Abstract
Background
There is significant racial disparity in prostate cancer (PCa) in terms of incidence, treatment, and outcomes. Racial diversity and compliance with FDA race reporting guidelines in PCa drug registration trials are unknown. We analyzed racial diversity and race reporting in drug licensing trials for PCa.
Methods
New drug authorizations for PCa from 2006 to 2020 were identified. The corresponding licensing trial publications were analyzed to check compliance with current FDA recommendations for race reporting. If race was unreported, the clinical trial report was analyzed to determine participant recruitment by race and lead the recruiting country.
Results
During the study period, 17 new drug registrations for the management of PCa involving ten unique drugs were identified. In total, 18,455 participants were included in FDA registration trials, of which 76.3% were white or Caucasian, 7.9% Asian, 2.9% Black or African American, 0.5% American Indian or Alaskan Native, 0.1% Native Hawaiian or other Pacific Islander, 1.8% other or multiple races and 10.5% unknown. 53% of trials reported race in the licensing publication, however of this only 55% met current FDA recommendations. When the race was unreported in the licensing publication, 88% of studies had further information in the clinical study report.
Conclusion
We found a significant under-representation of non-white participants in FDA drug registration trials for PCa. Race reporting in licensing publication is inconsistent and both FDA and International Committee of Medical Journal Editors guidelines are not being universally followed. Given the disproportionality of the disease burden of PCa, recruitment of Black and other minority participants to trials should be a research priority.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is the second most common cancer in men and shows significant disproportionally in racial prevalence [1]. In the USA, black men have a PCa incidence of 163.8 per 100,000 men, compared to 96.7 and 52.0 for white and American Indians/Alaskan natives, respectively [2]. Age-adjusted death rates are also highest in black men at 36.4 per 100,000 men, more than double that seen in white men (17.8) [2]. Causes for the racial disparity are likely multifactorial, including environmental, socioeconomic, and the underrepresentation of racial minorities in clinical trials.
Current US Food and Drug Administration (FDA) recommendations for clinical trials, introduced in 2016 recommend race reporting with a minimum of five categories; White, American Indian/Alaskan Native, Asian, Black/African American, and White Hawaiian/Pacific Islander [3]. Implementation and subsequent reporting in licensing publications of this recommendation are unknown. Furthermore, for licensing trials for which the USA is the lead participant contributor, it is unknown if these trials are representative of the PCa population. We analyzed racial representation in PCa registration trials for a period of 15 years (10 years before and 5 years after the introduction of FDA guidance) to analyze compliance with current FDA race reporting recommendations.
Methods
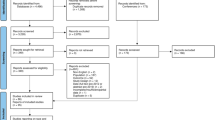
A retrospective review of all new molecular entities and subsequent marketing authorizations of PCa drugs from January 2006 to July 2020 was conducted using the FDA website. Clinical trials cited on the drug licensing label for market authorization were identified using the national clinical trial identifier (NCT). The corresponding licensing trial publication was identified through clinicaltrials.gov or PubMed.
We determined whether race was reported in the corresponding licensing publication, including supplementary appendices and compliance with FDA guidance. If race was unreported or only partially reported (defined ≤3 categories), then the study report on clinicaltrials.gov or FDA website was analyzed. Additional information on participant race and recruitment by lead country was obtained to assess proportional representation based on disease population.
Results
We identified 17 new drug registrations (with corresponding licensing publication) for the management of PCa involving ten unique drugs, including degarelix (one new license), cabazitaxel (two new licenses), denosumab (one new license), abiraterone (three new licenses), enzalutamide (four new licenses), radium-223 (one new license), apalutamide (two new licenses), darolutamide (one new license), rucaparib (one new license) and olaparib (one new license). Table 1 shows further information about licensing indication, clinical trial information, race reporting, and overall racial demographics.
The race was reported in 9 (52.9%) licensing publications. However, 4 (23.5%) provided limited information (e.g., only reporting frequency of Caucasian participants). Two of these trials had further information in the trial report and two had no further data available. For 8 (47.1%) licensing publications where no race information was reported, seven had further information within the trial report. Precise subgroup analysis by race was performed in only 2 (11.7%) studies, however, a further 9 (52.9%) studies did analyze trial participants by recruitment site continent from which some race data could be extrapolated.
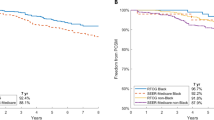
Of the 18,455Footnote 1 participants included in PCa licensing trials, 14,106 (76.3%) were white or Caucasian, 1454 (7.9%) Asian, 528 (2.9%) Black or African American, 88 (0.5%) American Indian or Alaskan Native, 12 (0.1%) Native Hawaiian or other Pacific Islander, 332 (1.8%) other or multiple races and 1949 (10.5%) unknown. Recruitment by country was reported in 11 (64.7%) out of 17 trials. The USA was the leading participant recruiter in 7 (41.1%) trials, which involved 8562 participants. Of which 6998 (81.7%) were white or Caucasian, 641 (7.5%) Asian, 291 (3.4%) Black or African American, 103Footnote 2 (1.2%) other and 529 (6.2%) unknown.
