β chains318,319. To minimize these mismatches, investigators introduced cysteine residues into the C-terminal domains of α and β chains and utilized the disulfide bond that formed between the cysteine residues to facilitate the assembly of the transgenic TCR α and β chains320,321. Other strategies have also been employed to prevent mispairing, such as mutating the C-terminal domains of the transgenic α and β chain to promote correct binding322. In addition, endogenous TCRs can be knocked out, which eliminates issues caused by TCR mispairing323,324. Indeed, endogenous TCRs also interfere with the function of CAR-T cells. When encountering p-HLA, the downstream signaling pathway initiated by CAR binding with the antigen was inhibited325. Thus endeavors have been made to replace the endogenous TCR gene with the CAR gene by a gene-editing tool to eliminate the interference due to TCRs, ensure uniform CAR expression, and increase antitumor potency68,326. In addition to removing TCRs, using a gene-editing tool to remove molecules governing immunological rejection, such as the β-2 microglobulin of HLA and other immune resistance factors, generated universal CAR-T cells with promising capacity for the treatment of hematological and solid tumors327,328,329, but more clinical evidence is needed to demonstrate the safety and efficacy of universal CAR-T cells330.
Infusion strategy
The infusion strategy is important for the efficacy and safety of genetically engineered T cells. The intravenous infusion of CD19 CAR-T cell therapy has achieved success in targeting CD19+ B cell lymphoma24,77,83, and other CAR-T cells have targeted CD20, CD30, CD33, or BCMA for the treatment of hematological malignancies via intravenous infusion23. CAR-T cells were also intravenously infused in pilot trials for treating solid tumors. Their efficacy was restricted in the treatment of solid tumors331; it was found that intravenously infused CAR-T cells accumulated in the lung, liver, and kidney29, and only a small proportion of the infused cells homed in to the tumor site. The local delivery of CAR-T cells is thus encouraging. Multiple intracranial infusions of IL13Rα CAR-T cells, by enhancing infiltration and persistence, resulted in glioblastoma regression31. Recently, Smith et al. produced a novel cell biopolymer device, in which CAR-T cells proliferated robustly, and transplanted the device with CAR-T cells directly into tumor tissue, significantly improving CAR-T cell trafficking and infiltration332.
Other challenges for engineered T therapy
Genetically engineered T cells have benefited patients in the treatment of tumors, but other obstacles beyond the issues discussed above have challenged the application of genetically modified T cells, even in the treatment of hematological malignancies28,77,81,213,271,333,334,335,336,337.
Failure of CAR-T cell generation
Although the total number of CAR-T cells used for infusion is small (108–109), the failure to produce sufficient engineered T cells has been observed, which challenges the pharmaceutics of this therapy. Many factors can result in the failure of CAR-T production. Cancer patients generally undergo chemotherapy and other regimens, leading to lymphopenia, which affects the quality and quantity of harvested T cells. Leukapheresis improves the quality and purity of T cells, but poor expansion of CAR-T cells after engineering impedes the generation of sufficient numbers of modified T cells. T cells with different phenotypes exert distinct functions. Previous studies demonstrated that CAR-T cells with a central memory T cell phenotype (CD8+CD45RA−CD45RO+CCR7+) are closely related to antitumor activity225,338,339,340. In addition to the central memory phenotype, the CD4+/CD8+ ratio and the differential expression of other molecules in the infused T cells might affect the antitumor capability of CAR-T therapy341,342. Therefore, obtaining enough CAR-T cells for cancer immunotherapy means not only generating a sufficient number of T cells but also producing a defined phenotype and a specified ratio of subsets within the modified T cells.
There are many cell culture strategies used for the production of CAR-T cells. Magnetic beads coated with CD3/CD28 antibodies are often combined with cytokines, such as IL-2, IL-7, and IL-15, for the activation and expansion of CAR-T cells225,343,344. Artificial APCs are also used to greatly expand and generate antigen-specific CAR-T cells345. CD4- and CD8-positive cells can be enriched for producing CAR-T cells, and a defined ratio of CD4 and CD8 cells can ultimately be ensured341. In addition to differences in the expansion protocol, CAR-T cells with different intracellular costimulatory domains in their CAR constructs exhibited different expansion and phenotype properties263,286,304,307,308,346,347. The lengths of the CAR extracellular spacers used are critical to CAR-T cell therapy348. It is likely that formulating CAR-T cells with a defined composition of different subsets can enhance the uniformity of CAR-T products, which could be used in further investigations to draw more definitive conclusions.
Relapse after CAR-T therapy
Although CD19-targeted CAR-T cells achieved dramatic antitumor efficacy in treating hematological malignancies27,275,341,349,350, a considerable number of patients with CR have relapsed after CAR-T therapy. Although the mechanism of relapse has not been fully elucidated, antigen loss and poor CAR-T persistence are thought to contribute to the relapse of disease.
Tumors in a small proportion of pediatric and adult responders relapsed after treatment with CD19 CAR-T cells because of CD19 deletion and CD19 tumor cell growth81,351,352, which results from acquired resistance to CAR-T therapy due to antigen escape90,353,354. To prevent the recurrence of the tumor, broader immune activation is required to elicit a second wave of immunity in addition to CAR-T cell therapy355. Recently, other novel mechanisms accounting for the relapse of hematological malignancies after CD19 CAR-T cell therapy have been reported. CD19 molecules on tumor cells transduced with CAR vectors can be masked by CAR molecules, leading to their escape from attack by CAR-T cells and the recurrence of malignancy88. A target antigen recognized by CAR-T cells on the tumor cells can even be removed by CAR-T cells through trogocytosis, resulting in a reduction in the antigen and fratricide between CAR-T cells89. Another obstacle is the poor persistence of CAR-T cells. The efficacy of CAR-T cell therapy is closely related to the in vivo expansion and survival of cells356. Many strategies discussed above, including the optimization of the CAR construct and preconditioning and infusion strategies, can prolong the survival of CAR-T cells and enhance their antitumor efficacy.
Side effects
CAR-T cells target the antigen expressed on the surface of tumor cells. This antigen is generally expressed on normal cells and not exclusively on tumor cells. CAR-T cells attack normal tissues when clearing tumor cells, resulting in side effects in normal tissues, which are called “on-target, off-tumor” side effects. Clinical studies in which CAR-T cells infused for cancer therapy have recognized HER2188, CAIX357, CD19358,359, and mesothelin360 have shown damage to normal tissues. Many strategies have been developed for enhancing the safety of CAR-T cell therapy, such as the targeting of other antigens, such as CD1a, in place of CD19361, equipping CAR-T cells with safety switches by introducing suicide genes362,363,364,365, expressing a split CAR molecule366, constructing inhibitory CAR constructs367 or dual antigen-activated CAR-T cells to recognize tumor cells more specifically368, and tuning the affinity of the scFv used for the CAR construction244,245,369 to minimize the incidence of severe adverse effects. In addition, the local injection of CAR-T cells can also reduce off-target effects, since the CAR-T cells are regionally restricted212. Further research may seek to improve the safety of CAR-T cells, especially regarding the selection of the CAR-recognized antigen, the development of controllable CAR-T cells, and the optimization of infusion strategies.
