Abstract
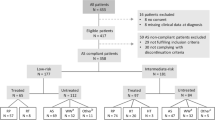
Recently, the use of targeted biopsy has been subject to critics, as it has been speculated that targeted biopsy might lead to overdiagnosis of clinically significant prostate cancer (PCa). In this study, we tried to evaluate whether targeted sampling in patients with organ-confined disease and ISUP 2 disease was associated with downgrading of the prostatectomy specimen, hence, leading to an unnecessary treatment, in terms of radical surgery. We relied on a prospectively-maintained multi-institutional database and identified 1293 patients with ISUP 2 disease on targeted biopsy only. Median (IQR) patients’ age at diagnosis was 65 (60, 70) years. Median PSA was 6.8 (5.0, 9.6) ng/ml. Overall, only 33 (2.6%) patients presented downgrading on their RP specimens. Patients who experienced downgrading were biopsied more frequently trans-rectally, had a lower total tumor length in mm and lower percentage of maximum core involvement and lower rates of cancer on systematic biopsy (all p ≤ 0.03). The strongest factors associated with reduced risk of downgrading were total tumor length, in mm, (OR: 0.71, 95% CI: 0.62,0.82, p < 0.001) and transperineal biopsy route (OR: 0.38, 95% CI: 0.14,1.00, p = 0.05).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
Data are available for bona fide researchers upon request.
Change history
20 January 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41391-022-00637-z
References
EAU Prostate Cancer Guidelines. 2020.
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–77.
Bass EJ, Pantovic A, Connor MJ, Loeb S, Rastinehad AR, Winkler M, et al. Diagnostic accuracy of magnetic resonance imaging targeted biopsy techniques compared to transrectal ultrasound-guided biopsy of the prostate: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2022;25:174–9.
Bass EJ, Pantovic A, Connor M, Gabe R, Padhani AR, Rockall A, et al. A systematic review and meta-analysis of the diagnostic accuracy of biparametric prostate MRI for prostate cancer in men at risk. Prostate Cancer Prostatic Dis. 2021;24:596–611.
van den Bergh RCN, Rouvière O, van der Kwast T, Briers E, Van den Broeck T, Cornford P, et al. Re: Andrew Vickers, Sigrid V. Carlsson, Matthew Cooperberg. Routine Use of Magnetic Resonance Imaging for Early Detection of Prostate Cancer Is Not Justified by the Clinical Trial Evidence. Eur Urol 2020;78:304–6: Prebiopsy MRI: Through the Looking Glass. Eur Urol. 2020;78:310–3.
Vickers A, Carlsson SV, Cooperberg M. Routine use of magnetic resonance imaging for early detection of prostate cancer is not justified by the clinical trial evidence. Eur Urol. 2020;78:304–6.
Enikeev D, Morozov A, Taratkin M, Barret E, Kozlov V, Singla N, et al. Active surveillance for intermediate-risk prostate cancer: systematic review and meta-analysis of current protocols and outcomes. Clin Genitourin Cancer. 2020;18:e739–e53.
Egevad L, Delahunt B, Srigley JR, Samaratunga H. International Society of Urological Pathology (ISUP) grading of prostate cancer—an ISUP consensus on contemporary grading. APMIS. 2016;124:433–5.
Spratt DE, Zhang J, Santiago-Jimenez M, Dess RT, Davis JW, Den RB, et al. Development and validation of a novel integrated clinical-genomic risk group classification for localized prostate cancer. J Clin Oncol. 2018;36:581–90.
Freedland SJ, Kane CJ, Amling CL, Aronson WJ, Terris MK, Presti JC Jr, et al. Upgrading and downgrading of prostate needle biopsy specimens: risk factors and clinical implications. Urology. 2007;69:495–9.
van Leenders G, van der Kwast TH, Grignon DJ, Evans AJ, Kristiansen G, Kweldam CF, et al. The 2019 International Society of Urological Pathology (ISUP) consensus conference on grading of prostatic carcinoma. Am J Surg Pathol. 2020;44:e87–e99.
Author information
Authors and Affiliations
Consortia
Contributions
Study concept and design: GP, AM. Acquisition of data: All authors. Analysis and interpretation of data: AM, GP. Drafting of the manuscript: AM, GP. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: AM. Supervision: GP.
Corresponding author
Ethics declarations
Competing interests
AM and GP own equities of Oltre Medical Consulting, Toulouse, France. The other authors have no conflicts.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In Table 1 of this article, a line was missing in the legend. It should have read ‘pT Stage is coded as follows: 0 = pT2, 1 = pT3a, 2 = pT3b and 3 = pT4’. In the sentence ‘We identified 1293 patients with complete data and no prior prostate biopsy.’ in this article should have read ‘We identified 1293 patients with complete data.’
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martini, A., Touzani, A., Mazzone, E. et al. Overdiagnosis and stage migration of ISUP 2 disease due to mpMRI-targeted biopsy: facts or fictions. Prostate Cancer Prostatic Dis 25, 794–796 (2022). https://doi.org/10.1038/s41391-022-00606-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-022-00606-6
This article is cited by
-
The impact of mpMRI-targeted vs systematic biopsy on the risk of prostate cancer downgrading at final pathology
World Journal of Urology (2024)
-
Reply to: Fact, overdiagnosis cannot be evaluated by comparing histological grading of prostate biopsy to prostatectomy specimen
Prostate Cancer and Prostatic Diseases (2023)
-
Fact, overdiagnosis cannot be evaluated by comparing histological grading of prostate biopsy to prostatectomy specimen
Prostate Cancer and Prostatic Diseases (2023)