Discussion and conclusion
Guidelines from the International Committee of Medical Journal Editors (ICMJE) recommend, that because the relevance of race is not always known authors should, at a minimum, provide descriptive data. This study found that race reporting in FDA licensing publications does not meet the ICMJE guidance and is reported in only 53% of licensing publicationsFootnote 3. This echoes the overall poor reporting rates observed in other phases 3 PCa clinical trials [4].
Following the introduction of new FDA guidance for race reporting in 2016, the reporting rate is relatively unchanged with 55% of studies meeting requirements. Reporting is also not uniform and harmonization of recording, following FDA guidance would greatly improve population analysis. Furthermore, this is consistent with a recent study by Rencsok et al which found 29 different race or ethnicity categories utilized in 72 PCa prevention, screening, and treatment trials from 1987 to 2016 [5].
A review by the FDA of new drug approvals between 2008 and 2013 found one-fifth of drugs demonstrate differences in exposure and/or response across racial/ethnic groups [3, 6]. Current guidance expects sponsors of clinical trials to enroll participants who reflect the demographics of the clinically relevant populations. This study concurs with the previous findings of significant under-enrollment of non-white participants in PCa trials [4, 5, 7]. Furthermore, in trials in which the USA was the leading participant recruiter, trial populations were not representative of the PCa population. Only one licensing publication acknowledged the underrepresentation of racial groups as a limitation, suggesting that racial disparity in PCa trials needs greater recognition [8]. Overall, there is a preponderance of white participants in PCa trials despite the known racial disproportionality of the disease burden. The recruitment of trial participants reflective of the burden of disease must be a research priority.
Notes
Race reporting in TRITON-2 was not mutually exclusive, thus totals in race breakdown exceed participant total
Includes White Hawaiian/Pacific Islander (ten patients) and American Indian/Alaskan Native (ten patients)
Journals (Journal of Clinical Oncology (n = 2)) and Clinical Cancer Research (n = 1) are not present on the ICJME list of journals stating compliance with guidance (http://icmje.org/journals-following-the-icmje-recommendations/#B)
References
al Hadidi S, Mims M, Miller-Chism CN, Kamble R. Participation of African American persons in clinical trials supporting U.S. food and drug administration approval of cancer drugs. Ann Intern Med. 2020. https://doi.org/10.7326/M20-0410.
USCS. Data visualizations—CDC. https://gis.cdc.gov/Cancer/USCS/DataViz.html. Accessed 28 Aug 2020.
HHS, Fda. Collection of race and ethnicity data in clinical trials guidance for industry and food and drug administration staff clinical medical preface public comment. 2016. http://www.regulations.gov. Accessed 31 Jul 2020.
Balakrishnan AS, Palmer NR, Fergus KB, Gaither TW, Baradaran N, Ndoye M, et al. Minority recruitment trends in phase III prostate cancer clinical trials (2003 to 2014): progress and critical areas for improvement. J Urol. 2019;201:259–67.
Rencsok EM, Bazzi LA, McKay RR, Huang FW, Friedant A, Vinson J, et al. Diversity of enrollment in prostate cancer clinical trials: current status and future directions. Cancer Epidemiol Biomark Prev. 2020;29:1374–80.
Ramamoorthy A, Pacanowski MA, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharmacol Therap. 2015;97:263–73.
Spratt DE, Osborne JR. Disparities in castration-resistant prostate cancer trials. J Clin Oncol. 2015;33:1101–3.
Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235–46.
Author information
Authors and Affiliations
Contributions
Concept and design: MPL. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: all authors. Critical revision of the manuscript: all authors. Administrative, technical, or material support: all authors.
Corresponding author
Ethics declarations
Ethics
Not required as all data available in the public domain (via FDA website).
Conflict of interest
No conflicts of interest declared. MPL and PS have no declarations. VP has received royalties from Johns Hopkins Press and Medscape; has received research funding from Arnold Ventures; has received honoraria for performing Grand Rounds and/or lectures from universities, medical centers, non-profits, and professional societies; has acted as a paid consultant for UnitedHealthcare; and has received speaking fees from eviCore and a plenary session podcast has Patreon backers. JK has received honoraria from Clovis Oncology, Tesaro, and AstraZeneca, outside the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lythgoe, M.P., Krell, J., Savage, P. et al. Race reporting and diversity in US food and drug administration (FDA) registration trials for prostate cancer; 2006–2020. Prostate Cancer Prostatic Dis 24, 1208–1211 (2021). https://doi.org/10.1038/s41391-021-00361-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00361-0
This article is cited by
-
Engaging communities to inform the development of a diverse cohort of cancer survivors: formative research for the eat move sleep study (EMOVES)
Research Involvement and Engagement (2023)
-
Disparities in prostate cancer diagnosis and management: recognizing that disparities exist at all junctures along the prostate cancer journey
Prostate Cancer and Prostatic Diseases (2023)
-
Current Status and Future Direction to Address Disparities in Diversity, Equity, and Inclusion in Prostate Cancer Care
Current Oncology Reports (2023)