Among the side effects of CAR-T cells, CRS is the most common adverse effect. CRS resulting from CD19 CAR-T cell therapy is tolerable if properly treated26,27,83,370. CRS is induced by the robust activation of the immune system during CAR-T cell therapy, triggering the release of a large number of cytokines, including IL-6, IL-10, IFN-y, TNF-α, GM-CSF, and other cytokines, and causing severe adverse effects371,372. The severity of the CRS-related side effects is closely related to the amounts of released cytokines373, tumor burden374,375, and the structure of the CAR272. mAbs against IL-6Ra (tocilizumab or sarilumab) are used for the treatment of CRS in addition to the use of corticosteroids to inhibit inflammatory reactions152. The blockade of IL-1β376 or TNF-α152 is another strategy used to treat CRS in addition to IL-6, but the benefits for patients are yet to be documented. GM-CSF-knockout CAR-T cells resulted in minimal incidence of CRS compared with GM-CSF-intact CAR-T cells372, indicating the pivotal role of CAR-T-derived GM-CSF in the occurrence of CRS.
In addition to CRS, varying degrees of neurotoxicity have occurred as a result of the clinical application of CAR-T cell therapy for treating hematological tumors377,378,379. Indeed, neurotoxicity is always closely related to CRS. Mild neurotoxicity is transient and reversible. The appearance of acute and severe neurotoxicity is usually accompanied by severe CRS symptoms due to the increased permeability of the blood–brain barrier. Although the mechanism of the incidence of neurotoxicity is largely unknown, many clues have indicated that inflammatory cytokines affect the function of the blood–brain barrier and endothelial cells, contributing to neurotoxicity380,381. In nonhuman primates, CAR-T cell-mediated neurotoxicity was also confirmed to be associated with pro-inflammatory CSF cytokines and pan-T-cell encephalitis382. For the treatment of neurotoxicity caused by CAR-T cell therapy, a regimen should be prescribed that is dependent on the severity. Anti-IL-6 therapy can reverse side effects at the early stage. For the treatment of late-stage neurotoxicity, corticosteroids are recommended. Patients with nonconvulsive and convulsive status epilepticus should be managed with benzodiazepines and additional antiepileptics383,384.
TCR-T cells target HLA-presented antigens on tumor cells. The potential side effects of TCR-T cell therapy are decreased compared to those of CAR-T cell therapy385. However, safety concerns should be paid more attention when the affinity or other characteristics of the TCR are modified. TCR-T cells with enhanced affinity always improve the recognition of the HLA-presented antigens, but side effects usually accompany this. In a pilot study using affinity-enhanced TCR-T therapy for the treatment of melanoma and other cancers, two of the nine patients died of leukoencephalopathy after treatment with MAGE-3 high-affinity TCR-T cells because of off-target toxicity386. In another clinical trial for the treatment of melanoma and myeloma, two patients died of cardiotoxicity after MAGE-3-specific, affinity-enhanced TCR-T cell therapy, mainly due to cross-reaction with the titin peptide, which is expressed in heart tissue141. Therefore, meticulous investigation of affinity-enhanced TCR-T therapy is needed before it can provide clinical benefit to cancer patients.
Outlook
Among cancer immunotherapies, CAR-T cell therapy has shown robust antitumor efficacy for the treatment of hematological malignancies. Upon the approval of CAR-T cell therapy for the treatment of leukemia and lymphoma, more patients will be able to benefit from this new therapy, but side effects, relapses after CR, and long-term monitoring should be of concern. CAR-T cell therapy for solid tumors is far from satisfactory. Endeavors should be made to overcome immunosuppression mediated by the microenvironment of solid tumors. TCR-T cells and other genetically engineered immune cells have produced effective immunity during cancer treatment in preclinical and clinical trials, and efforts should be made to enroll more patients to obtain the benefits of TCR-T therapy. It is hoped that another breakthrough in gene-engineered-T cell therapy for tumors is coming soon.
References
Hedrick, S. M., Cohen, D. I., Nielsen, E. A. & Davis, M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature 308, 149–153 (1984).
Yanagi, Y. et al. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature 308, 145–149 (1984).
Garcia, K. C. et al. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science 279, 1166–1172 (1998).
Rudolph, M. G. & Wilson, I. A. The specificity of TCR/pMHC interaction. Curr. Opin. Immunol. 14, 52–65 (2002).
Couzin-Frankel, J. Cancer immunotherapy. Science 342, 1432–1433 (2013).
Couzin-Frankel, J. Shaking up science. Science 339, 386–389 (2013).
Kelly, P. N. The cancer immunotherapy revolution. Science 359, 1344–1345 (2018).
June, C. H. et al. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Dudley, M. E. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854 (2002).
Ahmadzadeh, M. et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009).
Rosenberg, S. A. et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. New Engl. J. Med. 319, 1676–1680 (1988).
Attia, P. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J. Clin. Oncol. 23, 6043–6053 (2005).
Rosenberg, S. A. et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 8, 299–308 (2008).
Rosenberg, S. A. Cancer immunotherapy comes of age. Nat. Clin. Pract. Oncol. 2, 115 (2005).
Till, B. G. et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 112, 2261–2271 (2008).
Morgan, R. A. et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006).
Lotem, M. et al. Presentation of tumor antigens by dendritic cells genetically modified with viral and nonviral vectors. J. Immunother. 29, 616–627 (2006).
Kalos, M. & June, C. H. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 39, 49–60 (2013).
Kuwana, Y. et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 149, 960–968 (1987).
Gross, G., Waks, T. & Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. 86, 10024–10028 (1989).
Kaiser, A. D. et al. Towards a commercial process for the manufacture of genetically modified T cells for therapy. Cancer Gene Ther. 22, 72–78 (2015).
Brudno, J. N. & Kochenderfer, J. N. Chimeric antigen receptor T-cell therapies for lymphoma. Nat. Rev. Clin. Oncol. 15, 31–46 (2018).
Garfall, A. L. et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. New Engl. J. Med. 373, 1040–1047 (2015).
Grupp, S. A. et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. New Engl. J. Med. 368, 1509–1518 (2013).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New Engl. J. Med. 377, 2531–2544 (2017).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra138 (2013).
Kershaw, M. H. et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 12, 6106–6115 (2006).
Lamers, C. H. J. et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 24, e20–e22 (2006).
Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. New Engl. J. Med. 375, 2561–2569 (2016).
Jameson, S. C., Hogquist, K. A. & Bevan, M. J. Positive selection of thymocytes. Annu. Rev. Immunol. 13, 93–126 (1995).
Miller, J. F. Immunological function of the thymus. Lancet 2, 748–749 (1961).
Goldrath, A. W. & Bevan, M. J. Selecting and maintaining a diverse T-cell repertoire. Nature 402, 255–262 (1999).
Sykulev, Y. et al. Kinetics and affinity of reactions between an antigen-specific T cell receptor and peptide-MHC complexes. Immunity 1, 15–22 (1994).
Correale, P. et al. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J. Natl. Cancer Inst. 89, 293–300 (1997).
Eberlein, T. J., Rosenstein, M. & Rosenberg, S. A. Regression of a disseminated syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin 2. J. Exp. Med. 156, 385–397 (1982).
Chang, A. E. et al. Phase II trial of autologous tumor vaccination, anti-CD3-activated vaccine-primed lymphocytes, and interleukin-2 in stage IV renal cell cancer. J. Clin. Oncol. 21, 884–890 (2003).
Wen, Y. J. et al. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood 99, 3280–3285 (2002).
Hayashi, T. et al. Ex vivo induction of multiple myeloma-specific cytotoxic T lymphocytes. Blood 102, 1435–1442 (2003).
Himoudi, N. et al. Lack of T-cell responses following autologous tumour lysate pulsed dendritic cell vaccination, in patients with relapsed osteosarcoma. Clin. Transl. Oncol. 14, 271–279 (2012).
Bercovici, N. et al. Analysis and characterization of antitumor T-cell response after administration of dendritic cells loaded with allogeneic tumor lysate to metastatic melanoma patients. J. Immunother. 31, 101–112 (2008).
Bachleitner-Hofmann, T. et al. Pilot trial of autologous dendritic cells loaded with tumor lysate(s) from allogeneic tumor cell lines in patients with metastatic medullary thyroid carcinoma. Oncol. Rep. 21, 1585–1592 (2009).
Jenq, R. R. & van den Brink, M. R. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat. Rev. Cancer 10, 213–221 (2010).
Zeiser, R. & Blazar, B. R. Acute graft-versus-host disease - biologic process, prevention, and therapy. New Engl. J. Med. 377, 2167–2179 (2017).
Ferrara, J. L., Levine, J. E., Reddy, P. & Holler, E. Graft-versus-host disease. Lancet 373, 1550–1561 (2009).
Ash, R. C. et al. Successful allogeneic transplantation of T-cell-depleted bone marrow from closely HLA-matched unrelated donors. New Engl. J. Med. 322, 485–494 (1990).
Wagner, J. E. et al. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet 366, 733–741 (2005).
Urbano-Ispizua, A. et al. Rapid engraftment without significant graft-versus-host disease after allogeneic transplantation of CD34+ selected cells from peripheral blood. Blood 89, 3967–3973 (1997).
Smith, K. A. & Ruscetti, F. W. T-cell growth factor and the culture of cloned functional T cells. Adv. Immunol. 31, 137–175 (1981).
Vose, B. M. Expansion of autorecognitive cytotoxic effectors in human cancer by T cell growth factor (Interleukin 2)1. Arch. Geschwulstforsch 51, 317–326 (1981).
Pittari, G. et al. Revving up natural killer cells and cytokine-induced killer cells against hematological malignancies. Front. Immunol. 6, 230 (2015).
Guo, Y. & Han, W. Cytokine-induced killer (CIK) cells: from basic research to clinical translation. Chin. J. Cancer 34, 99–107 (2015).
Cappuzzello, E., Sommaggio, R., Zanovello, P. & Rosato, A. Cytokines for the induction of antitumor effectors: the paradigm of cytokine-induced killer (CIK) cells. Cytokine Growth Factor Rev. 36, 99–105 (2017).
Rosenberg, S. A., Spiess, P. & Lafreniere, R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 233, 1318–1321 (1986).
Rosenberg, S. A. et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. New Engl. J. Med. 319, 1676–1680 (1988).
Shimizu, J. et al. Antigen-presenting cells constitutively bind tumor antigens in the tumor-bearing state in vivo to construct an effective immunogenic unit. Jpn. J. Cancer Res. 82, 262–265 (1991).
Boon, T. & van der Bruggen, P. Human tumor antigens recognized by T lymphocytes. J. Exp. Med. 183, 725–729 (1996).
Staveley-O’Carroll, K. et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc. Natl Acad. Sci. USA 95, 1178–1183 (1998).
Restifo, N. P. et al. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J. Natl. Cancer Inst. 88, 100–108 (1996).
Gardner, R. et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 127, 2406–2410 (2016).
Blankenstein, T., Coulie, P. G., Gilboa, E. & Jaffee, E. M. The determinants of tumour immunogenicity. Nat. Rev. Cancer 12, 307–313 (2012).
Murphy, A. et al. Gene modification strategies to induce tumor immunity. Immunity 22, 403–414 (2005).
Hock, R. A. & Miller, A. D. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature 320, 275–277 (1986).
Schaefer-Ridder, M., Wang, Y. & Hofschneider, P. H. Liposomes as gene carriers: efficient transformation of mouse L cells by thymidine kinase gene. Science 215, 166–168 (1982).
Toneguzzo, F. & Keating, A. Stable expression of selectable genes introduced into human hematopoietic stem cells by electric field-mediated DNA transfer. Proc. Natl Acad. Sci. USA 83, 3496–3499 (1986).
Chang, C. W. et al. Modeling human severe combined immunodeficiency and correction by CRISPR/Cas9-enhanced gene targeting. Cell Rep. 12, 1668–1677 (2015).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
CRISPR meets CAR T-cell therapy. Cancer Discov. 7, OF6 (2017).
Rapoport, A. P. et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 21, 914–921 (2015).
Singh, N. et al. T cells targeting NY-ESO-1 demonstrate efficacy against disseminated neuroblastoma. Oncoimmunology 5, e1040216 (2016).
Abramson, J. S. et al. Anti-CD19 CAR T cells in CNS diffuse large-B-cell lymphoma. New Engl. J. Med. 377, 783–784 (2017).
Johnson, L. A. & June, C. H. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 27, 38–58 (2017).
LeBien, T. W. & Tedder, T. F. B lymphocytes: how they develop and function. Blood 112, 1570–1580 (2008).
Brentjens, R. J. et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 9, 279–286 (2003).
Lim, W. A. & June, C. H. The principles of engineering immune cells to treat cancer. Cell 168, 724–740 (2017).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New Engl. J. Med. 368, 1509–1518 (2013).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
Porter, D. L. et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. New. Engl. J. Med. 365, 725–733 (2011).
June, C. H. & Sadalaine, M. Chimeric antigen receptor therapy. New Engl. J. Med. 379, 64–73 (2018).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. New Engl. J. Med. 378, 439–448 (2018).
Porter, D. L. Randomized, phase II dose optimization study of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed, refractory CLL. Blood. 124, 1982 (2014).
Schuster, S. J. et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. New Engl. J. Med. 377, 2545–2554 (2017).
Klausen, U. et al. Cancer immune therapy for lymphoid malignancies: recent advances. Semin. Immunopathol. 41, 111–124 (2018).
Abramson, J. S. et al. High durable CR rates and preliminary safety profile for JCAR017 in R/R aggressive B-NHL (TRANSCEND NHL 001 Study): a defined composition CD19-directed CAR T cell product with potential for outpatient administration. Biol. Blood Marrow Transplant. 24, S25 (2018).
Ramos, C. A., Heslop, H. E. & Brenner, M. K. CAR-T cell therapy for lymphoma. Annu. Rev. Med. 67, 165 (2016).
Barrett, D. M., Grupp, S. A. & June, C. H. Chimeric antigen receptor- and TCR-modified T cells enter Main Street and Wall Street. J. Immunol. 195, 755–761 (2015).
Ruella, M. et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 24, 1499–1503 (2018).
Hamieh, M. et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568, 112–116 (2019).
Fry, T. J. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20–28 (2018).
Haso, W. et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121, 1165–1174 (2013).
Fesnak, A. D., June, C. H. & Levine, B. L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 16, 566–581 (2016).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra225 (2014).
Budde, L. E. et al. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS ONE 8, e82742–e82742 (2013).
Zhang, W. et al. Treatment of CD20-directed chimeric antigen receptor-modified T cells in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an early phase IIa trial report. Signal Transduct. Target. Ther. 1, 16002 (2016).
Till, B. G. et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 119, 3940–3950 (2012).
Yao, W. et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin. Immunol. 155, 160–175 (2014).
Dai, H., Wang, Y., Lu, X. & Han, W. Chimeric antigen receptors modified T-cells for cancer therapy. J. Natl Cancer Inst. 108, djv439 (2016).
Nerreter, T. et al. Super-resolution microscopy reveals ultra-low CD19 expression on myeloma cells that triggers elimination by CD19 CAR-T. Nat. Commun. 10, 3137 (2019).
Darce, J. R., Arendt, B. K., Wu, X. & Jelinek, D. F. Regulated expression of BAFF-binding receptors during human B cell differentiation. J. Immunol. 179, 7276–7286 (2007).
Tai, Y. T. et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 123, 3128–3138 (2014).
Seckinger, A. et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell 31, 396–410 (2017).
Carpenter, R. O. et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin. Cancer Res. 19, 2048–2060 (2013).
O’Connor, B. P. et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199, 91–98 (2004).
Novak, A. J. et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood 103, 689–694 (2004).
Raje, N. S. et al. Phase 1 study of tabalumab, a human anti-B-cell activating factor antibody, and bortezomib in patients with relapsed/refractory multiple myeloma. Clin. Cancer Res. 22, 5688–5695 (2016).
Zhao, W. H. et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 11, 141 (2018).
Bernfield, M. et al. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell Biol. 8, 365 (1992).
O’Connell, F. P., Pinkus, J. L. & Pinkus, G. S. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. Am. J. Clin. Pathol. 121, 254–263 (2004).
Saar, G. & June, C. H. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunological Rev. 263, 68–89 (2015).
Manz, M. G., Toshihiro, M., Koichi, A. & Weissman, I. L. Prospective isolation of human clonogenic common myeloid progenitors. Proc. Natl Acad. Sci. USA 99, 11872–11877 (2002).
Michael, H. et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood 116, 4532–4541 (2010).
Sivasubramanian, B. et al. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clin. Cancer Res. 14, 396–404 (2008).
Daneshmanesh, A. H. et al. Ror1, a cell surface receptor tyrosine kinase is expressed in chronic lymphocytic leukemia and may serve as a putative target for therapy. Int. J. Cancer 123, 1190–1195 (2008).
Gentile, A. et al. Ror1 is a pseudokinase that is crucial for Met-driven tumorigenesis. Cancer Res. 71, 3132–3141 (2011).
Berger, C. et al. Safety of targeting ROR1 in primates with chimeric antigen receptor-modified T cells. Cancer Immunol. Res. 3, 206 (2015).
Juan, V. et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 108, 3890–3897 (2006).
Ramos, C. A. et al. Clinical responses in patients infused with T lymphocytes redirected to target kappa-light immunoglobulin chain. Biol. Blood Marrow Transplant. 20, S26 (2014).
Ramos, C. A. et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J. Clin. Investig. 127, 3462–3471 (2017).
Wang, C. M. et al. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory hodgkin lymphoma: an open-label phase I trial. Clin. Cancer Res. 23, 1156–1166 (2016).
Saar, G. et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood 123, 2343–2354 (2014).
Mardiros, A. et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood 122, 3138–3148 (2013).
Frankel, A. E. et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood 124, 385–392 (2014).
Pizzitola, I. et al. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia 28, 1596–1605 (2014).
Quan-Shun, W. et al. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol. Ther. 23, 184–191 (2015).
Ritchie, D. S. et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol. Ther. 21, 2122–2129 (2013).
Ping, Y., Liu, C. & Zhang, Y. T-cell receptor-engineered T cells for cancer treatment: current status and future directions. Protein Cell 9, 254–266 (2018).
Liu, M. & Guo, F. Recent updates on cancer immunotherapy. Precis. Clin. Med. 1, 65–74 (2018).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62 (2015).
Harris, D. T. & Kranz, D. M. Adoptive T cell therapies: a comparison of T cell receptors and chimeric antigen receptors. Trends Pharmacol. Sci. 37, 220–230 (2016).
Johnson, L. A. et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–546 (2009).
Parkhurst, M. R. et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 19, 620–626 (2011).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 (2011).
Robbins, P. F. et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin. Cancer Res. 21, 1019 (2015).
Ikeda, H. T-cell adoptive immunotherapy using tumor-infiltrating T cells and genetically engineered TCR-T cells. Int. Immunol. 28, 349–353 (2016).
Kohm, A. P., Turley, D. M. & Miller, S. D. Targeting the TCR: T-cell receptor and peptide-specific tolerance-based strategies for restoring self-tolerance in CNS autoimmune disease. Int. Rev. Immunol. 24, 361–392 (2005).
Hammerl, D. et al. Adoptive T cell therapy: new avenues leading to safe targets and powerful allies. Trends Immunol. 39, 921–936 (2018).
San Miguel, J. F., Paiva, B. & Lasarte, J. J. Engineering anti-myeloma responses using affinity-enhanced TCR-engineered T cells. Cancer Cell 28, 281–283 (2015).
Kunert, A. et al. T-cell receptors for clinical therapy: in vitro assessment of toxicity risk. Clin. Cancer Res. 23, 6012–6020 (2017).
Zhang, L. & Morgan, R. A. Genetic engineering with T cell receptors. Adv. Drug Deliv. Rev. 64, 756–762 (2012).
Linette, G. P. et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122, 863–871 (2013).
Thakur, A., Huang, M. & Lum, L. G. Bispecific antibody based therapeutics: Strengths and challenges. Blood Rev. 32, 339–347 (2018).
Choi, B. D. et al. Bispecific antibodies engage T cells for antitumor immunotherapy. Expert Opin. Biol. Ther. 11, 843–853 (2011).
Vasekar, M. et al. Novel immunotherapies for hematological malignancies. Curr. Mol. Pharmacol. 9, 264–271 (2016).
Velasquez, M. P. & Bonifant, C. L. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 131, 30–38 (2018).
LoFfler, A. et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 95, 2098–2103 (2000).
Dreier, T. et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int. J. Cancer 100, 690–697 (2002).
Viardot, A. & Bargou, R. Bispecific antibodies in haematological malignancies. Cancer Treat. Rev. 65, 87–95 (2018).
Batlevi, C. L., Matsuki, E., Brentjens, R. J. & Younes, A. Novel immunotherapies in lymphoid malignancies. Nat. Rev. Clin. Oncol. 13, 25–40 (2016).
Teachey, D. T. et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 6, 664–679 (2016).
Topp, M. S. et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J. Clin. Oncol. 32, 4134 (2014).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
Matthias, K. et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood 119, 6226–6233 (2012).
Topp, M. S. et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood 120, 5185–5187 (2012).
Hipp, S. et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 31, 1743–1751 (2017).
Moore, G. L. et al. Tuning T cell affinity improves efficacy and safety of Anti-CD38 × Anti-CD3 bispecific antibodies in monkeys - a potential therapy for multiple myeloma. Blood. 126, 1798 (2015).
Bhutani, D. & Lum, L. G. Activated T cells armed with bispecific antibodies kill tumor targets. Curr. Opin. Hematol. 22, 476–483 (2015).
Fang, F., Xiao, W. & Tian, Z. NK cell-based immunotherapy for cancer. Semin. Immunol. 31, S1044532317300325 (2017).
Zhang, J., Zheng, H. & Diao, Y. Natural killer cells and current applications of chimeric antigen receptor-modified NK-92 cells in tumor immunotherapy. Int. J. Mol. Sci. 20, E317 (2019).
Vivier, E. et al. Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49 (2011).
Vivier, E. et al. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 12, 239–252 (2012).
Chu, Y., Gardenswartz, A., Termuhlen, A. M. & Cairo, M. S. Advances in cellular and humoral immunotherapy - implications for the treatment of poor risk childhood, adolescent, and young adult B-cell non-Hodgkin lymphoma. Br. J. Haematol. 185, 1055–1070 (2019).
Mehta, R. S. & Rezvani, K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front. Immunol. 9, 283 (2018).
Hu, Y., Tian, Z. G. & Zhang, C. Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol. Sin. 39, 167–176 (2018).
Ames, E. & Murphy, W. J. Advantages and clinical applications of natural killer cells in cancer immunotherapy. Cancer Immunol. Immunother. 63, 21–28 (2014).
Crosby, C. M. & Kronenberg, M. Tissue-specific functions of invariant natural killer T cells. Nat. Rev. Immunol. 18, 559–574 (2018).
Smyth, M. J. et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191, 661–668 (2000).
Nowak, M. et al. Defective NKT cell activation by CD1d+ TRAMP prostate tumor cells is corrected by interleukin-12 with α-galactosylceramide. PLoS ONE 5, e11311 (2012).
Tian, G. et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J. Clin. Invest. 126, 2341–2355 (2016).
Kershaw, M. H., Westwood, J. A. & Darcy, P. K. Gene-engineered T cells for cancer therapy. Nat. Rev. Cancer 13, 525–541 (2013).
Wei, G. et al. Advances of CD19-directed chimeric antigen receptor-modified T cells in refractory/relapsed acute lymphoblastic leukemia. Exp. Hematol. Oncol. 6, 10 (2017).
Cao, J. et al. Potent anti-leukemia activities of humanized CD19-targeted Chimeric antigen receptor T (CAR-T) cells in patients with relapsed/refractory acute lymphoblastic leukemia. Am. J. Hematol. 93, 851–858 (2018).
Fraietta, J. A. et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 24, 563–571 (2018).
Sadelain, M., Riviere, I. & Riddell, S. Therapeutic T cell engineering. Nature 545, 423–431 (2017).
Zhang, C. et al. Phase I escalating-dose trial of CAR-T therapy targeting CEA(+) metastatic colorectal cancers. Mol. Ther. 25, 1248–1258 (2017).
Wang, L. et al. Efficient tumor regression by adoptively transferred CEA-specific CAR-T cells associated with symptoms of mild cytokine release syndrome. Oncoimmunology 5, e1211218 (2016).
Schuberth, P. C. et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J. Transl. Med. 11, 187 (2013).
Chen, Y. et al. Eradication of neuroblastoma by T cells redirected with an optimized GD2-specific chimeric antigen receptor and interleukin-15. Clin. Cancer Res. 25, 2915–2924 (2019).
Golinelli, G. et al. Targeting GD2-positive glioblastoma by chimeric antigen receptor empowered mesenchymal progenitors. Cancer Gene Ther. https://doi.org/10.1038/s41417-018-0062-x (2018).
Yu, J. et al. Anti-GD2/4-1BB chimeric antigen receptor T cell therapy for the treatment of Chinese melanoma patients. J. Hematol. Oncol. 11, 1 (2018).
Stroncek, D. F. et al. Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells. Cytotherapy 18, 893–901 (2016).
Ahmed, N. et al. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 33, 1688–1696 (2015).
Hassan, R. et al. Mesothelin immunotherapy for cancer: ready for prime time? J. Clin. Oncol. 34, 4171–4179 (2016).
Krenciute, G. et al. Characterization and functional analysis of scFv-based chimeric antigen receptors to redirect T cells to IL13Ralpha2-positive glioma. Mol. Ther. 24, 354–363 (2016).
Whilding, L. M. et al. Targeting of aberrant alphavbeta6 integrin expression in solid tumors using chimeric antigen receptor-engineered T Cells. Mol. Ther. 25, 2427 (2017).
Liu, H. et al. Targeting alpha-fetoprotein (AFP)-MHC complex with CAR T-cell therapy for liver cancer. Clin. Cancer Res. 23, 478–488 (2017).
Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 14, 1264–1270 (2008).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Ahmed, N. et al. HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol. 3, 1094–1101 (2017).
Feng, K. et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci. China Life Sci. 59, 468–479 (2016).
Katz, S. C. et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin. Cancer Res. 21, 3149–3159 (2015).
Zhang, B. L. et al. Hurdles of CAR-T cell-based cancer immunotherapy directed against solid tumors. Sci. China Life Sci. 59, 340–348 (2016).
Craddock, J. A. et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 33, 780–788 (2010).
Moon, E. K. et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 17, 4719–4730 (2011).
Di Stasi, A. et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood 113, 6392–6402 (2009).
Adusumilli, P. S. et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 6, 261ra151 (2014).
Louveau, A., Harris, T. H. & Kipnis, J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 36, 569–577 (2015).
Choi, B. D. et al. Intracerebral delivery of a third generation EGFRvIII-specific chimeric antigen receptor is efficacious against human glioma. J. Clin. Neurosci. 21, 189–190 (2014).
Han, J. et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci. Rep. 5, 11483 (2015).
Priceman, S. J. et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2(+) breast cancer metastasis to the brain. Clin. Cancer Res. 24, 95–105 (2018).
Biswas, S. K. & Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11, 889–896 (2010).
Liyanage, U. K. et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 169, 2756–2761 (2002).
Schmid, M. C. et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell 19, 715–727 (2011).
Swartz, M. A. et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 72, 2473–2480 (2012).
Lindau, D. et al. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138, 105–115 (2013).
Koehler, H., Kofler, D., Hombach, A. & Abken, H. CD28 costimulation overcomes transforming growth factor-beta-mediated repression of proliferation of redirected human CD4+ and CD8+ T cells in an antitumor cell attack. Cancer Res. 67, 2265–2273 (2007).
Lee, J. C. et al. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 71, 2871–2881 (2011).
Loskog, A. et al. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia 20, 1819–1828 (2006).
Chmielewski, M. & Abken, H. CAR T cells releasing IL-18 convert to T-Bet(high) FoxO1(low) effectors that exhibit augmented activity against advanced solid tumors. Cell Rep. 21, 3205–3219 (2017).
Long, A. H. et al. Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunol. Res. 4, 869–880 (2016).
Noman, M. Z. et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 211, 781–790 (2014).
Katz, S. C. et al. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther. 23, 142–148 (2016).
Rabinovich, G. A., Gabrilovich, D. & Sotomayor, E. M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25, 267–296 (2007).
Huang, C. Y. et al. Transforming growth factor beta is a poor prognostic factor and inhibits the favorable prognostic value of CD8+ CTL in human hepatocellular carcinoma. J. Immunother. 40, 175–186 (2017).
Kloss, C. C. et al. Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T Cell proliferation and augments prostate cancer eradication. Mol. Ther. 26, 1855–1866 (2018).
Chmielewski, M. & Abken, H. TRUCKs: the fourth generation of CARs. Expert Opin. Biol. Ther. 15, 1145–1154 (2015).
Chmielewski, M., Hombach, A. A. & Abken, H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol. Rev. 257, 83–90 (2014).
Chmielewski, M. & Abken, H. CAR T cells transform to trucks: chimeric antigen receptor-redirected T cells engineered to deliver inducible IL-12 modulate the tumour stroma to combat cancer. Cancer Immunol. Immunother. 61, 1269–1277 (2012).
Curtsinger, J. M., Lins, D. C. & Mescher, M. F. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 197, 1141–1151 (2003).
Nishio, N. & Dotti, G. Oncolytic virus expressing RANTES and IL-15 enhances function of CAR-modified T cells in solid tumors. Oncoimmunology 4, e988098 (2015).
Wang, W. et al. Specificity redirection by CAR with human VEGFR-1 affinity endows T lymphocytes with tumor-killing ability and anti-angiogenic potency. Gene Ther. 20, 970–978 (2013).
Leen, A. M. et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol. Ther. 22, 1211–1220 (2014).
Papa, S., van Schalkwyk, M. & Maher, J. Clinical evaluation of ErbB-targeted CAR T-cells, following intracavity delivery in patients with ErbB-expressing solid tumors. Methods Mol. Biol. 1317, 365–382 (2015).
Markley, J. C. & Sadelain, M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood 115, 3508–3519 (2010).
Xu, Y. et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123, 3750–3759 (2014).
Koneru, M. et al. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors. Oncoimmunology 4, e994446 (2015).
Chinnasamy, D. et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin. Cancer Res. 18, 1672–1683 (2012).
Burkhardt, U. E. et al. IL-15 augments antitumoral activity of an ErbB2/HER2 cancer vaccine targeted to professional antigen-presenting cells. Cancer Immunol. Immunother. 61, 1473–1484 (2012).
Singh, H. et al. Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Cancer Res. 71, 3516–3527 (2011).
Chong, E. A. et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood 129, 1039–1041 (2017).
Cherkassky, L. et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Investig. 126, 3130–3144 (2016).
Ren, J. et al. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 8, 17002–17011 (2017).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl. J. Med. 366, 2443–2454 (2012).
John, L. B. et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin. Cancer Res. 19, 5636–5646 (2013).
Rafiq, S. et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 36, 847–856 (2018).
Lamers, C. H. et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol. Ther. 21, 904–912 (2013).
Gong, M. C. et al. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia 1, 123–127 (1999).
Jackson, H. J., Rafiq, S. & Brentjens, R. J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 13, 370–383 (2016).
Gross, G. & Eshhar, Z. Endowing T cells with antibody specificity using chimeric T cell receptors. FASEB J. 6, 3370–3378 (1992).
Eshhar, Z., Waks, T., Gross, G. & Schindler, D. G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl Acad. Sci. USA 90, 720–724 (1993).
De Genst, E., Saerens, D., Muyldermans, S. & Conrath, K. Antibody repertoire development in camelids. Dev. Comp. Immunol. 30, 187–198 (2006).
Xie, Y. J. et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc. Natl Acad. Sci. USA 116, 7624–7631 (2019).
Hassani, M. et al. Construction of a chimeric antigen receptor bearing a nanobody against prostate a specific membrane antigen in prostate cancer. J. Cell. Biochem. 120, 10787–10795 (2019).
Caruso, H. G. et al. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 75, 3505–3518 (2015).
Liu, X. et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 75, 3596–3607 (2015).
Held, G. et al. MHC/peptide-specific interaction of the humoral immune system: a new category of antibodies. J. Immunol. 195, 4210–4217 (2015).
Ellebrecht, C. T. et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353, 179 (2016).
Krebs, S. et al. T cells redirected to interleukin-13Rα2 with interleukin-13 mutein–chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1. Cytotherapy 16, 1121–1131 (2014).
Kawakami, Y. et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J. Immunol. 154, 3961–3968 (1995).
Xia, Y. et al. Treatment of metastatic non-small cell lung cancer with NY-ESO-1 specific TCR engineered-T cells in a phase I clinical trial: a case report. Oncol. Lett. 16, 6998–7007 (2018).
Vujanovic, L. et al. Molecular mimicry of MAGE-A6 and Mycoplasma penetrans HF-2 epitopes in the induction of antitumor CD8(+) T-cell responses. Oncoimmunology 3, e954501 (2014).
Tawara, I. et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood 130, 1985–1994 (2017).
Dudley, M. E. et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 26, 5233–5239 (2008).
Cameron, B. J. et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 5, 197ra103 (2013).
Thaxton, J. E. & Li, Z. To affinity and beyond: harnessing the T cell receptor for cancer immunotherapy. Hum. Vaccin. Immunother. 10, 3313–3321 (2014).
Jensen, M. C. et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transplant. 16, 1245–1256 (2010).
Bertram, E. M., Dawicki, W. & Watts, T. H. Role of T cell costimulation in anti-viral immunity. Semin. Immunol. 16, 185–196 (2004).
Croft, M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 14, 265–273 (2003).
Ramos, C. A. & Dotti, G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin. Biol. Ther. 11, 855–873 (2011).
Kowolik, C. M. et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 66, 10995–11004 (2006).
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
Zhang, J. P. et al. Sequential allogeneic and autologous CAR-T-cell therapy to treat an immune-compromised leukemic patient. Blood Adv. 2, 1691–1695 (2018).
Hombach, A. A. et al. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology 1, 458–466 (2012).
Shen, C. J. et al. Chimeric antigen receptor containing ICOS signaling domain mediates specific and efficient antitumor effect of T cells against EGFRvIII expressing glioma. J. Hematol. Oncol. 6, 33 (2013).
Sallman, D. A. et al. NKG2D-based chimeric antigen receptor therapy induced remission in a relapsed/refractory acute myeloid leukemia patient. Haematologica 103, e424–e426 (2018).
Nair, S. et al. Functional improvement of chimeric antigen receptor through intrinsic interleukin-15Ralpha signaling. Curr. Gene Ther. 19, 40–53 (2018).
Mata, M. et al. Inducible activation of MyD88 and CD40 in CAR T cells results in controllable and potent antitumor activity in preclinical solid tumor models. Cancer Discov. 7, 1306–1319 (2017).
Lai, Y. et al. Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T cells. Leukemia 32, 801–808 (2018).
Golumba-Nagy, V., Kuehle, J., Hombach, A. A. & Abken, H. CD28-zeta CAR T cells resist TGF-beta repression through IL-2 signaling, which can be mimicked by an engineered IL-7 autocrine loop. Mol. Ther. 26, 2218–2230 (2018).
Zolov, S. N., Rietberg, S. P. & Bonifant, C. L. Programmed cell death protein 1 activation preferentially inhibits CD28.CAR-T cells. Cytotherapy 20, 1259–1266 (2018).
Kochenderfer, J. N. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012).
van der Stegen, S. J. C., Hamieh, M. & Sadelain, M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 14, 499–509 (2015).
Savoldo, B. et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Investig. 121, 1822–1826 (2011).
Cheng, Z. et al. In vivo expansion and antitumor activity of coinfused CD28- and 4-1BB-engineered CAR-T cells in patients with B cell leukemia. Mol. Ther. 26, 976–985 (2018).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. New Engl. J. Med. 371, 1507–1517 (2014).
Diehn, M. et al. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc. Natl Acad. Sci. USA 99, 11796–11801 (2002).
Smeets, R. L. et al. Molecular pathway profiling of T lymphocyte signal transduction pathways; Th1 and Th2 genomic fingerprints are defined by TCR and CD28-mediated signaling. BMC Immunol. 13, 12 (2012).
Tang, X. Y. et al. Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: a non-randomised, open-label phase I trial protocol. BMJ Open 6, e013904 (2016).
Quintarelli, C. et al. Choice of costimulatory domains and of cytokines determines CAR T-cell activity in neuroblastoma. Oncoimmunology 7, e1433518 (2018).
Yeku, O. O. & Brentjens, R. J. Armored CAR T-cells: utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochemical Soc. Trans. 44, 412–418 (2016).
Chmielewski, M., Kopecky, C., Hombach, A. A. & Abken, H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 71, 5697–5706 (2011).
Priceman, S. J. et al. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. Oncoimmunology 7, e1380764 (2018).
Salter, A. I. et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci. Signal. 11, eaat6753 (2018).
Wang, J. et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J. Hematol. Oncol. 11, 7 (2018).
Kloss, C. C. et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 31, 71–75 (2013).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Maher, J. et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002).
Imai, C. et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 18, 676–684 (2004).
Hombach, A. et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J. Immunol. 167, 6123–6131 (2001).
Haynes, N. M. et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood 100, 3155–3163 (2002).
Li, S. et al. CD33-specific chimeric antigen receptor T cells with different co-stimulators showed potent anti-leukemia efficacy and different phenotype. Hum. Gene Ther. 29, 626–639 (2018).
Zhao, Z. et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28, 415–428 (2015).
Li, G. et al. 4-1BB enhancement of CAR T function requires NF-kappaB and TRAFs. JCI Insight. 3, 121322 (2018).
Frigault, M. J. et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol. Res. 3, 356–367 (2015).
Jang, I. K. et al. Human 4-1BB (CD137) signals are mediated by TRAF2 and activate nuclear factor-kappa B. Biochem. Biophys. Res. Commun. 242, 613–620 (1998).
Rothe, M. et al. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 83, 1243–1252 (1995).
Chu, Z. L. et al. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc. Natl Acad. Sci. USA 94, 10057–10062 (1997).
Cannons, J. L. et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol. 167, 1313–1324 (2001).
Zhong, X. S. et al. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol. Ther. 18, 413–420 (2010).
Carpenito, C. et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl Acad. Sci. 106, 3360–3365 (2009).
Ramos, C. A. et al. In vivo fate and activity of second- versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin’s lymphomas. Mol. Ther. 26, 2727–2737 (2018).
Haso, W. et al. Anti-CD22–chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121, 1165–1174 (2013).
Finney, H. M., Akbar, A. N. & Lawson, A. D. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J. Immunol. 172, 104–113 (2004).
Guedan, S. et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood 124, 1070–1080 (2014).
Fos, C. et al. ICOS ligation recruits the p50alpha PI3K regulatory subunit to the immunological synapse. J. Immunol. 181, 1969–1977 (2008).
Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia; chimeric antigen receptor-modified T cells for acute lymphoid leukemia; chimeric antigen receptor T cells for sustained remissions in leukemia. New Engl. J. Med. 374, 998 (2016).
Milone, M. C. et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 17, 1453–1464 (2009).
Song, D. G. et al. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood 119, 696–706 (2012).
Song, D. G. et al. Chimeric NKG2D CAR-expressing T cell-mediated attack of human ovarian cancer is enhanced by histone deacetylase inhibition. Hum. Gene Ther. 24, 295–305 (2013).
Lehner, M. et al. Redirecting T cells to Ewing’s sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PLoS ONE 7, e31210 (2012).
Zhang, T., Barber, A. & Sentman, C. L. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res. 66, 5927–5933 (2006).
Barber, A. et al. Chimeric NKG2D receptor-bearing T cells as immunotherapy for ovarian cancer. Cancer Res. 67, 5003–5008 (2007).
Barber, A., Zhang, T. & Sentman, C. L. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J. Immunol. 180, 72–78 (2008).
Chandran, S. S. et al. Persistence of CTL clones targeting melanocyte differentiation antigens was insufficient to mediate significant melanoma regression in humans. Clin. Cancer Res. 21, 534–543 (2015).
Miyao, K. et al. Introduction of genetically modified CD3zeta improves proliferation and persistence of antigen-specific CTLs. Cancer Immunol. Res. 6, 733–744 (2018).
Garcia, K. C. & Adams, E. J. How the T cell receptor sees antigen–a structural view. Cell 122, 333–336 (2005).
Rudolph, M. G., Stanfield, R. L. & Wilson, I. A. How TCRs bind MHCs, peptides, and coreceptors. Annu. Rev. Immunol. 24, 419–466 (2006).
Bendle, G. M. et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 16, 565–570 (2010).
van Loenen, M. M. et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc. Natl Acad. Sci. USA 107, 10972–10977 (2010).
Voss, R. H. et al. Coexpression of the T-cell receptor constant alpha domain triggers tumor reactivity of single-chain TCR-transduced human T cells. Blood 115, 5154–5163 (2010).
Cohen, C. J. et al. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 67, 3898–3903 (2007).
Shilyansky, J. et al. Identification of a T-cell receptor from a therapeutic murine T-cell clone. J. Immunother. 20, 247–255 (1997).
Legut, M. et al. CRISPR-mediated TCR replacement generates superior anticancer transgenic T cells. Blood 131, 311–322 (2018).
Ochi, T. et al. Novel adoptive T-cell immunotherapy using a WT1-specific TCR vector encoding silencers for endogenous TCRs shows marked antileukemia reactivity and safety. Blood 118, 1495–1503 (2011).
Davenport, A. J. et al. CAR-T cells inflict sequential killing of multiple tumor target cells. Cancer Immunol. Res. 3, 483–494 (2015).
Torikai, H. et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119, 5697–5705 (2012).
Universal, C. A. R. T cells treat leukemia. Cancer Discov. 7, 342 (2017).
Qasim, W. et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 9, eaaj2013 (2017).
Ren, J. et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 23, 2255 (2017).
Yang, Y., Jacoby, E. & Fry, T. J. Challenges and opportunities of allogeneic donor-derived CAR T cells. Curr. Opin. Hematol. 22, 509–515 (2015).
Newick, K., O’Brien, S., Moon, E. & Albelda, S. M. CAR T cell therapy for solid tumors. Annu. Rev. Med. 68, 139–152 (2017).
Smith, T. T. et al. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J. Clin. Investig. 127, 2176–2191 (2017).
Jiang, L. & Wang, W. Genetically modified immune cells for cancer immunotherapy. Sci. China Life Sci. 61, 1277–1279 (2018).
Wei, J. & Han, W. CART trials are going ahead. Sci. China Life Sci. 60, 1276–1279 (2017).
Li, D. & Wang, W. Booming cancer immunotherapy fighting tumors. Sci. China Life Sci. 60, 1445–1449 (2017).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Wang, D. & DuBois, R. N. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 36, 1085–1093 (2015).
Gattinoni, L. et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 115, 1616–1626 (2005).
Hinrichs, C. S. et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl Acad. Sci. USA 106, 17469–17474 (2009).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Turtle, C. J. et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Investig. 126, 2123–2138 (2016).
Radvanyi, L. G. et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 18, 6758–6770 (2012).
Lee, D. W. et al. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin. Cancer Res. 18, 2780–2790 (2012).
Huang, Y. et al. Interleukin-armed chimeric antigen receptor-modified T cells for cancer immunotherapy. Gene Ther. 25, 192–197 (2018).
Butler, M. O. & Hirano, N. Human cell-based artificial antigen-presenting cells for cancer immunotherapy. Immunol. Rev. 257, 191–209 (2014).
Altvater, B. et al. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin. Cancer Res. 15, 4857–4866 (2009).
Song, D. G. et al. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB). Cancer Res. 71, 4617–4627 (2011).
Chang, Z. L. et al. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat. Chem. Biol. 14, 317–324 (2018).
Porter, D. L. et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 7, 303ra139 (2015).
Kochenderfer, J. N. et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 33, 540–549 (2015).
Oh, H. M., Park, J. H., Song, D. H. & Chung, M. E. Efficacy and safety of a new botulinum toxin type A free of complexing proteins. Toxins (Basel) 8, E4 (2015).
Bhoj, V. G. et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 128, 360–370 (2016).
Shalabi, H. et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica 103, e215–e218 (2018).
Yu, H. et al. Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma. Am. J. Hematol. 92, E11–E13 (2017).
Jackson, H. J. & Brentjens, R. J. Overcoming antigen escape with CAR T-cell therapy. Cancer Discov. 5, 1238–1240 (2015).
Adachi, K. et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 36, 346–351 (2018).
Lamers, C. H. J. et al. Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol. Immun. 56, 1875–1883 (2007).
Brentjens, R. J. et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118, 4817–4828 (2011).
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 (2011).
Beatty, G. L. et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce antitumor activity in solid malignancies. Cancer Immunol. Res. 2, 112–120 (2014).
Sanchez-Martinez, D. et al. Fratricide-resistant CD1a-specific CAR T-cells for the treatment of cortical T-cell acute lymphoblastic leukemia. Blood 133, 2291–2304 (2019).
Russo, V. et al. A dual role for genetically modified lymphocytes in cancer immunotherapy. Trends Mol. Med. 18, 193–200 (2012).
Bole-Richard, E., Deschamps, M., Ferrand, C. & Robinet, E. Improving the safety of cell therapy products by suicide gene transfer. Front. Pharmacol. 6, 174 (2015).
Straathof, K. C. et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 105, 4247–4254 (2005).
Wang, X. L. et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118, 1255–1263 (2011).
Wu, C. Y. et al. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 350, aab4077 (2015).
Fedorov, V. D., Themeli, M. & Sadelain, M. PD-1-and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 5, 215ra172 (2013).
Wilkie, S. et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J. Clin. Immunol. 32, 1059–1070 (2012).
Song, D. G. et al. A fully human chimeric antigen receptor with potent activity against cancer cells but reduced risk for off-tumor toxicity. Oncotarget 6, 21533–21546 (2015).
Abboud, R. et al. Severe cytokine-release syndrome after T cell-replete peripheral blood haploidentical donor transplantation is associated with poor survival and anti-IL-6 therapy is safe and well tolerated. Biol. Blood Marrow Transplant. 22, 1851–1860 (2016).
Shimabukuro-Vornhagen, A. et al. Cytokine release syndrome. J. Immunother. Cancer 6, 56 (2018).
Sachdeva, M. et al. Granulocyte-macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators. J. Biol. Chem. 294, 5430–5437 (2019).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra25 (2014).
Maude, S. L. et al. Efficacy and safety of CTL019 in the first US phase II multicenter trial in pediatric relapsed/refractory acute lymphoblastic leukemia: results of an interim analysis. Blood. 128, 2801 (2016).
Park, J. H. et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. New Engl. J. Med. 378, 449–459 (2018).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Hunter, B. D. & Jacobson, C. A. CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djz017 (2019).
Shalabi, H. et al. Systematic evaluation of neurotoxicity in children and young adults undergoing CD22 chimeric antigen receptor T-cell therapy. J. Immunother. 41, 350–358 (2018).
Karschnia, P. et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T-cells. Blood 133, 2212–2221 (2019).
Gust, J. et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 (2017).
Mackall, C. L. & Miklos, D. B. CNS endothelial cell activation emerges as a driver of CAR T cell-associated neurotoxicity. Cancer Discov. 7, 1371–1373 (2017).
Taraseviciute, A. et al. Chimeric antigen receptor T cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 8, 750–763 (2018).
Gust, J., Taraseviciute, A. & Turtle, C. J. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs 32, 1091–1101 (2018).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018).
Harris, D. T. et al. Comparison of T cell activities mediated by human TCRs and CARs that use the same recognition domains. J. Immunol. 200, 1088–1100 (2018).
Morgan, R. A. et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 36, 133–151 (2013).
Chapuis, A. G. et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med. 25, 1064–1072 (2019).
Beatty, G. L. et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 155, 29–32 (2018).
Tchou, J. et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol. Res. 5, 1152–1161 (2017).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
Thistlethwaite, F. C. et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 66, 1425–1436 (2017).
Louis, C. U. et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011).
Wang, Y. et al. CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. Oncoimmunology 7, e1440169 (2018).
Sahin, U. & Tureci, O. Personalized vaccines for cancer immunotherapy. Science 359, 1355–1360 (2018).
Haga-Friedman, A., Horovitz-Fried, M. & Cohen, C. J. Incorporation of transmembrane hydrophobic mutations in the TCR enhance its surface expression and T cell functional avidity. J. Immunol. 188, 5538–5546 (2012).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFC1303403), the National Natural and Scientific Foundation of China (81572981/H1611 and 81672397/H1617), the National High-Tech R&D Program (863 Program; 2014AA020704), and the Science and Technology Major Project of Sichuan Province of China (2017SZDZX0012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
W.W. is a scientific co-founder of Cygenpeutics and CarEne and holds equity in both companies. Z.-H.L. is a co-founder of CARsgen Therapeutics and holds equity in the company. The other authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, D., Li, X., Zhou, WL. et al. Genetically engineered T cells for cancer immunotherapy. Sig Transduct Target Ther 4, 35 (2019). https://doi.org/10.1038/s41392-019-0070-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-019-0070-9
This article is cited by
-
High-throughput cell optoporation system based on Au nanoparticle layers mediated by resonant irradiation for precise and controllable gene delivery
Scientific Reports (2024)
-
Revolutionizing cancer treatment: comprehensive insights into immunotherapeutic strategies
Medical Oncology (2024)
-
Distinct functions of CAR-T cells possessing a dectin-1 intracellular signaling domain
Gene Therapy (2023)
-
G9a/GLP inhibition during ex vivo lymphocyte expansion increases in vivo cytotoxicity of engineered T cells against hepatocellular carcinoma
Nature Communications (2023)
-
Responsive biomaterials: optimizing control of cancer immunotherapy
Nature Reviews Materials (2023)